Influence of Laser Scanning Speed on Wear and Corrosion Resistance of Aluminum–Nickel Coating on Magnesium Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Laser Cladding
2.3. Microstructural Characterization and Property Measurement
3. Results and Discussion
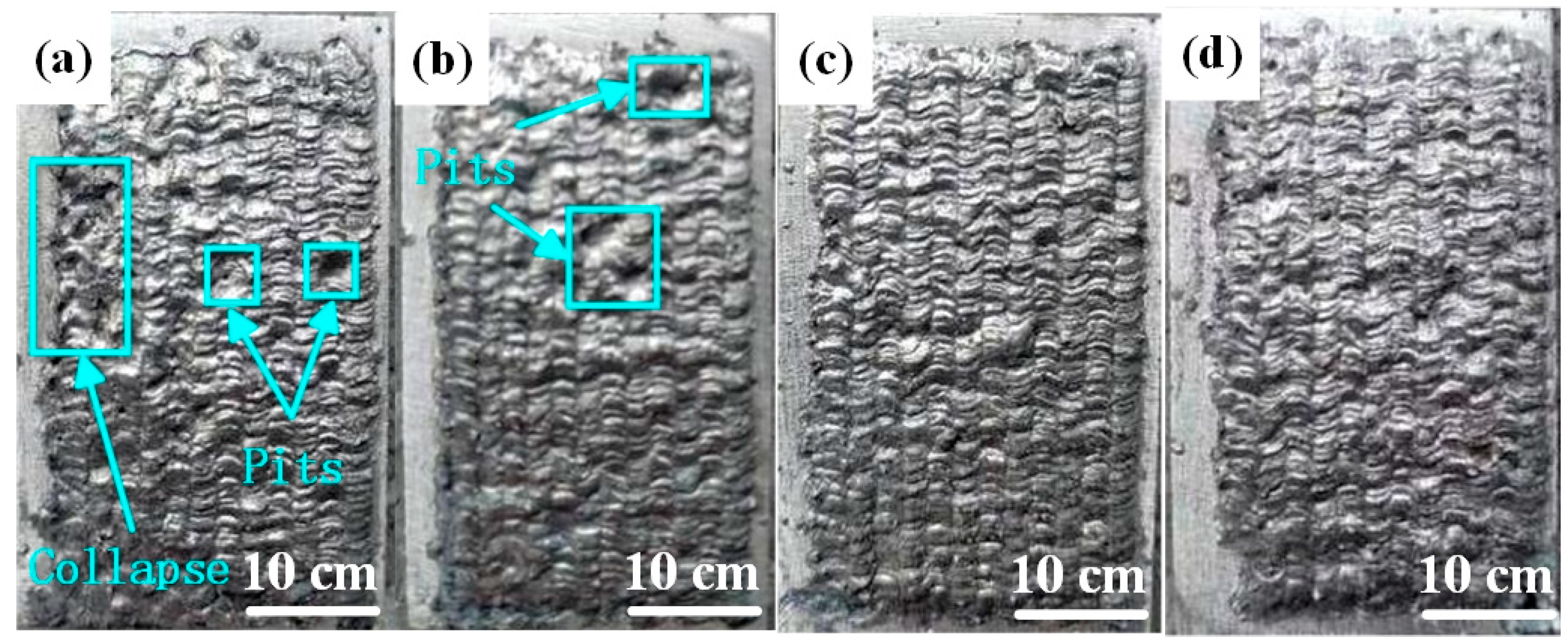
