Use of Cold Plasma as an Alternative to Improve Corn Starch-Based Films: Effect of the Plasma Application Strategy
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Plasma Application
2.3. Starch-Based Film Preparation
2.4. Characterization of the Film Properties
2.4.1. Solubility and Water Absorption Index (WAI)
2.4.2. Hydrophobicity
2.4.3. Amylose Content
2.4.4. Chemical Groups and Molecular Structure
2.4.5. Surface Morphology
2.5. Statistical Analysis
3. Results and Discussion
3.1. Amylose Content
3.2. Solubility and Water Absorbance Index
3.3. Hydrophobicity
3.4. Molecular Structure
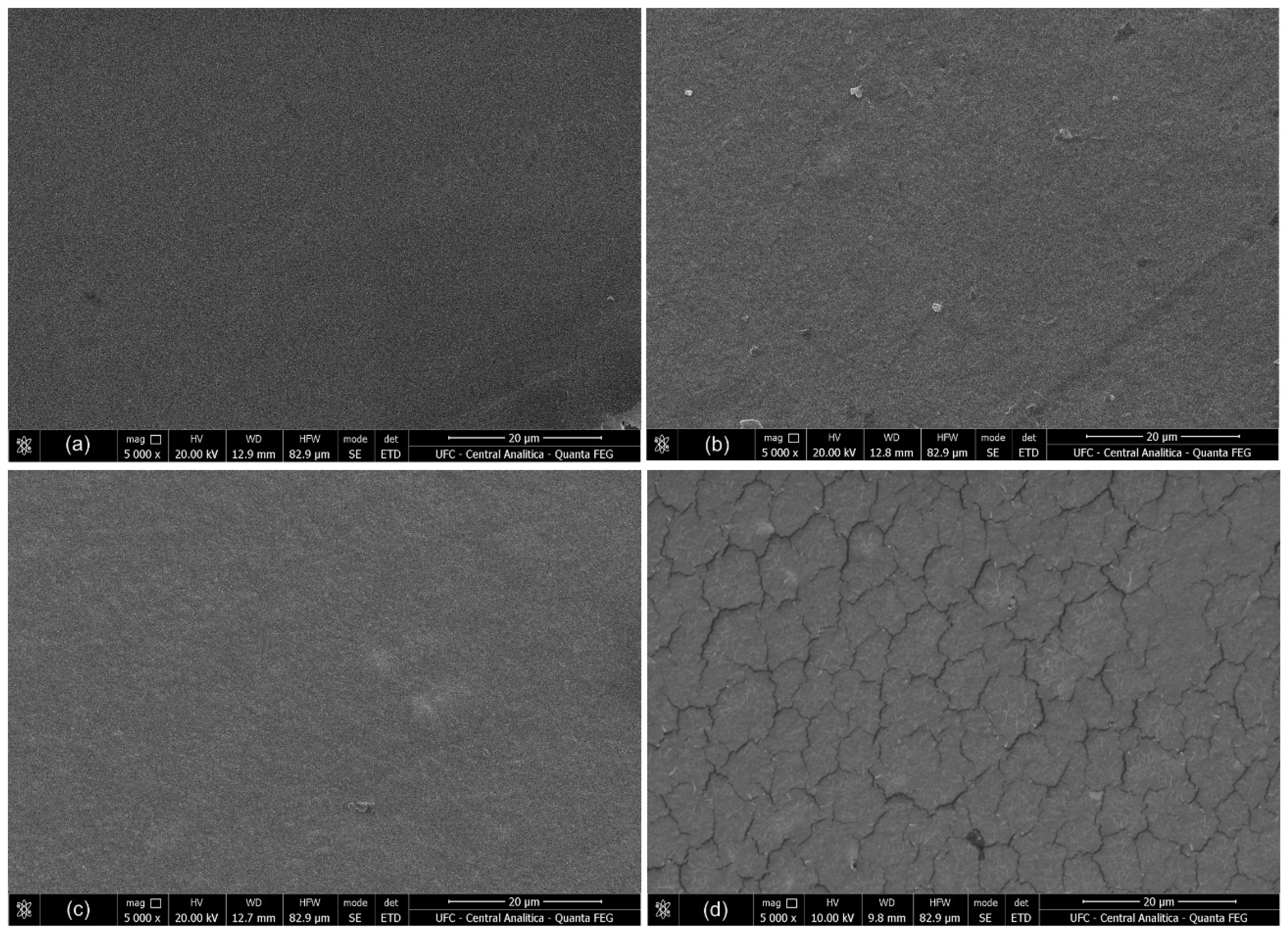
3.5. Surface Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, G.; Yang, B.; Wang, J.; Zhang, Y.; Li, S.; Chen, Y. Facile Production of Low-viscosity Mung Bean Starch through Cold Plasma Treatment: Improvement of Film Formability. J. Food Sci. 2023, 88, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Okyere, A.Y.; Rajendran, S.; Annor, G.A. Cold Plasma Technologies: Their Effect on Starch Properties and Industrial Scale-up for Starch Modification. Curr. Res. Food Sci. 2022, 5, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S. Major Factors Affecting the Characteristics of Starch Based Biopolymer Films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Wan, S.; Liu, Q.; Yang, D.; Guo, P.; Gao, Y.; Mo, R.; Zhang, Y. Characterization of High Amylose Corn Starch-Cinnamaldehyde Inclusion Films for Food Packaging. Food Chem. 2022, 403, 134219. [Google Scholar] [CrossRef] [PubMed]
- Marenco-Orozco, G.A.; Rosa, M.F.; Fernandes, F.A.N. Effects of Multiple-step Cold Plasma Processing on Banana (Musa sapientum) Starch-based Films. Packag. Technol. Sci. 2022, 35, 589–601. [Google Scholar] [CrossRef]
- Wang, J.; Guo, K.; Fan, X.; Feng, G.; Wei, C. Physicochemical Properties of C-Type Starch from Root Tuber of Apios fortunei in Comparison with Maize, Potato, and Pea Starches. Molecules 2018, 23, 2132. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.M.G.; Barros, D.R.; da Silva, L.S.; Sanches, E.A.; da Costa Pinto, C.; de Souza, S.M.; Clerici, M.T.P.S.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Dielectric Barrier Atmospheric Cold Plasma Applied to the Modification of Ariá (Goeppertia allouia) Starch: Effect of Plasma Generation Voltage. Int. J. Biol. Macromol. 2021, 182, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.V.; da Silva, A.P.M.; Barros, M.O.; de sá, M. Souza Filho, M.; Rosa, M.F.; Azeredo, H.M.C. Nanocomposite Films from Mango Kernel or Corn Starch with Starch Nanocrystals. Starch Stärke 2018, 70, 1800028. [Google Scholar] [CrossRef]
