Acmella oleracea Metabolite Extraction Using Natural Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of NADESs
2.2.2. Physicochemical Characterisation of NADESs
2.2.3. Fourier Transform Infrared Spectroscopy Characterisation of NADESs before and after Extraction
2.2.4. Extraction Methods
2.2.5. Determination of Total Polyphenol Content (TPC) and Flavonoids (TFC)
3. Results
3.1. Characterisation of NADESs
3.1.1. Physical Characterisation of NADES Extraction Solvents
3.1.2. FTIR Characterisation
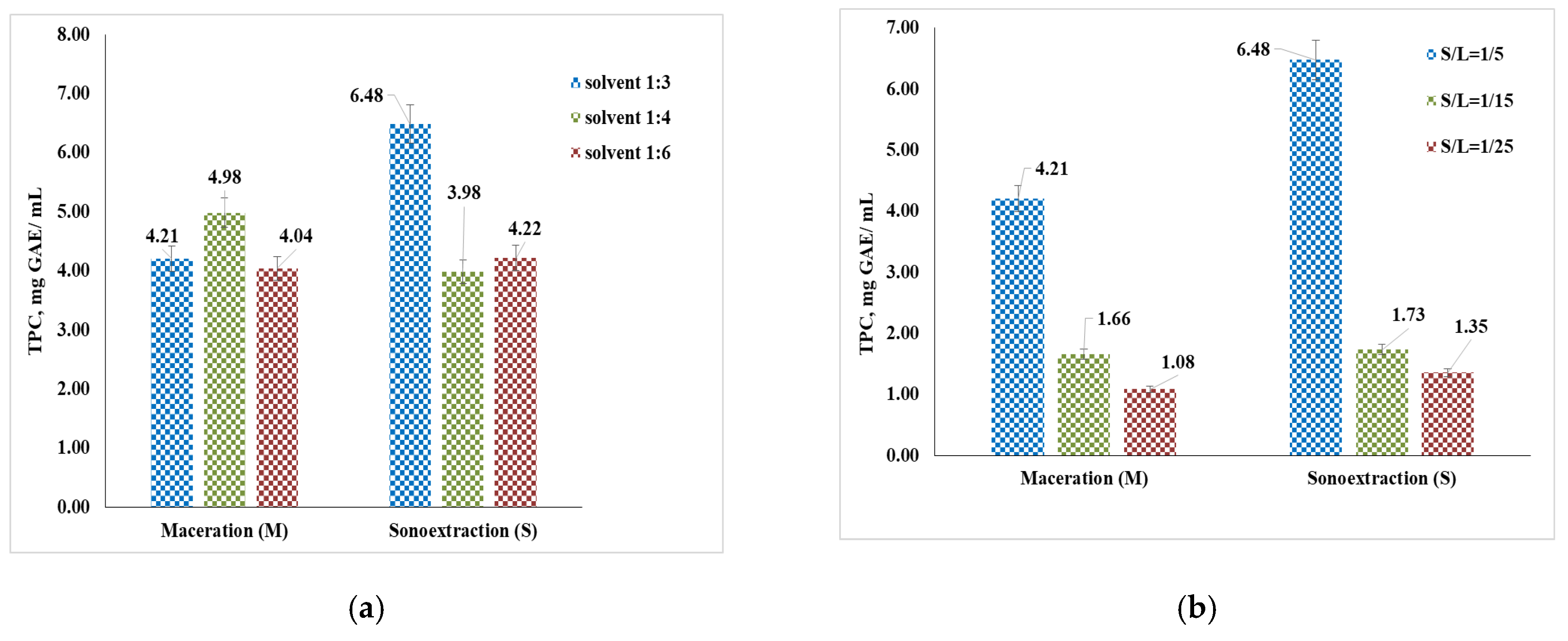
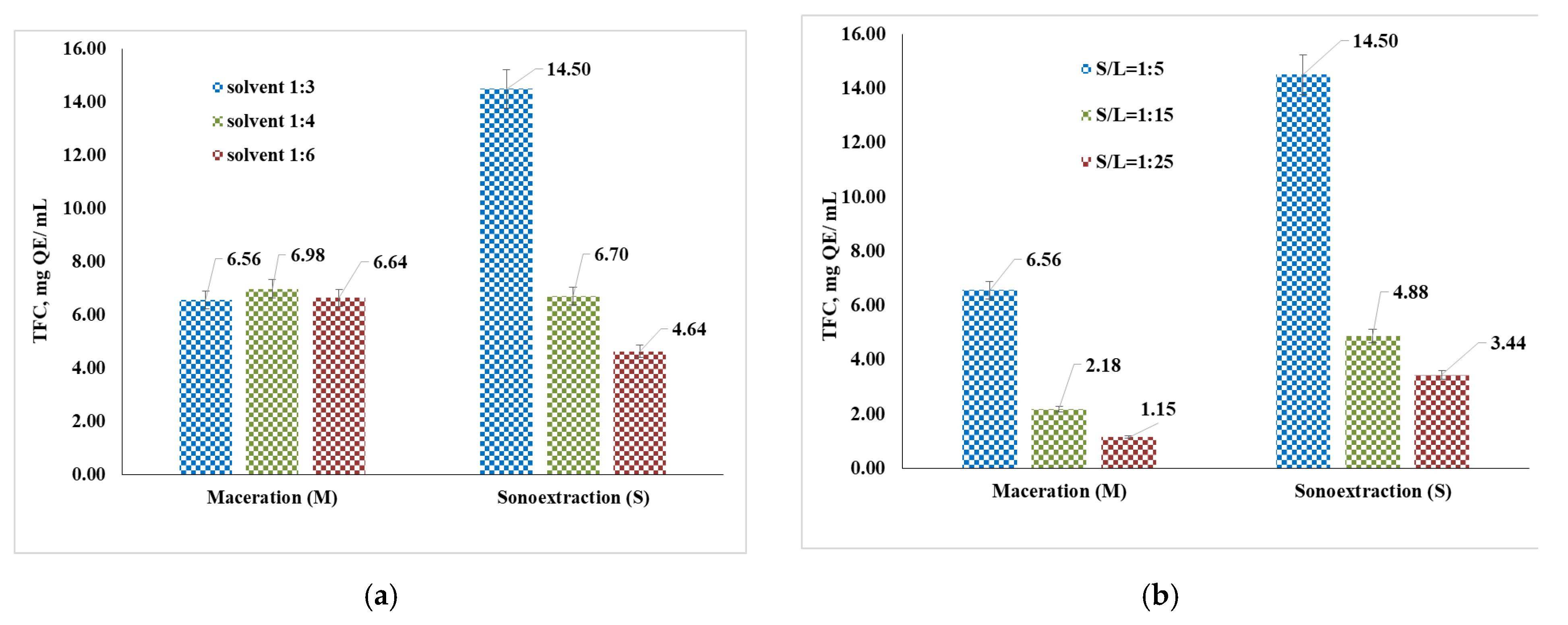
3.2. Total Polyphenol Content (TPC) and Flavonoids (TFC)
4. Discussion
4.1. Physical Characterisation of NADES Extraction Solvents
4.2. FTIR Characterisation
4.3. Determination of Total Polyphenol Content (TPC) and Flavonoids (TFC)
- Depending on the S/L ratio (Figure 6b), it can be seen that the best results were recorded in the case of sonoextraction (7.52 mg QE/g), followed by maceration (6.56 mg QE/g), using the 1:5 ratio. This result is in accordance with data from the literature, as sonoextraction usually provides a high extraction yield with the use of less solvent and energy compared to maceration.
- Considering the extraction time (Figure 6c), under the selected conditions, the best values of the flavonoid content were obtained in the case of sonoextraction for 60 min and S/L = 1:5 (14.5 mg QE/g). It can be observed that with the increase in the extraction time, in the case of both methods, an increase in the amount of extracted flavonoids is also observed.