3.1. Effect of Laser Scanning Speed on Macroscopic Morphology of Al-Ni Coatings
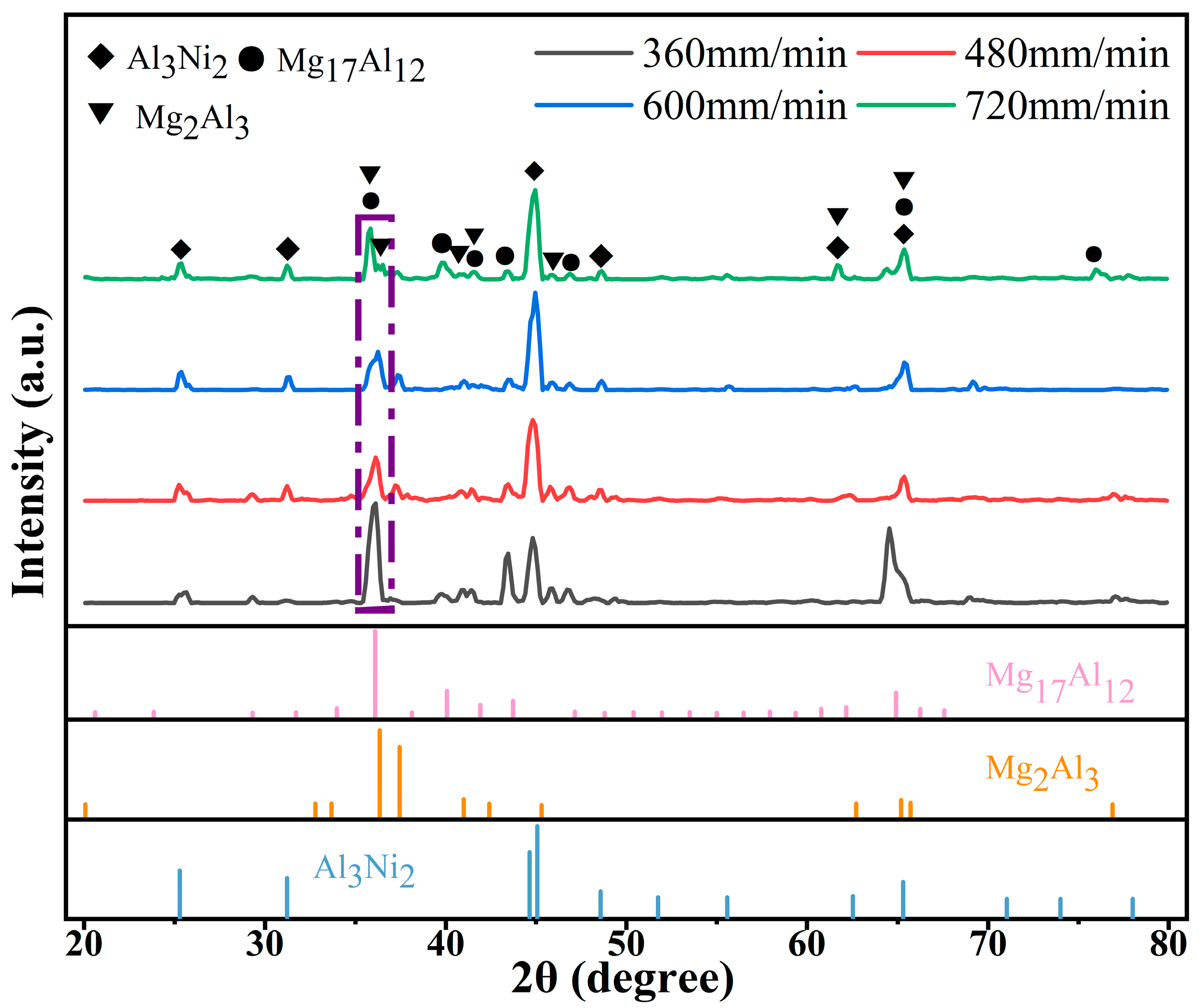
3.2. Effect of Laser Scanning Speed on Phase Composition of Al-Ni Coatings
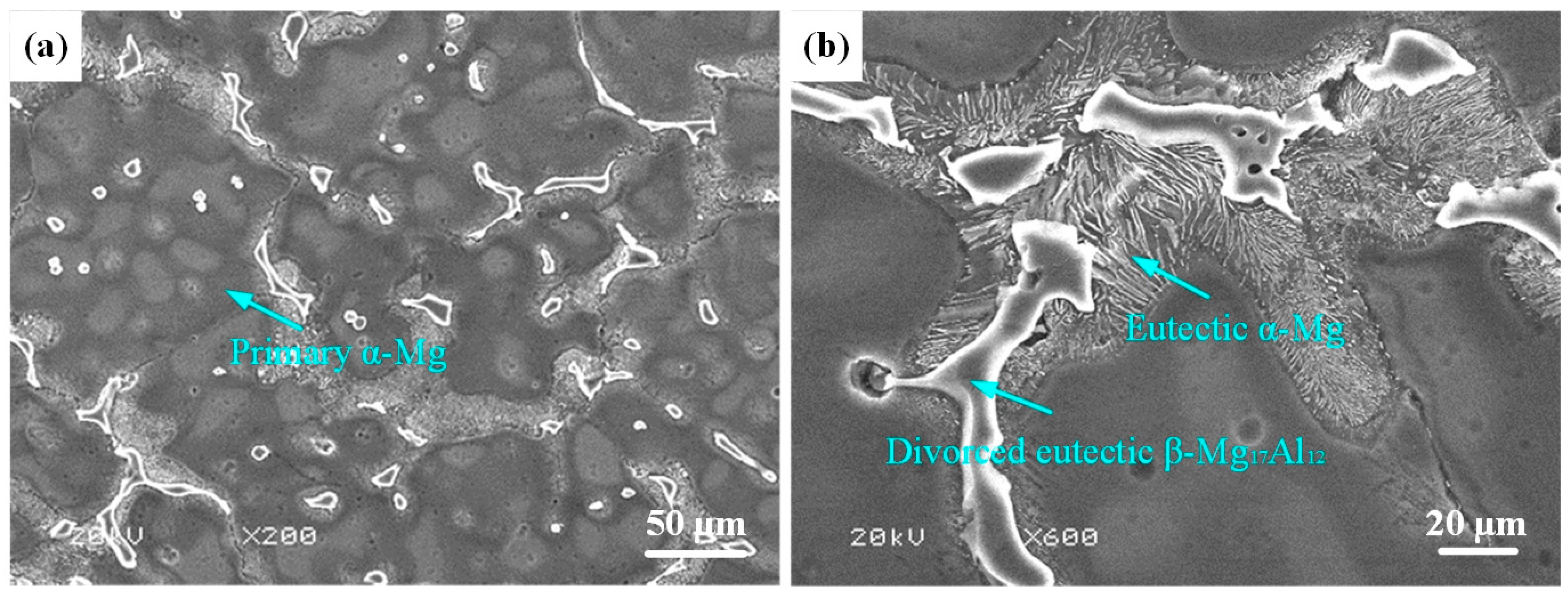
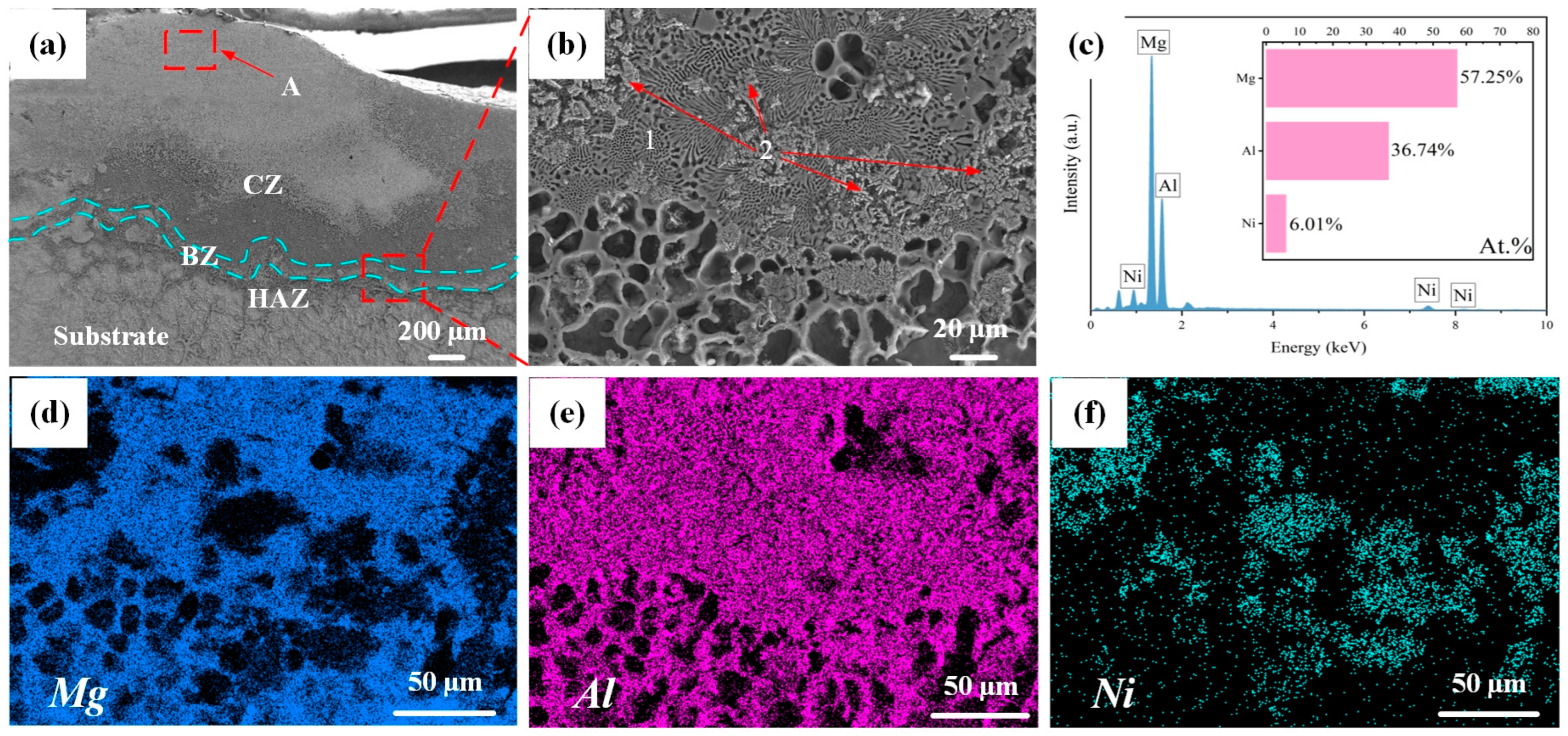