- Luo, W.; Li, B.; Zhang, Y.; Tan, L.; Hu, C.; Huang, C.; Chen, Z.; Huang, L. Unveiling the Retrogradation Mechanism of a Novel High Amylose Content Starch-Pouteria Campechiana Seed. Food Chem. X 2023, 18, 100637. [Google Scholar] [CrossRef]
- Mehboob, S.; Ali, T.M.; Sheikh, M.; Hasnain, A. Effects of Cross Linking and/or Acetylation on Sorghum Starch and Film Characteristics. Int. J. Biol. Macromol. 2020, 155, 786–794. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Stability and Composting Behaviour of PLA–Starch Laminates Containing Active Extracts and Cellulose Fibres from Rice Straw. Polymers 2024, 16, 1474. [Google Scholar] [CrossRef]
- Otálora González, C.M.; Felix, M.; Bengoechea, C.; Flores, S.; Gerschenson, L.N. Development and Characterization of Edible Films Based on Cassava Starch Modified by Corona Treatment. Foods 2024, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Krümmel, A.; Pagno, C.H.; Malheiros, P.d.S. Active Films of Cassava Starch Incorporated with Carvacrol Nanocapsules. Foods 2024, 13, 1141. [Google Scholar] [CrossRef]
- Mangaraj, S.; Thakur, R.R.; Yadav, A. Development and Characterization of PLA and Cassava Starch-based Novel Biodegradable Film Used for Food Packaging Application. J. Food Process. Preserv. 2022, 46, e16314. [Google Scholar] [CrossRef]
- Beuter, D.A.; Meza, B.E.; Brumovsky, L.A.; Peralta, J.M. Effect of Yerba Mate (Ilex paraguariensis St. Hil.) Extract on the Drying Behavior of Cassava Starch Films Enriched with Rebaudioside A. J. Food Process. Preserv. 2022, 46, e17159. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Q.; Luo, Y.; Murad, M.S.; Zhu, L.; Mu, G. Improved Packing Performance and Structure-Stability of Casein Edible Films by Dielectric Barrier Discharges (DBD) Cold Plasma. Food Packag. Shelf Life 2020, 24, 100471. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljevic, V.; O’Donnell, C.P.; Bourke, P.; Cullen, P.J. Applications of Cold Plasma Technology in Food Packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Pankaj, S.K.K.; Bueno-Ferrer, C.; Misra, N.N.N.; O’Neill, L.; Tiwari, B.K.K.; Bourke, P.; Cullen, P.J.J. Dielectric Barrier Discharge Atmospheric Air Plasma Treatment of High Amylose Corn Starch Films. LWT—Food Sci. Technol. 2015, 63, 1076–1082. [Google Scholar] [CrossRef]
- Hernandez-Perez, P.; Flores-Silva, P.C.; Velazquez, G.; Morales-Sanchez, E.; Rodríguez-Fernández, O.; Hernández-Hernández, E.; Mendez-Montealvo, G.; Sifuentes-Nieves, I. Rheological Performance of Film-Forming Solutions Made from Plasma-Modified Starches with Different Amylose/Amylopectin Content. Carbohydr. Polym. 2021, 255, 117349. [Google Scholar] [CrossRef]
