Impact of Spray Drying on the Properties of Grape Pomace Extract Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Grape Pomace Drying
2.2. Ultrasound-Assisted Extraction (UAE) of Grape Pomace Anthocyanins
2.3. Production of Anthocyanin Powder by Spray Dryer
2.4. Process Characterization
2.5. Physico-Chemical Properties of the Liquid and Powder Extracts
2.5.1. Anthocyanin Content and Anthocyanin Retention
2.5.2. Moisture Content and Water Activity (aw)
2.5.3. Particle Morphology
2.6. Powder Flowability
2.7. Statistical Analysis
3. Results
3.1. Process Characterization: Outlet Air Temperature and Process Yield
3.2. Powder Characterization
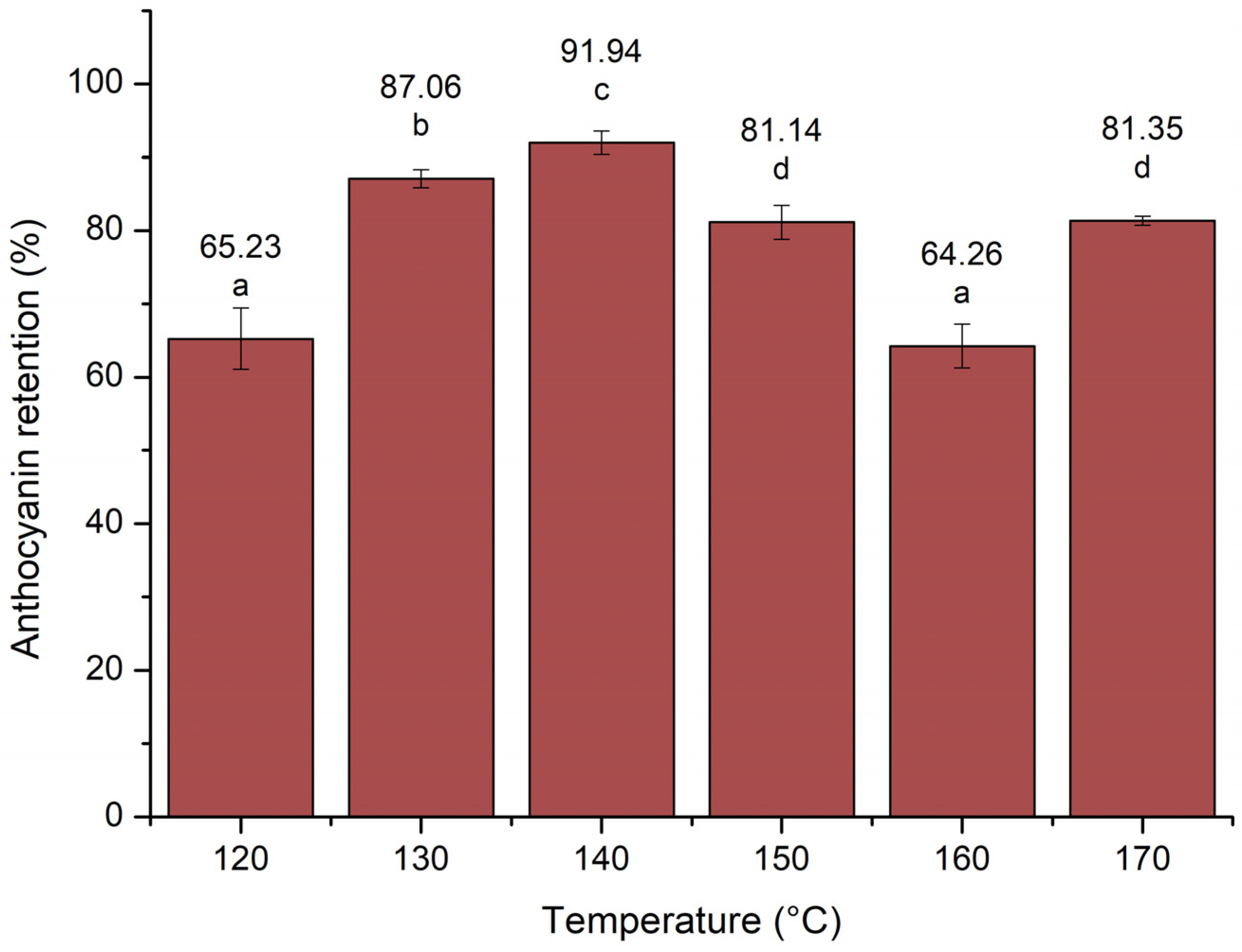
3.2.1. Anthocyanin Retention
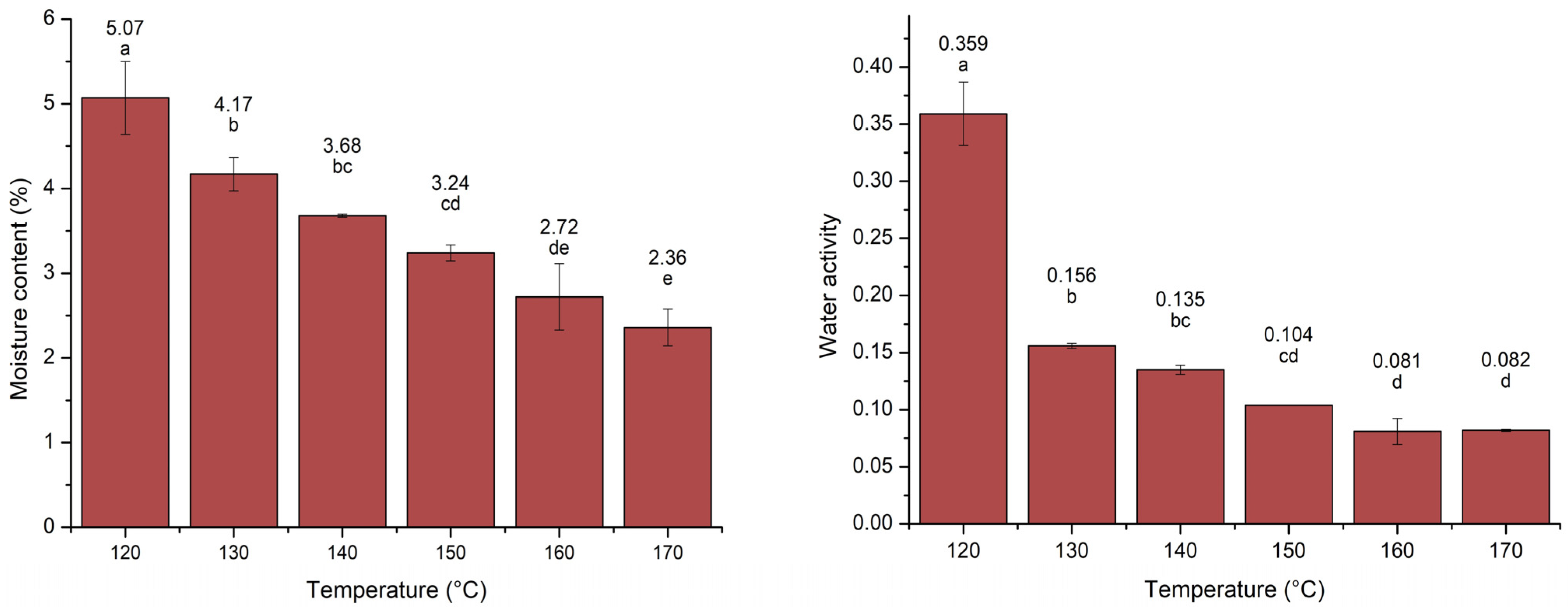
3.2.2. Powder’s Moisture Content and Water Activity (aw)
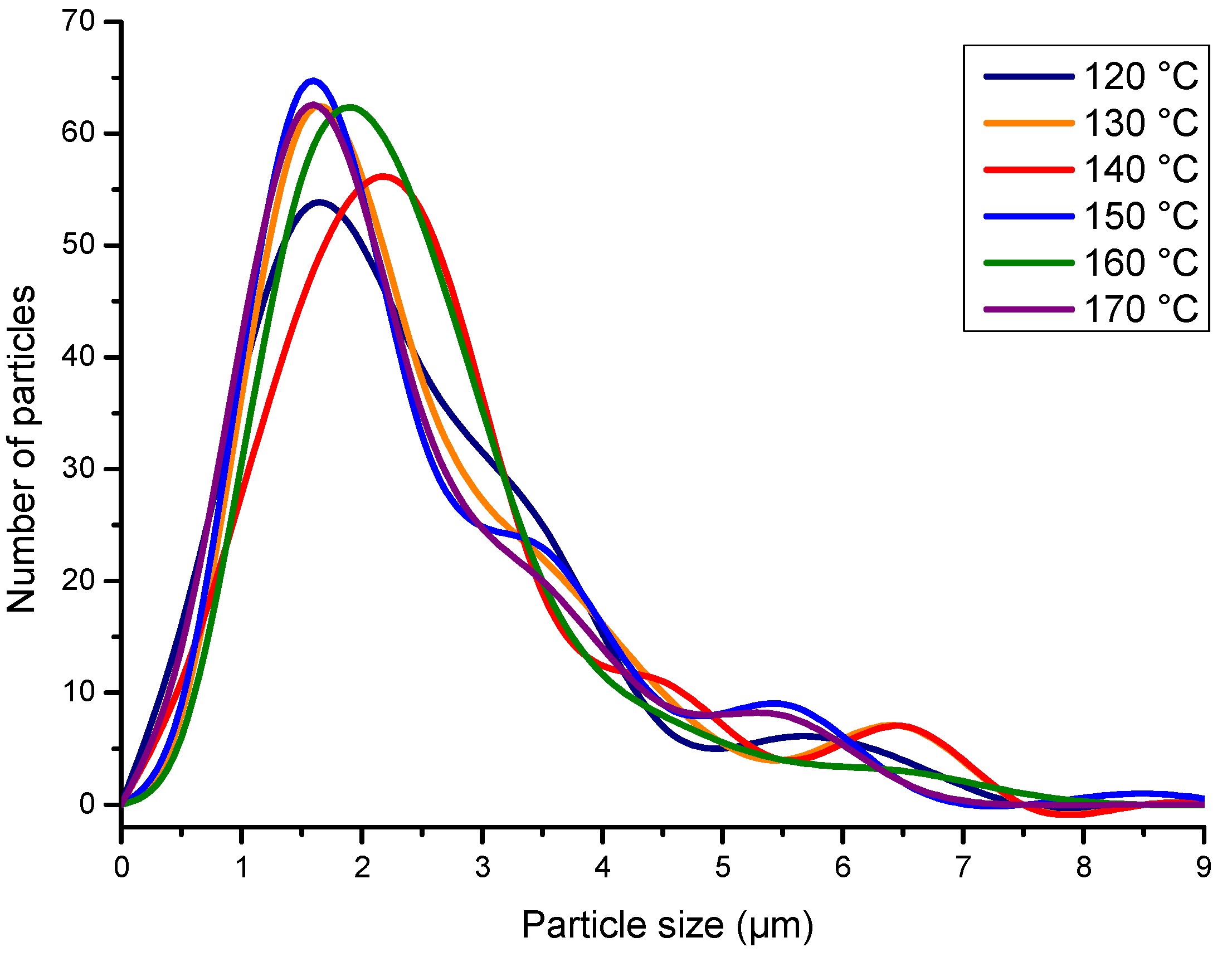
3.2.3. Particle Morphology
3.3. Powder Flowability
3.3.1. Bulk Density
3.3.2. Carr Index (CI), and Hausner Ratio (H.R.)
3.3.3. Flow Index (IF)
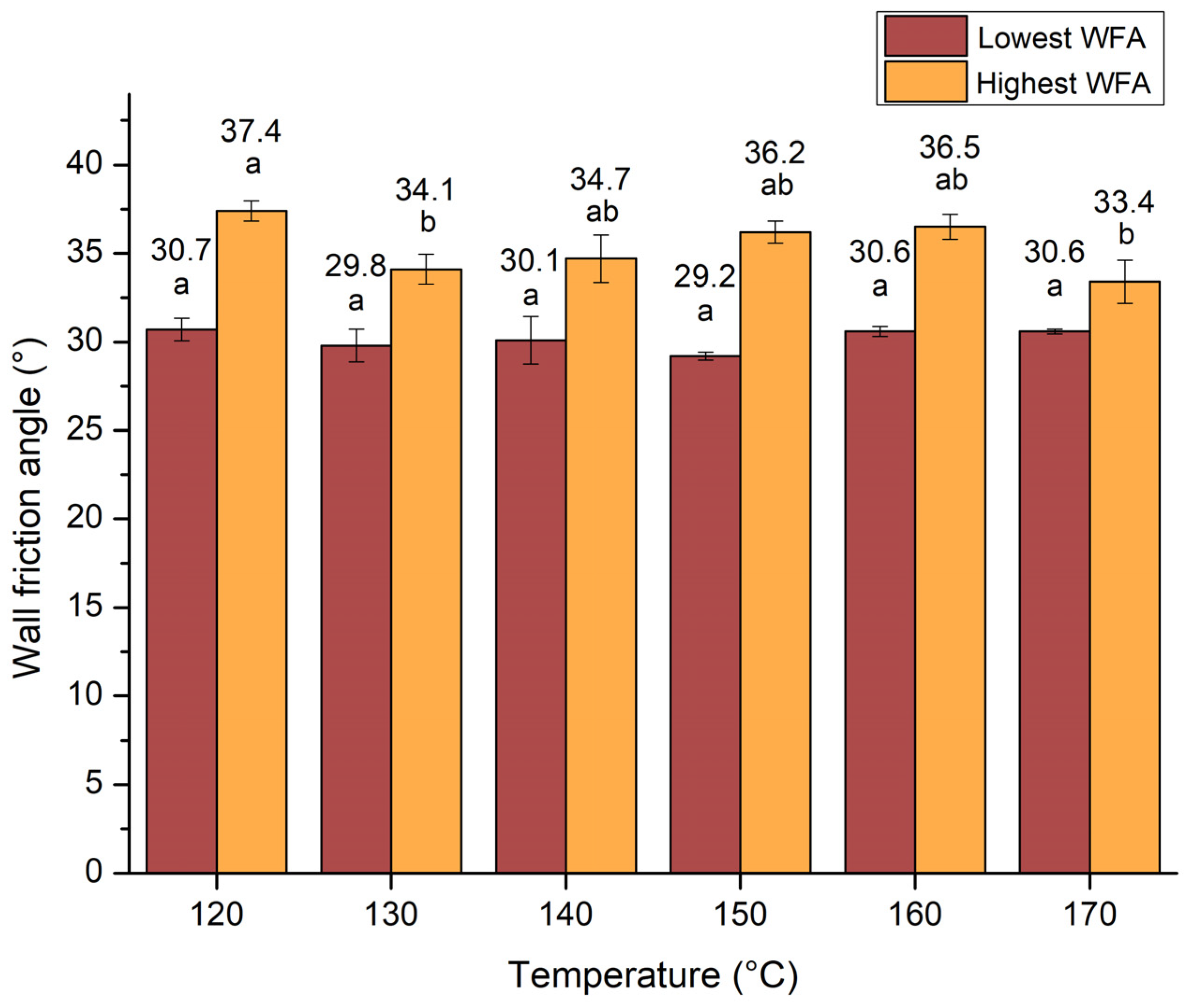
3.3.4. Wall Friction Angles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Todorović, A.; Šturm, L.; Salević-Jelić, A.; Lević, S.; Osojnik Črnivec, I.G.; Prislan, I.; Skrt, M.; Bjeković, A.; Poklar Ulrih, N.; Nedović, V. Encapsulation of Bilberry Extract with Maltodextrin and Gum Arabic by Freeze-Drying: Formulation, Characterisation, and Storage Stability. Processes 2022, 10, 1991. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Alves Rezende, C.; Alexandre Rodrigues, R.; Fernández Barbero, G.; de Tarso Vieira e Rosa, P.; Martínez, J. Encapsulation of Anthocyanin-Rich Extract from Blackberry Residues by Spray-Drying, Freeze-Drying and Supercritical Antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Feitosa, B.F.; Decker, B.L.A.; de Brito, E.S.; Rodrigues, S.; Mariutti, L.R.B. Microencapsulation of Anthocyanins as Natural Dye Extracted from Fruits—A Systematic Review. Food Chem. 2023, 424, 136361. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of Grape Pomace: Drying Behavior and Ultrasound Extraction of Phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Coelho, M.C.; Ghalamara, S.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Innovation and Winemaking By-Product Valorization: An Ohmic Heating Approach. Processes 2023, 11, 495. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P.; Mcphee, D.J. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Classification of Fruits Based on Anthocyanin Types and Relevance to Their Health Effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ju, H.; Xu, G.-S.; Wu, Y.-C.; Chen, X.; Li, H.-J. Recent Development of Carrier Materials in Anthocyanins Encapsulation Applications: A Comprehensive Literature Review. Food Chem. 2024, 439, 138104. [Google Scholar] [CrossRef] [PubMed]
- Millinia, B.L.; Mashithah, D.; Nawatila, R.; Kartini, K. Microencapsulation of Roselle (Hibiscus sabdariffa L.) Anthocyanins: Effects of Maltodextrin and Trehalose Matrix on Selected Physicochemical Properties and Antioxidant Activities of Spray-Dried Powder. Future Foods 2024, 9, 100300. [Google Scholar] [CrossRef]
- Silva, J.T.d.P.; Borges, M.H.; de Souza, C.A.C.; Fávaro-Trindade, C.S.; Sobral, P.J.d.A.; de Oliveira, A.L.; Martelli-Tosi, M. Grape Pomace Rich-Phenolics and Anthocyanins Extract: Production by Pressurized Liquid Extraction in Intermittent Process and Encapsulation by Spray-Drying. Foods 2024, 13, 279. [Google Scholar] [CrossRef]
- Machado, M.H.; da Rosa Almeida, A.; Maciel, M.V.d.O.B.; Vitorino, V.B.; Bazzo, G.C.; da Rosa, C.G.; Sganzerla, W.G.; Mendes, C.; Barreto, P.L.M. Microencapsulation by Spray Drying of Red Cabbage Anthocyanin-Rich Extract for the Production of a Natural Food Colorant. Biocatal. Agric. Biotechnol. 2022, 39, 102287. [Google Scholar] [CrossRef]
- Almeida, R.F.; Gomes, M.H.G.; Kurozawa, L.E. Rice Bran Protein Increases the Retention of Anthocyanins by Acting as an Encapsulating Agent in the Spray Drying of Grape Juice. Food Res. Int. 2023, 172, 113237. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.I.; Chen, W. Trends of Spray Drying: A Critical Review on Drying of Fruit and Vegetable Juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- Chuwattanakula, V.; Yaviracha, K.; Eiamsa-ardb, S. Influences of Spray Drying Conditions on the Physiochemical Properties of Karanda Fruit. Chem. Eng. Trans. 2023, 102, 223–230. [Google Scholar] [CrossRef]
- Rosales-Chimal, S.; Navarro-Cortez, R.O.; Bello-Perez, L.A.; Vargas-Torres, A.; Palma-Rodríguez, H.M. Optimal Conditions for Anthocyanin Extract Microencapsulation in Taro Starch: Physicochemical Characterization and Bioaccessibility in Gastrointestinal Conditions. Int. J. Biol. Macromol. 2023, 227, 83–92. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.B.; Thomazini, M.; Balieiro, J.C.d.C.; Fávaro-Trindade, C.S. Effect of Spray Drying on the Physicochemical Properties and Color Stability of the Powdered Pigment Obtained from Vinification Byproducts of the Bordo Grape (Vitis labrusca). Food Bioprod. Process 2015, 93, 39–50. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Dupas, J.; Baldeweck, F.; Meunier, V. Experimental Study on the Impact of Key Material Properties on Flowability of Sucrose and Maltodextrin. J. Food Eng. 2024, 364, 111802. [Google Scholar] [CrossRef]
- Lima, A.C.d.S.; Afonso, M.R.A.; Rodrigues, S.; de Aquino, A.C. Flowability of Spray-dried Sapodilla Pulp Powder. J. Food Process Eng. 2022, 45, e14092. [Google Scholar] [CrossRef]
- Silva, M.F.S.; Silva, L.M.A.; Quintela, A.L.; dos Santos, A.G.; Silva, F.A.N.; de Oliveira, F.d.C.E.; Alves Filho, E.G.; de Brito, E.S.; Canuto, K.M.; Pessoa, C.; et al. UPLC-HRMS and NMR Applied in the Evaluation of Solid-Phase Extraction Methods as a Rational Strategy of Dereplication of Phyllanthus spp. Aiming at the Discovery of Cytotoxic Metabolites. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1120, 51–61. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Chandra Singh, M.; Price, W.E.; Kelso, C.; Charlton, K.; Probst, Y. Impact of Molar Absorbance on Anthocyanin Content of the Foods. Food Chem. 2022, 386, 132855. [Google Scholar] [CrossRef]
- Nixdorf, S.L.; Hermosín-Gutiérrez, I. Brazilian Red Wines Made from the Hybrid Grape Cultivar Isabel: Phenolic Composition and Antioxidant Capacity. Anal. Chim. Acta 2010, 659, 208–215. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of Ultrasound Processing on Anthocyanins and Color of Red Grape Juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.A.M.; Tucker, I.G.; Doyle, C.S.; Denman, J.A.; Das, S.C. Manipulation of Spray-Drying Conditions to Develop Dry Powder Particles with Surfaces Enriched in Hydrophobic Material to Achieve High Aerosolization of a Hygroscopic Drug. Int. J. Pharm. 2018, 543, 318–327. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating Flow Properties of Solids. Chem. Eng. J. 1965, 72, 163–168. [Google Scholar]
- Hausner, H.H. Friction Conditions in a Mass of Metal Powder. J. Powder Metall. 1967, 3, 7–13. [Google Scholar]
- Lopes Neto, J.P.; do Nascimento, J.W.B.; da Silva, V.R.; Lopes, F.F.d.M. Propriedade de Fluxo e Característica de Escoabilidade de Rações Avícolas Para Dimensionamento de Silos. Ciência Agrotecnol. 2007, 31, 851–859. [Google Scholar] [CrossRef][Green Version]