3.3. Effect of Laser Scanning Speed on Microstructure of Al-Ni Coatings
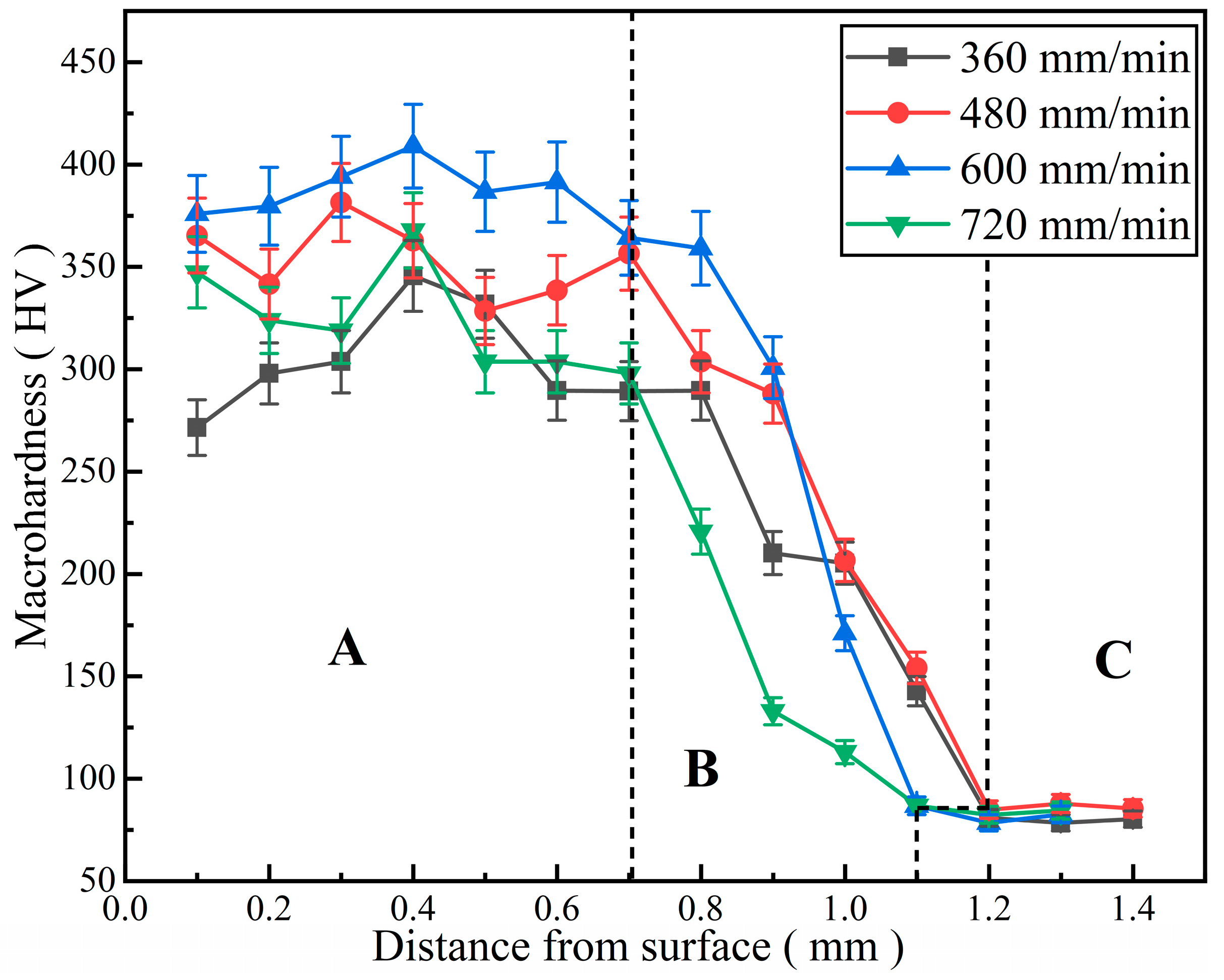
3.4. Effect of Laser Scanning Speed on Microhardness of Al-Ni Coatings
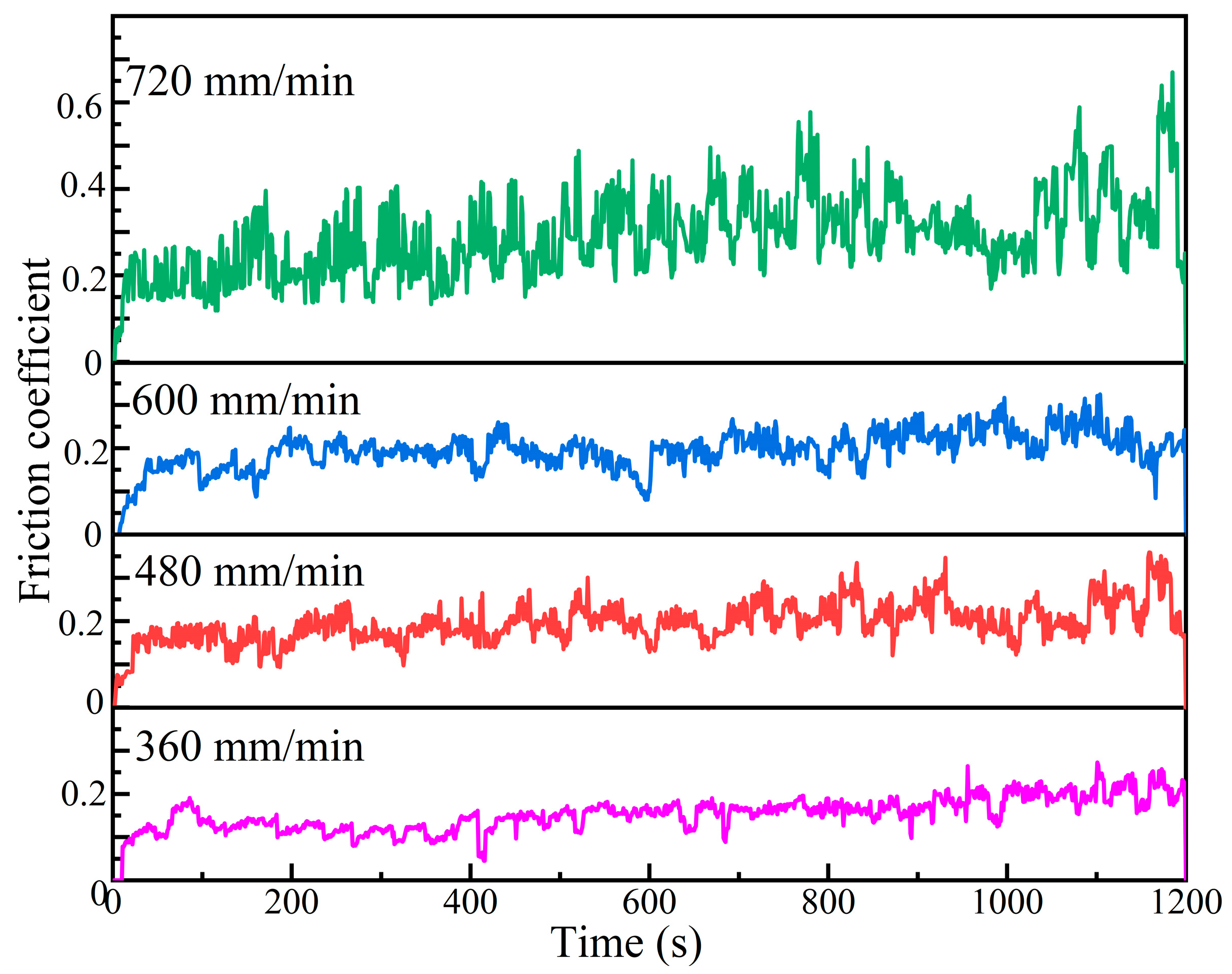
3.5. Effect of Laser Scanning Speed on Wear Resistance of Al-Ni Coatings
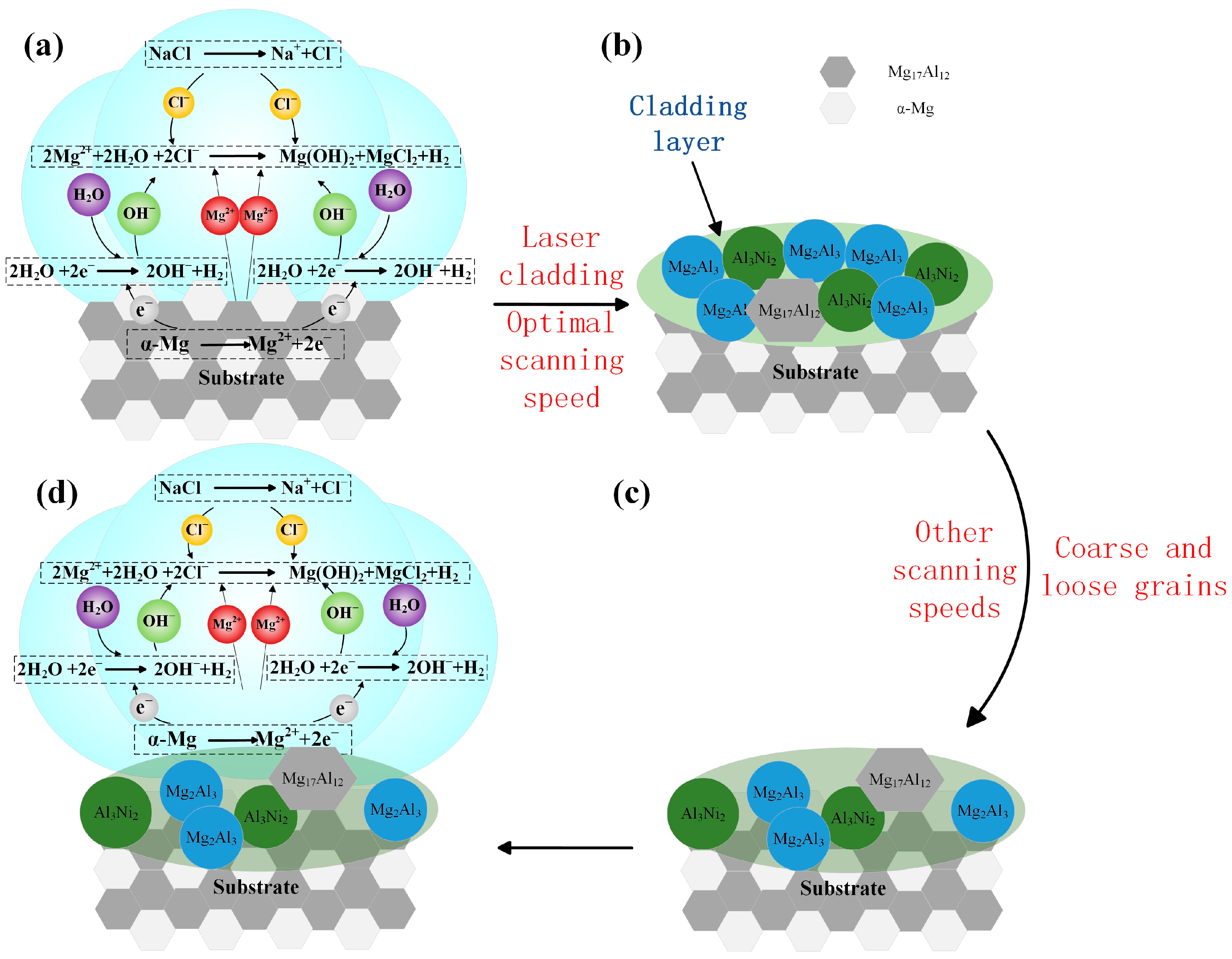
3.6. Effect of Laser Scanning Speed on Corrosion Resistance of Al-Ni Coatings
4. Conclusions
- At all laser scanning speeds, the morphological quality of coatings is good, the fish scale trajectory is obvious and there are no major defects. The coating is the smoothest at the laser scanning speed of 600 mm/min.