- Sifuentes-Nieves, I.; Hernández-Hernández, E.; Neira-Velázquez, G.; Morales-Sánchez, E.; Mendez-Montealvo, G.; Velazquez, G. Hexamethyldisiloxane Cold Plasma Treatment and Amylose Content Determine the Structural, Barrier and Mechanical Properties of Starch-Based Films. Int. J. Biol. Macromol. 2019, 124, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gou, Q.; Yang, L.; Yu, Q.; Han, L. Dielectric Barrier Discharge Plasma: A Green Method to Change Structure of Potato Starch and Improve Physicochemical Properties of Potato Starch Films. Food Chem. 2022, 370, 130992. [Google Scholar] [CrossRef] [PubMed]
- Goiana, M.L.; de Brito, E.S.; Alves Filho, E.G.; de Castro Miguel, E.; Fernandes, F.A.N.; de Azeredo, H.M.C.; de Freitas Rosa, M. Corn Starch Based Films Treated by Dielectric Barrier Discharge Plasma. Int. J. Biol. Macromol. 2021, 183, 2009–2016. [Google Scholar] [CrossRef]
- Fang, C.; Huang, J.; Pu, H.; Yang, Q.; Chen, Z.; Zhu, Z. Cold-Water Solubility, Oil-Adsorption and Enzymolysis Properties of Amorphous Granular Starches. Food Hydrocoll. 2021, 117, 106669. [Google Scholar] [CrossRef]
- D5725-99; Standard Test Method for Surface Wettability and Adsorbency of Sheeted Materials Using an Automated Contact Angle Tester. ASTM: West Conshohocken, PA, USA, 2008.
- Hu, J.; Cheng, F.; Lin, Y.; Zhao, K.; Zhu, P. Dissolution of Starch in Urea/NaOH Aqueous Solutions. J. Appl. Polym. Sci. 2016, 133, 43390. [Google Scholar] [CrossRef]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared Spectroscopy as a Tool to Characterise Starch Ordered Structure—A Joint FTIR–ATR, NMR, XRD and DSC Study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Tiwari, B.K.; Brito, E.S.; Rodrigues, S.; Fernandes, F.A.N. Evaluation of Plasma, High-Pressure and Ultrasound Processing on the Stability of Fructooligosaccharides. Int. J. Food Sci. Technol. 2016, 51, 2034–2040. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Gomes, W.F.; Cavalcante, R.S.; Tiwari, B.K.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.N.; Rodrigues, S. Fructooligosaccharides Integrity after Atmospheric Cold Plasma and High-Pressure Processing of a Functional Orange Juice. Food Res. Int. 2017, 102, 282–290. [Google Scholar] [CrossRef]
- Talja, R.A.; Peura, M.; Serimaa, R.; Jouppila, K. Effect of Amylose Content on Physical and Mechanical Properties of Potato-Starch-Based Edible Films. Biomacromolecules 2008, 9, 658–663. [Google Scholar] [CrossRef]
- Wu, W.-C.; Hsiao, P.-Y.; Huang, Y.-C. Effects of Amylose Content on Starch-Chitosan Composite Film and Its Application as a Wound Dressing. J. Polym. Res. 2019, 26, 137. [Google Scholar] [CrossRef]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Physical Action of Nonthermal Cold Plasma Technology for Starch Modification. Food Phys. 2024, 1, 100011. [Google Scholar] [CrossRef]