- Goula, A.M.; Adamopoulos, K.G. Spray Drying of Tomato Pulp in Dehumidified Air: I. The Effect on Product Recovery. J. Food Eng. 2005, 66, 25–34. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, M.M.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 2019, 8914852. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Spray Drying Performance of a Laboratory Spray Dryer for Tomato Powder Preparation. Dry. Technol. 2003, 21, 1273–1289. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems Associated with Spray Drying of Sugar-Rich Foods. Dry. Technol. 1997, 15, 671–684. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghalegi Ghalenoei, M.; Dehnad, D. Influence of Spray Drying on Water Solubility Index, Apparent Density, and Anthocyanin Content of Pomegranate Juice Powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Laokuldilok, T.; Kanha, N. Effects of Processing Conditions on Powder Properties of Black Glutinous Rice (Oryza sativa L.) Bran Anthocyanins Produced by Spray Drying and Freeze Drying. LWT-Food Sci. Technol. 2015, 64, 405–411. [Google Scholar] [CrossRef]
- Shrestha, A.K.; Ua-arak, T.; Adhikari, B.P.; Howes, T.; Bhandari, B.R. Glass Transition Behavior of Spray Dried Orange Juice Powder Measured by Differential Scanning Calorimetry (DSC) and Thermal Mechanical Compression Test (TMCT). Int. J. Food Prop. 2007, 10, 661–673. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of Spray Drying Conditions and Feed Composition on the Physical Properties of Black Mulberry Juice Powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; de Oliveira, I.R.N. Parameter Optimization for Spray-Drying Microencapsulation of Jaboticaba (Myrciaria jaboticaba) Peel Extracts Using Simultaneous Analysis of Responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- Lu, W.; Yang, X.; Shen, J.; Li, Z.; Tan, S.; Liu, W.; Cheng, Z. Choosing the Appropriate Wall Materials for Spray-Drying Microencapsulation of Natural Bioactive Ingredients: Taking Phenolic Compounds as Examples. Powder Technol. 2021, 394, 562–574. [Google Scholar] [CrossRef]
- Furuta, T.; Neoh, T.L. Microencapsulation of Food Bioactive Components by Spray Drying: A Review. Dry. Technol. 2021, 39, 1800–1831. [Google Scholar] [CrossRef]
- Santos, S.S.; Rodrigues, L.M.; Costa, S.C.; Madrona, G.S. Antioxidant Compounds from Blackberry (Rubus fruticosus) Pomace: Microencapsulation by Spray-Dryer and PH Stability Evaluation. Food Packag. Shelf Life 2019, 20, 100177. [Google Scholar] [CrossRef]