- The coatings are composed of Al3Ni2, Mg17Al12 and Mg2Al3. The microstructure of Al-Ni coatings comprises dendrite crystals, and the grain size first decreases and then increases with the increase in the laser scanning speed.
- The microhardness of Al-Ni coatings at different scanning speeds is 3.3~4.8 times that of the substrate. When the laser scanning speed is 600 mm/min, the average microhardness of the coating is the highest due to the effect of fine grain strengthening and dispersion strengthening. The wear volume of the coating is 49.62% of the matrix.
- Because of the large amount of Al2O3 formed on the coating surface and the pairs with low potential difference during the corrosion experiment, the corrosion resistance of the coating is improved. When the laser scanning speed is 600 mm/min, the corrosion resistance of the coating is the best. The self-corrosion current density is one order of magnitude lower than that of the substrate, and the self-corrosion potential increases by 0.52 V. In addition, with the increase in laser scanning speed, the corrosion resistance of Al-Ni coatings first increases and then decreases.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meng, G.Y.; Protasova, N.A.; Kruglov, E.P.; Lin, X.; Ding, X. Solidification behavior and morphological evolution in laser surface forming of AlCoCrCuFeNi multi-layer high-entropy alloy coatings on AZ91D. J. Alloys Compd. 2019, 772, 994–1002. [Google Scholar] [CrossRef]
- Mao, P.; Wang, J.X.; Bai, S.; Liu, Z.Y. Effects of ultrasonic treatment on the microstructures and hardening of Al–Cu–Mg–Ag alloys. J. Mater. Res. Technol. 2024, 30, 6064–6067. [Google Scholar] [CrossRef]
- Wang, S.; Pan, H.C.; Xie, D.S.; Zhang, D.D.; Li, J.R.; Xie, H.B. Grain refinement and strength enhancement in Mg wrought alloys: A review. J. Magnes. Alloy. 2023, 11, 4128–4145. [Google Scholar] [CrossRef]
- Yang, L.Q.; Li, Z.Y.; Zhang, Y.Q.; Wei, S.Z.; Wang, Y.J.; Kang, Y. In-situ TiC-Al3Ti reinforced Al-Mg composites with Y2O3 addition formed by laser cladding on AZ91D. Surf. Coat. Technol. 2020, 383, 125249. [Google Scholar] [CrossRef]
- Pei, X.L.; Li, Z.Y.; Zhang, Y.Q.; Wei, S.Z.; Yang, L.Q.; Wang, Y.J. A gradient Al/Ni-Cr-Al layer formed by direct current pulse metal inert gas welding combined laser cladding on AZ91D magnesium alloy. Vacuum 2019, 165, 86–95. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Kim, J.Y.; Li, M.; Gao, Y.; Hu, Y.B.; Jiang, B.; Pan, F.S. Effects of Nd content on the microstructures and mechanical properties of ZK60 Mg alloy and corresponding strengthening mechanisms. Mater. Sci. Eng. A-Struct. 2024, 901, 146504. [Google Scholar] [CrossRef]
- Song, Y.W.; Dai, J.M.; Sun, S. A comparative study on the corrosion behavior of AZ80 and EW75 Mg alloys in industrial atmospheric environment. Mater. Today 2024, 38, 108263. [Google Scholar] [CrossRef]