- Depending on the temperature (Figure 6d), it can be seen that better results were obtained in the case of sonoextraction at 60 °C (5.8 mg QE/g). The temperature also influences the amount of extracted flavonoids through the action of changing the viscosity of the solvent and increasing the permeability of the cell membrane of the plant, and a high temperature also promotes the formation of more cavitation bubbles, which expand the solid–solvent contact area and diffusion.
- If we also take into account the composition of the solvent, pure or mixed with water in different percentages (Figure 6e), we can consider the results in the case of using the NADES 1:3 mixture as an extraction solvent in combination with different percentages of water. It is observed that in the case of maceration, the amount of flavonoids increases with the increase in the percentage of water in the extractant. In the case of sonoextraction, the largest quantity of extracted flavonoids is seen in the case of using the simple solvent without water. The percentage of water added influences the flavonoids extracted in a favourable way but without reaching the values obtained in the case of the solvent without water. As in the case of polyphenols, it can be said that the introduction of water into the extraction solvent has an important role depending on the extraction method used. Solvents can influence a solute’s solubility and stability by facilitating or hindering particular molecular interactions. An ideal hydrophilicity may be reached with a particular volume of water; the extraction or solubilisation of flavonoids does not follow a solvent’s increasing hydrophilicity in a proportionate manner, as certain phenolics require particular water levels for efficient extraction. This demonstrates that the type of NADES components at the proper mixing ratio and the water content in a NADES may be changed to alter the solvent’s ability to dissolve target chemicals in plants.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea plant; identification, applications and use as an emerging food source–review. Food Rev. Int. 2021, 37, 399–414. [Google Scholar] [CrossRef]
- Dallazen, J.L.; da Luz, B.B.; Maria-Ferreira, D.; Nascimento, A.M.; Cipriani, T.R.; de Souza, L.M.; Geppetti, P.; de Paula Werner, M.F. Local effects of natural alkylamides from Acmella oleracea and synthetic isobutylalkyl amide on neuropathic and postoperative pain models in mice. Fitoterapia 2022, 160, 105224. [Google Scholar] [CrossRef] [PubMed]
- Jerônimo, L.B.; Santos, P.V.L.; Pinto, L.C.; da Costa, J.S.; de Aguiar Andrade, E.H.; Setzer, W.N.; do Rosário da Silva, J.K.; Carvalho de Araújo, J.A.; Figueiredo, P.L.B. Acmella oleracea (L.) RK Jansen essential oils: Chemical composition, antioxidant, and cytotoxic activities. Biochem. Syst. Ecol. 2024, 112, 104775. [Google Scholar] [CrossRef]