- Nazarudin, N.; Ulyarti, U.; Pratama, I.A.; Yuwono, S.D. Improving the Characteristics of Edible Film Using Modified Cassava Starch Over Ethanol Precipitation. Sci. Technol. Indones. 2023, 8, 32–37. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Rhim, J.W.; Roh, H.J.; Kim, S.S. Preparation and Physical Properties of Zein-Coated High-Amylose Corn Starch Film. LWT 2002, 35, 680–686. [Google Scholar] [CrossRef]
- Hosseini, P.; Hojjatoleslamy, M.; Molavi, H. Investigation of the Mixing Ratio of Quince Seed Gum, Potato Starch and Gellan Gum on the Properties of the Resulting Film by Mixture Design. Int. J. Biol. Macromol. 2023, 237, 123869. [Google Scholar] [CrossRef]
- Yadav, P.; Gautam, S.; Bosco, S.J.D. Amaranthus paniculatus (Rajgeera) a Non-Conventional Source of Starch: Effect of Oxidation and Heat Moisture Treatment and Its Application in Edible Film. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Shanbhag, C.; Shenoy, R.; Shetty, P.; Srinivasulu, M.; Nayak, R. Formulation and Characterization of Starch-Based Novel Biodegradable Edible Films for Food Packaging. J. Food Sci. Technol. 2023, 60, 2858–2867. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Armada, M.; Gottifredi, J.C. Physicochemical Characterization of Starch Based Films. J. Food Eng. 2007, 82, 17–25. [Google Scholar] [CrossRef]
- Hoque, M.; McDonagh, C.; Tiwari, B.K.; Kerry, J.P.; Pathania, S. Effect of Cold Plasma Treatment on the Packaging Properties of Biopolymer-Based Films: A Review. Appl. Sci. 2022, 12, 1346. [Google Scholar] [CrossRef]
- Cui, C.; Gao, L.; Dai, L.; Ji, N.; Qin, Y.; Shi, R.; Qiao, Y.; Xiong, L.; Sun, Q. Hydrophobic Biopolymer-Based Films: Strategies, Properties, and Food Applications. Food Eng. Rev. 2023, 15, 360–379. [Google Scholar] [CrossRef]
- Petronilho, S.; Oliveira, A.; Domingues, M.R.; Nunes, F.M.; Coimbra, M.A.; Gonçalves, I. Hydrophobic Starch-Based Films Using Potato Washing Slurries and Spent Frying Oil. Foods 2021, 10, 2897. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, J.; Zhao, H.; Song, X.; Ji, Z.; Xie, C.; Chen, F.; Meng, Y. Biomimetic Robust Starch Composite Films with Super-Hydrophobicity and Vivid Structural Colors. Int. J. Mol. Sci. 2022, 23, 5607. [Google Scholar] [CrossRef]
- Karim, A.; Norziah, M.H.; Seow, C.C. Methods for the Study of Starch Retrogradation. Food Chem. 2000, 71, 9–36. [Google Scholar] [CrossRef]
- Lu, H.; Ma, R.; Chang, R.; Tian, Y. Evaluation of Starch Retrogradation by Infrared Spectroscopy. Food Hydrocoll. 2021, 120, 106975. [Google Scholar] [CrossRef]
- Deeyai, P.; Suphantharika, M.; Wongsagonsup, R.; Dangtip, S. Characterization of Modified Tapioca Starch in Atmospheric Argon Plasma under Diverse Humidity by FTIR Spectroscopy. Chin. Phys. Lett. 2013, 30, 018103. [Google Scholar] [CrossRef]