- Betz, M.; Kulozik, U. Whey Protein Gels for the Entrapment of Bioactive Anthocyanins from Bilberry Extract. Int. Dairy. J. 2011, 21, 703–710. [Google Scholar] [CrossRef]
- Koh, J.; Xu, Z.; Wicker, L. Blueberry Pectin and Increased Anthocyanins Stability under in Vitro Digestion. Food Chem. 2020, 302, 125343. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Dadmohammadi, Y.; Lee, M.C.; Abbaspourrad, A. Combination of Copigmentation and Encapsulation Strategies for the Synergistic Stabilization of Anthocyanins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3164–3191. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Cánovas, G.V.; Fontana, A.J.; Schmidt, S.J.; Labuza, T.P. (Eds.) Water Activity in Foods; Wiley: Hoboken, NJ, USA, 2007; ISBN 9780813824086. [Google Scholar]
- Tonon, R.V.; Freitas, S.S.; Hubinger, M.D. Spray Drying of Açai (Euterpe oleraceae Mart.) Juice: Effect of Inlet Air Temperature and Type of Carrier Agent. J. Food Process Preserv. 2011, 35, 691–700. [Google Scholar] [CrossRef]
- Nguyen, Q.; Dang, T.; Nguyen, T.; Nguyen, T.; Nguyen, N. Microencapsulation of Roselle (Hibiscus sabdariffa L.) Anthocyanins: Effects of Drying Conditions on Some Physicochemical Properties and Antioxidant Activities of Spray-dried Powder. Food Sci. Nutr. 2022, 10, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Şahin-Nadeem, H.; Dinçer, C.; Torun, M.; Topuz, A.; Özdemir, F. Influence of Inlet Air Temperature and Carrier Material on the Production of Instant Soluble Sage (Salvia fruticosa Miller) by Spray Drying. LWT-Food Sci. Technol. 2013, 52, 31–38. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; de Aguirre, J.M. Effects of Spray-Drying Conditions on the Physicochemical Properties of Blackberry Powder. Dry. Technol. 2012, 30, 154–163. [Google Scholar] [CrossRef]
- da Silva Carvalho, A.G.; da Costa Machado, M.T.; da Silva, V.M.; Sartoratto, A.; Rodrigues, R.A.F.; Hubinger, M.D. Physical Properties and Morphology of Spray Dried Microparticles Containing Anthocyanins of Jussara (Euterpe edulis Martius) Extract. Powder Technol. 2016, 294, 421–428. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Y.; Zhu, L.; Fang, Z.; Shi, Q. Effect of Carrier Types on the Physicochemical and Antioxidant Properties of Spray-Dried Black Mulberry Juice Powders. J. Food Meas. Charact. 2020, 14, 1201–1212. [Google Scholar] [CrossRef]