- Rahim, A.R.; Jayusman, P.A.; Muhammad, N.; Mohamed, N.; Lim, V.; Ahmad, N.H.; Mohamad, S.; Abdul Hamid, Z.A.; Ahmad, F.; Mokhtar, N.; et al. Potential antioxidant and anti-inflammatory effects of Spilanthes acmella and its health beneficial effects: A review. Int. J. Environ. Res. Public Health 2021, 18, 3532. [Google Scholar] [CrossRef] [PubMed]
- Kaoui, S.; Chebli, B.; Zaidouni, S.; Basaid, K.; Mir, Y. Deep eutectic solvents as sustainable extraction media for plants and food samples: A review. Sustain. Chem. Pharm. 2023, 31, 100937. [Google Scholar] [CrossRef]
- Bahia, P.V.B.; dos Reis Lago Brandão, B.; Machado, M.E. Deep eutectic solvent for the extraction of polycyclic aromatic compounds in fuel, food and environmental samples. Talanta 2024, 277, 126418. [Google Scholar] [CrossRef] [PubMed]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 73. [Google Scholar] [CrossRef]
- Yahaya, N.; Mohamed, A.H.; Sajid, M.; Zain, N.N.M.; Liao, P.C.; Chew, K.W. Deep eutectic solvents as sustainable extraction media for extraction of polysaccharides from natural sources: Status, challenges and prospects. Carbohydr. Polym. 2024, 338, 122199. [Google Scholar] [CrossRef]
- Bragagnolo, F.S.; Strieder, M.M.; Pizani, R.S.; de Souza Mesquita, L.M.; González-Miquel, M.; Rostagno, M.A. Revisiting natural deep eutectic solvents (NADES) as extraction media and ready-to-use purposes. TrAC Trends Anal. Chem. 2024, 175, 117726. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasoundassisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2019, 60, 2564–2592. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Cabrera, L.; Xavier, L.; Zecchi, B. Extraction of phenolic compounds with antioxidant activity from olive pomace using natural deep eutectic solvents: Modelling and optimization by response surface methodology. Discov. Food 2024, 4, 29. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Miličević, N.; Panić, M.; Valinger, D.; Bubalo, M.C.; Benković, M.; Jurina, T.; Kljusurić, J.G.; Redovniković, I.R.; Tušek, A.J. Development of continuously operated aqueous two-phase microextraction process using natural deep eutectic solvents. Sep. Purif. Technol. 2020, 244, 116746. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef] [PubMed]
- Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Cvetanović Kljakić, A.; Pojić, M.; Mandić, A.; Stupar, A.; Santos, F.; Duarte, A.R.C.; Mišan, A. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules 2022, 27, 1508. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.A.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Villa, C.; Caviglia, D.; Robustelli della Cuna, F.S.; Zuccari, G.; Russo, E. NaDES Application in Cosmetic and Pharmaceutical Fields: An Overview. Gels 2024, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Caprin, B.; Charton, V.; Vogelgesang, B. The use of NaDES to support innovation in the cosmetic Industry. Adv. Bot. Res. 2021, 97, 309–332. [Google Scholar]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharm. Biomed. Anal. 2018, 161, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.F.; Carvalho, M.G.D.; Smith, R.E.; Sabaa-Srur, A.U. Spilanthol: Occurrence, extraction, chemistry and biological activities. Rev. Bras. Farmacogn. 2016, 26, 128–133. [Google Scholar] [CrossRef]