- Wen, X.; Cui, X.F.; Jin, G.; Jiao, Y.L.; Fang, Y.C. A novel Ni2MnCuSnAl0.1 multi-principal element alloy coating to enhance the wear resistance and corrosion resistance of Mg-Li alloy. Opt. Laser Technol. 2021, 142, 107243. [Google Scholar] [CrossRef]
- Wu, M.J.; Wu, F.F.; Long, M.J.; Ye, P.C.; Jiang, F.; Jiang, J.Y. Effect of oxidation treatment on the structure and composition of oxide film on Al-Mg-Sc alloy surface and microstructure of Al substrate near interface. Mater. Lett. 2022, 325, 132819. [Google Scholar] [CrossRef]
- Liu, Y.R.; Zhang, K.M.; Zou, J.X.; Yan, P.; Zhang, X.; Song, L.X. Microstructure and property modifications in surface layers of a Mg-4Sm-2Al-0.5Mn alloy induced by pulsed electron beam treatments. J. Magnes. Alloy. 2021, 9, 216–224. [Google Scholar] [CrossRef]
- Dong, F.; Peng, X.Q.; Lai, T.; Guan, C.L.; Li, G.H.; Liu, J.F.; Dai, Y.F. Improving the surface quality and its mechanism in ultraprecision machining of Al–Mg–Si alloy by multiple high energy shot peening pretreatment. J. Mater. Res. Technol. 2024, 30, 7051–7064. [Google Scholar] [CrossRef]
- Mohammadreza, D.; Hamid, R.B.; Abbas, S.; Mahmood, R.; Ashish, K.K.; Seeram, R.; Pradeep, L.M.; Manoranjan, M.; Ahmad, F.I.; Safian, S.; et al. Surface modification of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses. J. Magnes. Alloy. 2022, 10, 2025–2061. [Google Scholar]
- Mohammad, D.; Pang, X.; Hamid, J. The effect of pure aluminum cold spray coating on corrosion and corrosion fatigue of magnesium (3% Al-1% Zn) extrusion. Surf. Coat. Technol. 2017, 309, 423–435. [Google Scholar]
- Usman, A.; Lui, Y.; Lu, F.F.; Muhammad, Y.; Li, C.J.; Li, C.X. Enhancement of Corrosion Resistance and Tribological Properties of LA43M Mg Alloy by Cold-Sprayed Aluminum Coatings Reinforced with Alumina and Carbon Nanotubes. J. Therm. Spray Technol. 2021, 30, 668–679. [Google Scholar]
- Su, Y.P.; Yue, T.M. Microstructures of the bonding area in laser cladded Zr-based amorphous alloy coating on magnesium. Mater. Today Commun. 2020, 25, 101715. [Google Scholar] [CrossRef]
- Tan, C.L.; Zhu, H.M.; Kuang, T.C.; Shi, J.; Liu, H.W.; Liu, Z.W. Laser cladding Al-based amorphous-nanocrystalline composite coatings on AZ80 magnesium alloy under water cooling condition. J. Alloys Compd. 2017, 690, 108–115. [Google Scholar] [CrossRef]
- Cen, L.; Du, W.; Gong, M.C.; Lu, Y.; Zhang, C.; Gao, M. Effect of high-frequency beam oscillation on microstructures and cracks in laser cladding of Al-Cu-Mg alloys. Surf. Coat. Technol. 2022, 447, 128852. [Google Scholar] [CrossRef]
- Li, N.; Wei, Z.T.; Zhao, W.J.; Yan, S.; Liu, D.Z.; Jiao, Q.J. Improved ignition and combustion performance of Al-Zn-Mg ternary alloys by incorporating Mg into Al-Zn alloys. Chem. Eng. J. 2024, 494, 153237. [Google Scholar] [CrossRef]