- Gawałek, J. Spray Drying of Chokeberry Juice—Antioxidant Phytochemicals Retention in the Obtained Powders versus Energy Consumption of the Process. Foods 2022, 11, 2898. [Google Scholar] [CrossRef]
- Bednarska, M.A.; Janiszewska-Turak, E. The Influence of Spray Drying Parameters and Carrier Material on the Physico-Chemical Properties and Quality of Chokeberry Juice Powder. J. Food Sci. Technol. 2020, 57, 564–577. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Dellarosa, N.; Tylewicz, U.; Laghi, L.; Romani, S.; Dalla Rosa, M.; Witrowa-Rajchert, D. The Influence of Carrier Material on Some Physical and Structural Properties of Carrot Juice Microcapsules. Food Chem. 2017, 236, 134–141. [Google Scholar] [CrossRef]
- Moreira, G.É.G.; Maia Costa, M.G.; de Souza, A.C.R.; de Brito, E.S.; de Medeiros, M.d.F.D.; de Azeredo, H.M.C. Physical Properties of Spray Dried Acerola Pomace Extract as Affected by Temperature and Drying Aids. LWT-Food Sci. Technol. 2009, 42, 641–645. [Google Scholar] [CrossRef]
- Lumay, G.; Boschini, F.; Traina, K.; Bontempi, S.; Remy, J.-C.; Cloots, R.; Vandewalle, N. Measuring the Flowing Properties of Powders and Grains. Powder Technol. 2012, 224, 19–27. [Google Scholar] [CrossRef]
- Suhag, R.; Kellil, A.; Razem, M. Factors Influencing Food Powder Flowability. Powders 2024, 3, 65–76. [Google Scholar] [CrossRef]
- Nayak, C.A.; Rastogi, N.K. Effect of Selected Additives on Microencapsulation of Anthocyanin by Spray Drying. Dry. Technol. 2010, 28, 1396–1404. [Google Scholar] [CrossRef]
- Righi da Rosa, J.; Nunes, G.L.; Motta, M.H.; Fortes, J.P.; Cezimbra Weis, G.C.; Rychecki Hecktheuer, L.H.; Muller, E.I.; Ragagnin de Menezes, C.; Severo da Rosa, C. Microencapsulation of Anthocyanin Compounds Extracted from Blueberry (Vaccinium spp.) by Spray Drying: Characterization, Stability and Simulated Gastrointestinal Conditions. Food Hydrocoll. 2019, 89, 742–748. [Google Scholar] [CrossRef]
- Shah, D.S.; Moravkar, K.K.; Jha, D.K.; Lonkar, V.; Amin, P.D.; Chalikwar, S.S. A Concise Summary of Powder Processing Methodologies for Flow Enhancement. Heliyon 2023, 9, e16498. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of Natural Dyes with Biopolymers for Application in Food: A Review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Landillon, V.; Cassan, D.; Morel, M.-H.; Cuq, B. Flowability, Cohesive, and Granulation Properties of Wheat Powders. J. Food Eng. 2008, 86, 178–193. [Google Scholar] [CrossRef]