- Marchesini, P.; Barbosa, A.F.; Franco, C.; Novato, T.; Sanches, M.N.G.; de Carvalho, M.G.; Monteiro, C.M.O. Activity of the extract of Acmella oleracea on immature stages of Amblyomma sculptum (Acari: Ixodidae). Vet. Parasitol. 2018, 254, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, E.; Ferrati, M.; Baldassarri, C.; Cappellacci, L.; Marmugi, M.; Caselli, A.; Benelli, G.; Maggi, F.; Petrelli, R. A Review of the Chemistry and Biological Activities of Acmella oleracea (“jambù”, Asteraceae), with a View to the Development of Bioinsecticides and Acaricides. Plants 2022, 11, 2721. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Berger, M.; de Cecco, B.S.; Mallmann, L.P.; Terraciano, P.B.; Driemeier, D.; Rodrigues, E.; Beys-da Silva, W.O.; Konrath, E.L. Chymase inhibition: A key factor in the anti-inflammatory activity of ethanolic extracts and spilanthol isolated from Acmella oleracea. J. Ethnopharmacol. 2021, 270, 113610. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Zonfrillo, B.; Maggini, V.; Bogani, P.; Gallo, E.; Firenzuoli, F.; Mulinacci, N.; Innocenti, M. Acmella oleracea (L.) R.K. Jansen: Alkylamides and phenolic compounds in aerial parts and roots of in vitro seedlings. J. Pharm. Biomed. Anal. 2022, 220, 114991. [Google Scholar] [CrossRef]
- Abeysinghe, D.; Wijerathne, S.; Dharmadasa, R. Secondary Metabolites Contents and Antioxidant Capacities of Acmella Oleraceae Grown under Different Growing Systems. World J. Agric. Res. 2014, 2, 163–167. [Google Scholar]
- Usmani, Z.; Sharma, M.; Tripathi, M.; Lukk, T.; Karpichev, Y.; Gathergood, N.; Singh, B.N.; Thakur, V.K.; Tabatabaei, M.; Gupta, V.K. Biobased natural deep eutectic system as versatile solvents: Structure, interaction and advanced applications. Sci. Total Environ. 2023, 881, 163002. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.Z.; Xu, K.J.; Huang, Y.H.; Wen, Q.; Ding, X.Q. Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 2016, 152, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; Della, P.S.; Dugo, L.; Gentili, A.; Mondello, L.; Gara, L.D. Choline-chloride and betaine-based deep eutectic solvents for green extraction of nutraceutical compounds from spent coffee ground. J. Pharmaceut. Biomed. 2020, 189, 113421. [Google Scholar] [CrossRef]
- Kučan, K.Z.; Perković, M.; Cmrk, K.; Nacinovic, D.; Rogosic, M. Betaine plus (glycerol or ethylene glycol or propylene glycol) deep eutectic solvents for extractive purification of gasoline. Chemistryselect 2018, 3, 12582–12590. [Google Scholar] [CrossRef]