- Shen, H.; Ge, X.; Zhang, B.; Su, C.; Zhang, Q.; Jiang, H.; Zhang, G.; Yuan, L.; Yu, X.; Li, W. Preparing Potato Starch Nanocrystals Assisted by Dielectric Barrier Discharge Plasma and Its Multiscale Structure, Physicochemical and Rheological Properties. Food Chem. 2022, 372, 131240. [Google Scholar] [CrossRef]
- Oh, Y.A.; Roh, S.H.; Min, S.C. Cold Plasma Treatments for Improvement of the Applicability of Defatted Soybean Meal-Based Edible Film in Food Packaging. Food Hydrocoll. 2016, 58, 150–159. [Google Scholar] [CrossRef]
- Wong, L.-W.; Hou, C.-Y.; Hsieh, C.-C.; Chang, C.-K.; Wu, Y.-S.; Hsieh, C.-W. Preparation of Antimicrobial Active Packaging Film by Capacitively Coupled Plasma Treatment. LWT 2020, 117, 108612. [Google Scholar] [CrossRef]


| Plasma Frequency (Hz) | Exposure Time (min) | Plasma-Treated Film | Starch-Treated Film | Film-Forming Solution-Treated Film |
|---|---|---|---|---|
| 0 | 0 | 23.4 ± 1.0 ab | 23.4 ± 1.0 ed | 23.4 ± 1.0 bc |
| 100 | 10 | 21.6 ± 0.4 b | 24.8 ± 0.4 d | 25.2 ± 0.1 b |
| 100 | 20 | 23.7 ± 0.9 ab | 22.3 ± 0.1 e | 20.0 ± 0.2 d |
| 200 | 10 | 22.1 ± 1.2 bc | 31.0 ± 0.3 b | 22.3 ± 0.3 c |
| 200 | 20 | 20.8 ± 0.9 c | 33.4 ± 0.1 a | 23.1 ± 0.1 c |
| 300 | 10 | 22.4 ± 0.4 b | 28.6 ± 0.3 bc | 24.8 ± 0.3 b |
| 300 | 20 | 23.7 ± 0.4 a | 27.2 ± 0.5 bc | 27.0 ± 0.7 a |
| Plasma Frequency (Hz) | Exposure Time (min) | Plasma-Treated Film | Starch-Treated Film | Film-Forming Solution-Treated Film |
|---|---|---|---|---|
| 0 | 0 | 1.97 ± 0.05 a | 1.97 ± 0.05 b | 1.97 ± 0.05 a |
| 100 | 10 | 2.08 ± 0.67 ab | 1.98 ± 0.11 b | 1.87 ± 0.04 a |
| 100 | 20 | 1.99 ± 0.56 ab | 1.97 ± 0.04 b | 1.88 ± 0.22 a |
| 200 | 10 | 2.01 ± 0.34 ab | 2.07 ± 0.47 ab | 1.93 ± 1.02 a |
| 200 | 20 | 2.07 ± 0.98 ab | 2.07 ± 0.16 ab | 1.87 ± 0.46 a |
| 300 | 10 | 1.87 ± 0.09 a | 2.10 ± 0.10 ab | 1.92 ± 0.34 a |
| 300 | 20 | 1.68 ± 0.11 b | 2.18 ± 0.08 a | 1.91 ± 0.03 a |
| Plasma Frequency (Hz) | Exposure Time (min) | Plasma-Treated Film | Starch-Treated Film | Film-Forming Solution-Treated Film |
|---|---|---|---|---|
| 0 | 0 | 90.0 ± 1.6 a | 90.0 ± 1.6 a | 90.0 ± 1.6 a |
| 100 | 10 | 83.8 ± 0.6 b | 83.8 ± 3.3 b | 79.1 ± 3.0 b |
| 100 | 20 | 83.7 ± 0.8 b | 86.2 ± 3.1 ab | 80.9 ± 3.3 b |
| 200 | 10 | 82.6 ± 1.4 ab | 75.8 ± 4.1 c | 79.2 ± 0.4 b |
| 200 | 20 | 84.0 ± 1.2 b | 76.5 ± 3.7 c | 81.8 ± 4.0 b |
| 300 | 10 | 83.3 ± 1.1 ab | 78.3 ± 4.0 c | 79.2 ± 2.3 b |
| 300 | 20 | 82.7 ± 0.8 ab | 79.5 ± 3.3 c | 79.4 ± 1.7 b |
| Plasma Frequency (Hz) | Exposure Time (min) | Plasma-Treated Film | Starch-Treated Film | Film-Forming Solution-Treated Film |
|---|---|---|---|---|
| 0 | 0 | 54.2 ± 1.1 e | 54.2 ± 1.1 b | 54.2 ± 1.1 c |
| 100 | 10 | 55.9 ± 1.1 de | 54.0 ± 1.7 b | 56.2 ± 1.4 c |
| 100 | 20 | 58.5 ± 0.9 cd | 53.2 ± 1.8 b | 61.0 ± 1.6 b |
| 200 | 10 | 57.1 ± 0.6 d | 53.5 ± 1.3 ab | 64.6 ± 1.1 a |