- Ribeiro, L.C.; da Costa, J.M.C.; Afonso, M.R.A. Flow Behavior of Cocoa Pulp Powder Containing Maltodextrin. Braz. J. Food Technol. 2020, 23, e2020034. [Google Scholar] [CrossRef]
- Jenike, A.W. Storage and Flow of Solids. Bulletin No. 123; Utah State University: Salt Lake City, UT, USA, 1964; Volume 53. [Google Scholar]
- Maciel, R.M.G.; Lima, S.B.; Costa, J.M.C.; Afonso, M.R.A. Influência Da Maltodextrina Nas Propriedades de Escoamento Do Pó Da Polpa de Cupuaçu. Braz. J. Dev. 2020, 6, 5829–5839. [Google Scholar] [CrossRef]
- Li, Q.; Rudolph, V.; Weigl, B.; Earl, A. Interparticle van Der Waals Force in Powder Flowability and Compactibility. Int. J. Pharm. 2004, 280, 77–93. [Google Scholar] [CrossRef]
- Rigolon, T.C.B.; Silva, R.R.A.; de Oliveira, T.V.; Nascimento, A.L.A.A.; de Barros, F.A.R.; Martins, E.; Campelo, P.H.; Stringheta, P.C. Exploring Anthocyanins-Polysaccharide Synergies in Microcapsule Wall Materials via Spray Drying: Interaction Characterization and Evaluation of Particle Stability. Meas. Food 2024, 13, 100126. [Google Scholar] [CrossRef]
- Turker, N.; Aksay, S.; Ekiz, H.I. Effect of Storage Temperature on the Stability of Anthocyanins of a Fermented Black Carrot (Daucus carota var. L.) Beverage: Shalgam. J. Agric. Food Chem. 2004, 52, 3807–3813. [Google Scholar] [CrossRef]
- Fitzpatrick, J.J.; Barry, K.; Cerqueira, P.S.M.; Iqbal, T.; O’Neill, J.; Roos, Y.H. Effect of Composition and Storage Conditions on the Flowability of Dairy Powders. Int. Dairy. J. 2007, 17, 383–392. [Google Scholar] [CrossRef]
- Afonso, M.R.A.; Rodrigues, B.K.M.; da Costa, J.M.C.; Rybka, A.C.P.; Wurlitzer, N.J. Microstructure and Flow Properties of Lyophilized Mango Pulp with Maltodextrin. Rev. Bras. Eng. Agrícola Ambient. 2019, 23, 133–137. [Google Scholar] [CrossRef]
- Lee, Y.J.; Yoon, W.B. Flow Behavior and Hopper Design for Black Soybean Powders by Particle Size. J. Food Eng. 2015, 144, 10–19. [Google Scholar] [CrossRef]


| Flowability | Compressibility Index (%) | Hausner Ratio |
|---|---|---|
| Excellent | <10 | 1.00–1.11 |
| Good | 11–15 | 1.12–1.18 |
| Adequate | 16–20 | 1.19–1.25 |
| Acceptable | 21–25 | 1.26–1.34 |
| Difficult | 26–30 | 1.35–1.45 |
| Very difficult | 32–37 | 1.46–1.59 |
| Excessively difficult | >38 | >1.60 |