- Yamasaki, M.; Shi, Z.; Atrens, A.; Furukawa, A.; Kawamura, Y. Influence of crystallographic orientation and Al alloying on the corrosion behaviour of extruded α-Mg/LPSO two-phase Mg-Zn-Y alloys with multimodal microstructure. Corros. Sci. 2022, 200, 110237. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, K.M.; Zou, J.X. Microstructures and properties in surface layers of Mg-6Zn-1Ca magnesium alloy laser-clad with Al-Si powders. Trans. Nonferr. Metal. Soc. 2018, 28, 96–102. [Google Scholar] [CrossRef]
- Lei, Y.W.; Sun, R.L.; Tang, Y.; Niu, W. Experimental and thermodynamic investigations into the microstructure of laser clad Al–Si coatings on AZ91D alloys. Surf. Coat. Technol. 2012, 207, 400–405. [Google Scholar] [CrossRef]
- Wan, S.M.; Cui, X.F.; Jin, G.; Ma, J.J.; Yang, Y.Y.; Liu, K.J.; Li, J.; Wang, S.; Wang, J.Y. Microstructure and properties characterization of laser-cladded Cu-Al alloy coatings on Mg-Li alloy. Surf. Coat. Technol. 2023, 460, 129430. [Google Scholar] [CrossRef]
- Gao, Y.L.; Wang, C.S.; Pang, H.J.; Liu, H.B.; Yao, M. Broad-beam laser cladding of Al–Cu alloy coating on AZ91HP magnesium alloy. Appl. Surf. Sci. 2007, 253, 4917–4922. [Google Scholar] [CrossRef]
- Nie, M.H.; Zhang, S.; Wang, Z.Y.; Zhang, C.H.; Chen, H.T.; Chen, J. Effect of laser power on microstructure and interfacial bonding strength of laser cladding 17-4PH stainless steel coatings. Mater. Chem. Phys. 2022, 275, 125236. [Google Scholar] [CrossRef]
- He, C.Y.; Du, Y.; Chen, H.L.; Ouyang, H.W. Measurement of the isothermal sections at 700 and 427 °C in the Al–Mg–Ni system. Int. J. Mater. Res. 2008, 99, 907–911. [Google Scholar] [CrossRef]
- Gan, Z.T.; Yu, G.; He, X.L.; Li, S.X. Numerical simulation of thermal behavior and multicomponent mass transfer in direct laser deposition of Co-base alloy on steel. Int. J. Heat. Mass. Tran. 2017, 104, 28–38. [Google Scholar] [CrossRef]
- Gao, Y.L.; Xiong, D.S.; Wang, C.S.; Chen, Y.Z. Influences of laser powers on microstructure and properties of the coatings on the AZ91HP magnesium alloy. Acta Metall. Sin.-Engl. Lett. 2009, 22, 167–173. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Y.P.; Hu, L.; Li, Y.K. Microstructure and mechanical properties laser cladding 304 stainless steel. Appl. Laser 2019, 39, 191–197. [Google Scholar]
- Luo, X.X.; Cao, J.; Meng, G.H.; Chuan, Y.Y.; Yao, Z.J.; Xie, H. Systematical investigation on the microstructures and tribological properties of Fe-Al laser cladding coatings. Appl. Surf. Sci. 2020, 516, 146121. [Google Scholar] [CrossRef]
- Zheng, B.J.; Chen, X.M.; Lian, J.S. Microstructure and wear property of laser cladding Al+SiC powders on AZ91D magnesium alloy. Opt. Laser Eng. 2010, 48, 526–532. [Google Scholar] [CrossRef]
- Taltavull, C.; Torres, B.; Lopez, A.J.; Rodrigo, P.; Otero, E.; Atrens, A.; Rams, J. Corrosion behaviour of laser surface melted magnesium alloy AZ91D. Mater. Des. 2014, 57, 40–50. [Google Scholar] [CrossRef]
- Bu, R.; Jin, A.X.; Sun, Q.; Zan, W.; He, R.L. Study on laser cladding and properties of AZ63-Er alloy for automobile engine. J. Mater. Res. Technol. 2020, 9, 5154–5160. [Google Scholar] [CrossRef]