| 200 | 20 | 64.3 ± 0.6 a | 55.4 ± 2.2 ab | 62.5 ± 1.2 b |
| 300 | 10 | 59.2 ± 0.4 c | 57.0 ± 1.4 a | 62.5 ± 1.8 b |
| 300 | 20 | 61.6 ± 0.5 b | 58.1 ± 1.7 a | 56.2 ± 1.4 c |
| Plasma Frequency (Hz) | Exposure Time (min) | Plasma-Treated Film | Starch-Treated Film | Film-Forming Solution-Treated Film |
|---|---|---|---|---|
| 930 cm−1 | ||||
| 0 | 0 | 0.14 ± 0.1 a | 0.35 ± 0.1 a | 0.35 ± 0.1 ab |
| 100 | 10 | 0.11 ± 0.1 bc | 0.32 ± 0.1 b | 0.33 ± 0.1 b |
| 100 | 20 | 0.10 ± 0.1 c | 0.27 ± 0.1 c | 0.35 ± 0.1 ab |
| 200 | 10 | 0.12 ± 0.1 b | 0.32 ± 0.1 b | 0.36 ± 0.1 a |
| 200 | 20 | 0.13 ± 0.1 a | 0.33 ± 0.1 ab | 0.33 ± 0.1 b |
| 300 | 10 | 0.12 ± 0.1 b | 0.29 ± 0.1 c | 0.35 ± 0.1 ab |
| 300 | 20 | 0.11 ± 0.1 bc | 0.16 ± 0.1 d | 0.34 ± 0.1 ab |
| 995 cm−1/1022 cm−1 | ||||
| 0 | 0 | 1.28 ± 0.1 ab | 1.46 ± 0.1 a | 1.46 ± 0.1 ab |
| 100 | 10 | 1.26 ± 0.1 b | 1.36 ± 0.1 bc | 1.39 ± 0.1 c |
| 100 | 20 | 1.28 ± 0.1 ab | 1.31 ± 0.1 cd | 1.47 ± 0.1 a |
| 200 | 10 | 1.29 ± 0.1 a | 1.37 ± 0.1 b | 1.48 ± 0.1 a |
| 200 | 20 | 1.27 ± 0.1 ab | 1.44 ± 0.1 a | 1.43 ± 0.1 b |
| 300 | 10 | 1.28 ± 0.1 ab | 1.34 ± 0.1 c | 1.41 ± 0.1 bc |
| 300 | 20 | 1.28 ± 0.1 ab | 1.21 ± 0.1 e | 1.43 ± 0.1 b |
| 1045 cm−1/1022 cm−1 | ||||
| 0 | 0 | 0.73 ± 0.1 a | 0.71 ± 0.1 c | 0.71 ± 0.1 a |
| 100 | 10 | 0.58 ± 0.1 b | 0.71 ± 0.1 c | 0.69 ± 0.1 a |
| 100 | 20 | 0.58 ± 0.1 b | 0.77 ± 0.1 a | 0.71 ± 0.1 a |
| 200 | 10 | 0.55 ± 0.1 c | 0.69 ± 0.1 c | 0.70 ± 0.1 a |
| 200 | 20 | 0.56 ± 0.1 c | 0.72 ± 0.1 bc | 0.70 ± 0.1 a |
| 300 | 10 | 0.57 ± 0.1 bc | 0.74 ± 0.1 b | 0.69 ± 0.1 a |
| 300 | 20 | 0.56 ± 0.1 c | 0.78 ± 0.1 a | 0.70 ± 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goiana, M.L.; Mattos, A.L.A.; Rosa, M.d.F.; Fernandes, F.A.N. Use of Cold Plasma as an Alternative to Improve Corn Starch-Based Films: Effect of the Plasma Application Strategy. Processes 2024, 12, 1429. https://doi.org/10.3390/pr12071429
Goiana ML, Mattos ALA, Rosa MdF, Fernandes FAN. Use of Cold Plasma as an Alternative to Improve Corn Starch-Based Films: Effect of the Plasma Application Strategy. Processes. 2024; 12(7):1429. https://doi.org/10.3390/pr12071429
Chicago/Turabian StyleGoiana, Mayara Lima, Adriano Lincoln Albuquerque Mattos, Morsyleide de Freitas Rosa, and Fabiano André Narciso Fernandes. 2024. "Use of Cold Plasma as an Alternative to Improve Corn Starch-Based Films: Effect of the Plasma Application Strategy" Processes 12, no. 7: 1429. https://doi.org/10.3390/pr12071429
APA StyleGoiana, M. L., Mattos, A. L. A., Rosa, M. d. F., & Fernandes, F. A. N. (2024). Use of Cold Plasma as an Alternative to Improve Corn Starch-Based Films: Effect of the Plasma Application Strategy. Processes, 12(7), 1429. https://doi.org/10.3390/pr12071429