| Inlet Air Temperature (°C) | Outlet Air Temperature (°C) | Process Yield (%) |
|---|---|---|
| 120 | 68.2 | 45.35 ± 2.75 |
| 130 | 76 | 43.02 ± 2.67 |
| 140 | 82.4 | 50.00 ± 3.06 |
| 150 | 90 | 45.35 ± 2.34 |
| 160 | 97.6 | 48.84 ± 3.80 |
| 170 | 103.5 | 48.84 ± 3.26 |
| Inlet Air Temperature (°C) | Carr Index (%) | Hausner Ratio |
|---|---|---|
| 120 | 44.99 ± 4.24 a | 1.82 ± 0.25 a |
| 130 | 43.58 ± 3.48 a | 1.77 ± 0.21 a |
| 140 | 45.25 ± 2.97 a | 1.83 ± 0.18 a |
| 150 | 43.91 ± 2.73 a | 1.78 ± 0.17 a |
| 160 | 42.29 ± 4.08 a | 1.73 ± 0.23 a |
| 170 | 48.07 ± 4.20 a | 1.93 ± 0.24 a |
| Inlet Air Temperature (°C) | |||||
|---|---|---|---|---|---|
| T = 120 °C | T = 130 °C | T = 140 °C | |||
| δc | δ1 | δc | δ1 | δc | δ1 |
| 1.674 | 1.338 | 1.360 | 1.351 | 1.350 | 1.261 |
| 3.014 | 2.635 | 2.936 | 2.529 | 2.808 | 2.291 |
| 5.819 | 4.044 | 5.746 | 4.249 | 5.608 | 3.907 |
| 11.714 | 7.511 | 11.654 | 7.575 | 11.279 | 6.992 |
| 23.596 | 12.781 | 22.964 | 13.528 | 22.853 | 11.877 |
| IF | 1.618 | IF | 1.528 | IF | 1.667 |
| Inlet Air Temperature (°C) | |||||
| T = 150 °C | T = 160 °C | T = 170 °C | |||
| δc | δ1 | δc | δ1 | δc | δ1 |
| 1.338 | 1.346 | 1.342 | 1.273 | 1.331 | 1.262 |
| 2.843 | 2.775 | 2.775 | 2.219 | 2.813 | 2.2 |
| 5.765 | 5.685 | 5.685 | 3.952 | 5.683 | 4.053 |
| 11.702 | 11.376 | 11.376 | 6.911 | 11.381 | 7.184 |
| 23.570 | 22.735 | 22.735 | 11.745 | 22.641 | 12.106 |
| IF | 1.611 | IF | 1.682 | IF | 1.636 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decker, B.L.A.; Miguel, E.d.C.; Fonteles, T.V.; Fernandes, F.A.N.; Rodrigues, S. Impact of Spray Drying on the Properties of Grape Pomace Extract Powder. Processes 2024, 12, 1390. https://doi.org/10.3390/pr12071390
Decker BLA, Miguel EdC, Fonteles TV, Fernandes FAN, Rodrigues S. Impact of Spray Drying on the Properties of Grape Pomace Extract Powder. Processes. 2024; 12(7):1390. https://doi.org/10.3390/pr12071390
Chicago/Turabian StyleDecker, Betina Louise Angioletti, Emilio de Castro Miguel, Thatyane Vidal Fonteles, Fabiano A. N. Fernandes, and Sueli Rodrigues. 2024. "Impact of Spray Drying on the Properties of Grape Pomace Extract Powder" Processes 12, no. 7: 1390. https://doi.org/10.3390/pr12071390
APA StyleDecker, B. L. A., Miguel, E. d. C., Fonteles, T. V., Fernandes, F. A. N., & Rodrigues, S. (2024). Impact of Spray Drying on the Properties of Grape Pomace Extract Powder. Processes, 12(7), 1390. https://doi.org/10.3390/pr12071390