- Sun, Y.P.; Yang, C.T.; Yang, C.G.; Xu, D.K.; Li, Q.; Yin, L.; Qiu, C.S.; Liu, D.; Yang, K. Stern–Geary Constant for X80 Pipeline Steel in the Presence of Different Corrosive Microorganisms. Acta Metall. Sin.-Engl. Lett. 2019, 32, 1483–1489. [Google Scholar] [CrossRef]
- Sun, F.Z.; Li, X.X.; Zheng, K.Y.; Bo, H.; Li, Y.; Zang, Y.; Pang, M. Study on the Influence of Graphene Content Variation on the Microstructure Evolution and Properties of Laser Additive Manufacturing Nickel-Based/SiC Composite Cladding Layer on Aluminum Alloy Surface. Materials 2022, 15, 8219. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Wang, C.S.; Lin, Q.; Liu, H.B.; Yao, M. Broad-beam laser cladding of Al–Si alloy coating on AZ91HP magnesium alloy. Surf. Coat. Technol. 2006, 201, 2701–2706. [Google Scholar] [CrossRef]
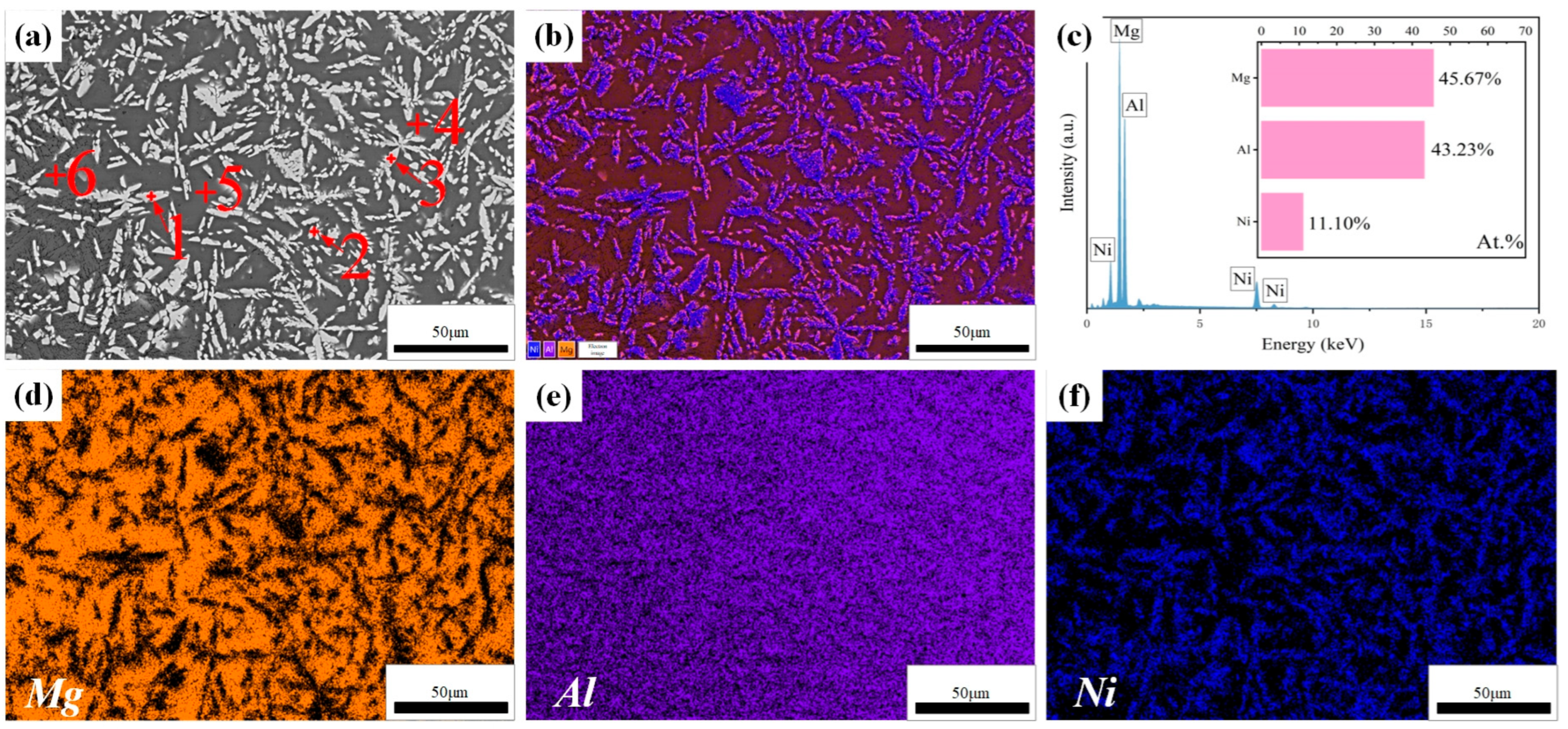
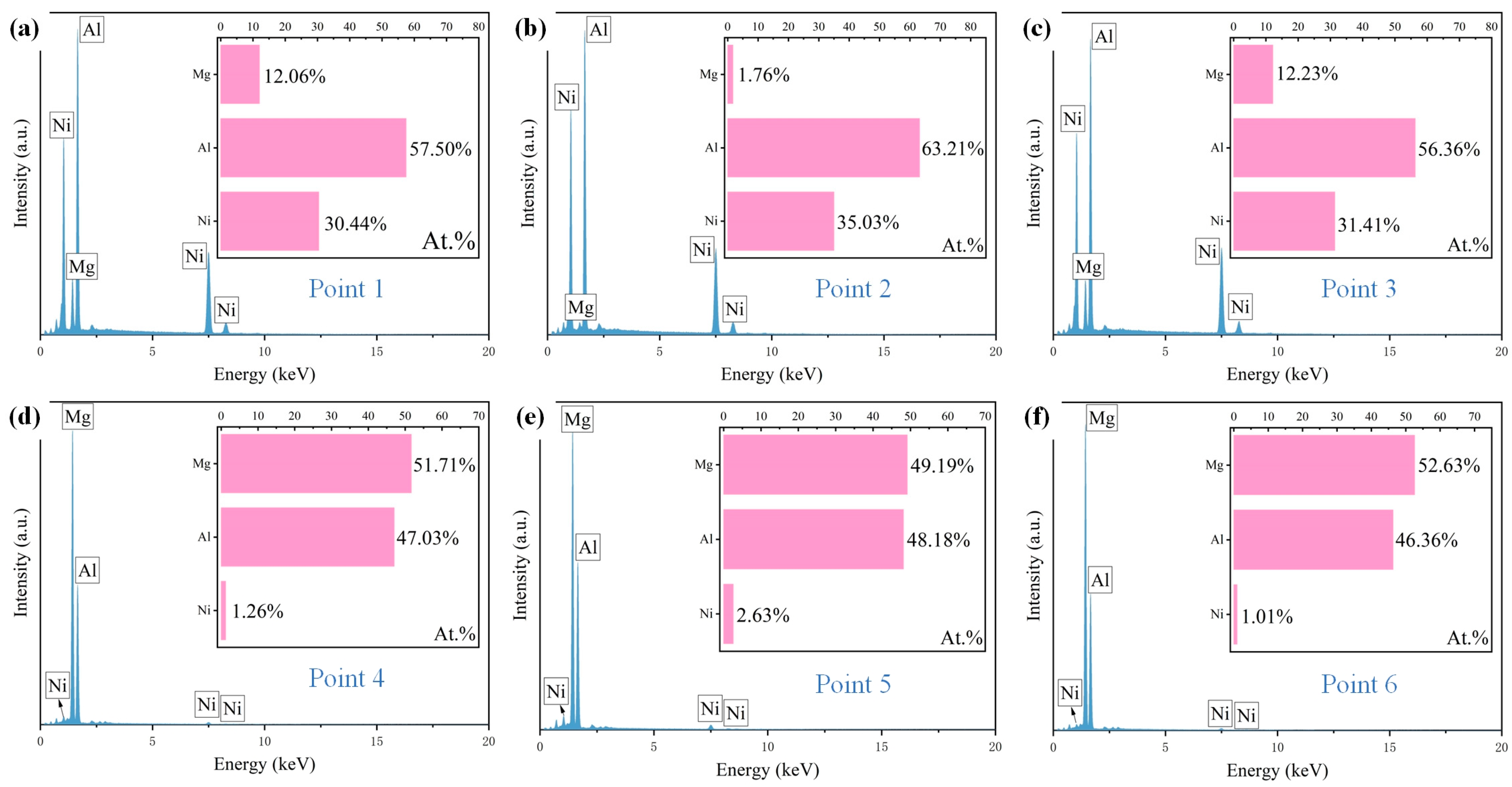



| Element | Al | Zn | Mn | Si | Fe | Cu | Ni | Be | Mg |
|---|---|---|---|---|---|---|---|---|---|
| Mass percentage | 8.8900 | 0.5620 | 0.2041 | 0.0443 | 0.0030 | 0.0034 | 0.0090 | 0.0012 | Bal. |
| Element | Al | Ni |
|---|---|---|
| Mass percentage (wt. %) | 64.8 | 35.2 |
| Laser Power (W) | Scanning Speed (mm/min) | Overlap Rate (%) | Laser Spot Diameter (mm) | Argon Flow Rate (L/min) |
|---|---|---|---|---|
| 900 | 360 | 30 | 3 | 5 |
| 900 | 480 | 30 | 3 | 5 |
| 900 | 600 | 30 | 3 | 5 |
| 900 | 720 | 30 | 3 | 5 |
| Scanning Speed (mm/min) | Diffraction Intensity | Peak Width at Half Height | Grain Size (nm) |
|---|---|---|---|
| 360 | 584 | 0.591 | 14.3 |
| 480 | 253 | 0.650 | 13.0 |
| 600 | 221 | 0.697 | 12.1 |
| 720 | 292 | 0.612 | 13.8 |
| Coating | Ecorr (V) | Icorr (A/cm2) | Cathodic Slope | Anodic Slope | Rp (Ω) |
|---|---|---|---|---|---|
| 360 mm/min | −1.29 | 3.548 × 10−3 | 5.875 | 6.331 | 10 |
| 480 mm/min | −0.955 | 1.075 × 10−4 | 6.876 | 3.564 | 387 |
| 600 mm/min | −0.918 | 1.261 × 10−4 | 9.541 | 2.262 | 292 |
| 720 mm/min | −1.298 | 2.587 × 10−3 | 8.068 | 5.247 | 13 |
| Mg alloy | −1.438 | 3.623 × 10−3 | 3.336 | 5.149 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Jiang, S.; Lu, P.; Bai, S.; Zhang, D.; Jie, M. Influence of Laser Scanning Speed on Wear and Corrosion Resistance of Aluminum–Nickel Coating on Magnesium Alloy. Processes 2024, 12, 1689. https://doi.org/10.3390/pr12081689
Gao Y, Jiang S, Lu P, Bai S, Zhang D, Jie M. Influence of Laser Scanning Speed on Wear and Corrosion Resistance of Aluminum–Nickel Coating on Magnesium Alloy. Processes. 2024; 12(8):1689. https://doi.org/10.3390/pr12081689
Chicago/Turabian StyleGao, Yali, Shan Jiang, Pengyong Lu, Sicheng Bai, Dongdong Zhang, and Meng Jie. 2024. "Influence of Laser Scanning Speed on Wear and Corrosion Resistance of Aluminum–Nickel Coating on Magnesium Alloy" Processes 12, no. 8: 1689. https://doi.org/10.3390/pr12081689