- Mohd, F.F.; Mohd, N.M. The formulation and physicochemical properties of betaine-based natural deep eutectic solvent. J. Mol. Liq. 2022, 360, 119392. [Google Scholar] [CrossRef]
- Pavun, L.; Đikanovic, P.; Jelikic-Stankov, M.; Đurdevic, D.; Uskokovic-Markovic, S. Determination of flavonoids and total polyphenol contents in commercial apple juices. Czech J. Food Sci. 2018, 36, 233–238. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Chen, Q.; He, N.; Fan, J.; Song, F. Physical Properties of Betaine-1,2-Propanediol-Based Deep Eutectic Solvents. Polymers 2022, 14, 1783. [Google Scholar] [CrossRef]
- Maxim, C.; Turcov, D.; Trifan, A.; Suteu, D.; Barna, A.S. Preliminary characterization of the phytoextracts from Acmella oleracea with therapeutic potential and applicability in active cosmetics. Sci. Study Res. 2024, 25, 169–182. [Google Scholar] [CrossRef]
- Abate, T.; Amabile, C.; Chianese, S.; Musmarra, D.; Muñoz, R. Solubility of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in sustainable and green solvents: Effect of HV content and comparison between experimental results and theoretical prediction. J. Mol. Liq. 2024, 393, 123640. [Google Scholar] [CrossRef]

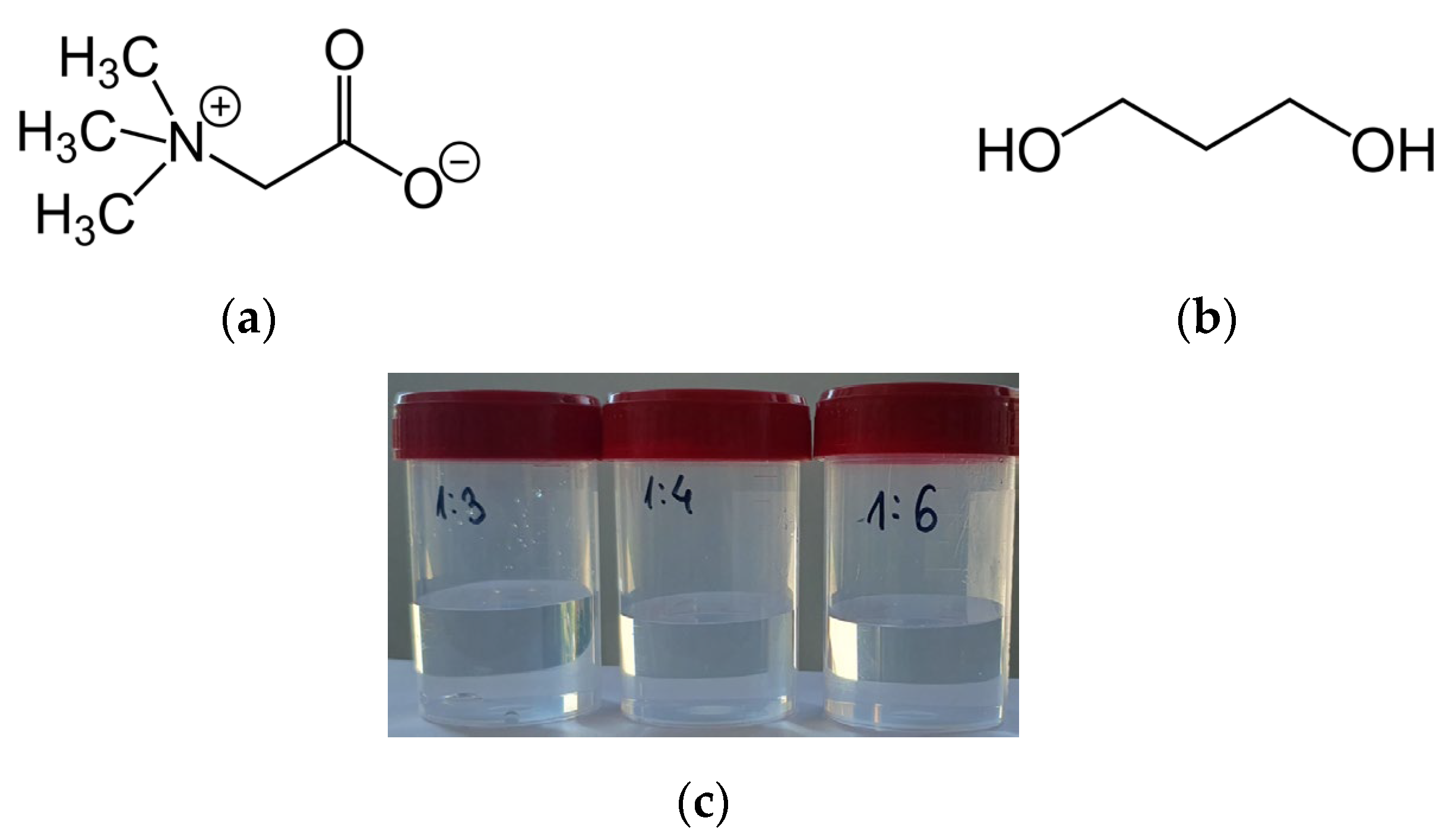




| NADESs | Physicochemical Properties | |||
|---|---|---|---|---|
| pH | Conductivity | Density (g/mL) | Optic Images | |
| NADES 1 (1:3 = betaine/propanediol) | 9.08 | 0.00 | 1.146 |  |
| NADES 2 (1:4 = betaine/propanediol) | 8.81 | 0.00 | 1.141 |  |
| NADES 3 (1:6 = betaine7propanediol) | 8.57 | 0.00 | 1.087 |  |
| Extracted Compound | Type of Solvent/Method Characteristics | |
|---|---|---|
| Propanediol | NADES (Betaine/Propanediol = 1:3) | |
| Polyphenols, mg GAE/g | 3.39 (M: 60% propanediol concentration, 7 days, S/L = 1:5) 3.00 (M: 40% propanediol concentration, 30 days, S/L = 1:5) | 6.48 (S: 60 min, S/L = 1:5, 25 °C) 4.21 (M: 15 min, S/L = 1:5, 25 °C) |
| Flavonoids, mg QE/g | 8.98 (M: 40% propanediol concentration, 30 days, S/L = 1:15) 8.37 (M: 60% propanediol concentration, 14 days, S/L = 1:20) | 14.5 (S: 60 min, S/L = 1:5, 25 °C) 8.66 (M: 45 min, S/L = 1:5, 25 °C) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxim, C.; Blaga, A.C.; Tataru-Farmus, R.-E.; Suteu, D. Acmella oleracea Metabolite Extraction Using Natural Deep Eutectic Solvents. Processes 2024, 12, 1686. https://doi.org/10.3390/pr12081686
Maxim C, Blaga AC, Tataru-Farmus R-E, Suteu D. Acmella oleracea Metabolite Extraction Using Natural Deep Eutectic Solvents. Processes. 2024; 12(8):1686. https://doi.org/10.3390/pr12081686
Chicago/Turabian StyleMaxim, Claudia, Alexandra Cristina Blaga, Ramona-Elena Tataru-Farmus, and Daniela Suteu. 2024. "Acmella oleracea Metabolite Extraction Using Natural Deep Eutectic Solvents" Processes 12, no. 8: 1686. https://doi.org/10.3390/pr12081686




