Abstract
Due to the serious scarcity of water resources and the aggravation of water pollution in northern China, replenishing reclaimed water can alleviate the water shortage problem in northern rivers to a certain extent, but has also become an important way for antibiotic resistance genes (ARGs) to spread into rivers. In order to study the characteristics of ARGs in reclaimed water recharge rivers (Wenyu River), the abundance and distribution of ARGs in a typical reclaimed water replenishment river were analyzed by metagenomic sequencing technology combined with river water quality. The results showed that: due to the input of reclaimed water, the water quality characteristics of downstream sampling points of the river were significantly different from those upstream. Water quality factors such as total organic carbon, chemical oxygen demand, chlorophyll, and total nitrogen tended to increase gradually. Quinolones and macrolides were the main types of antibiotics. A total of 1217 ARGs were detected in the reclaimed water river system, including multidrug resistance, macrolide-lincosamide-streptogramin (MLS), tetracycline, glycopeptide, peptide, aminocoumarin, etc. The average abundance of ARGs in reclaimed water was higher than that in rivers. Among them, multidrug ARGs existed most widely, which may gradually become the main trend of ARGs’ evolutionary variation. RDA results revealed that the environmental factors EC and DO, as well as tetracycline antibiotics (TCs), may be important environmental factors affecting the distribution of ARGs.
1. Introduction
The progression of societal development has driven industrialization and urbanization, which has been accompanied by a growing demand for water resources. Currently, over 400 cities in China are facing the predicament of inadequate water supply [1]. To alleviate this issue, numerous cities have emphasized the exploration and utilization of new water sources, with wastewater recycling and resource recovery being recognized as crucial avenues to mitigate water scarcity. As an emerging type of supplementary water source, reclaimed water is frequently utilized as the primary source for urban greening and river replenishment due to its abundant and relatively stable water volume [2,3]. However, this practice can have negative consequences. Previous research has reported that reclaimed wastewater can significantly affect the physicochemical properties and biota structure of receiving water bodies, and even pose ecological risks [4], such as the risk of eutrophication caused by excessive nitrogen input and ecological risk caused by environmental hormones [5,6]. Among these, the risk of resistance gene transmission deserves attention.
The increasing emergence and spread of antibiotic resistance genes (ARGs) has become one of the hot issues of global public health concern [7,8,9]. China, being a major producer and consumer of antibiotics, is facing a serious problem of antibiotic resistance [10]. The massive use of antibiotics induces and accelerates the risk of antibiotic resistance gene generation, transmission, and the formation of drug-resistant bacteria [11]. Antibiotic resistance has emerged in human and animal pathogenic and commensal bacteria [12], and the increase and spread of antibiotic resistance has become a huge problem in the treatment of diseases, resulting in no effective medicines in the face of some deadly infections [12,13,14]. Unlike traditional chemical pollutants, ARGs exhibit unique environmental behaviors due to their inherent biological properties, such as replicability, dissemination, and environmental persistence [15]. Once these ARGs enter into human or animal pathogenic bacteria, they will pose a potential threat to human public health [9,16]. Studies have shown that resistance genes are widely present in the environment in rivers, soils, and activated sludge from sewage treatment plants [17]. Among them, river ecosystems are the main sites for harboring land-based pollutants, such as the discharge of municipal sewage and farm effluents, leading to the possibility that rivers could be potential reservoirs for resistance genes [18]. Due to factors such as economic and technological development, ARGs carried in reclaimed water are discharged into rivers, which become an important pathway for the diffusion and spread of ARGs into the environment, causing environmental pollution and posing a potential threat to human health, and their environmental behaviors and characteristics have received extensive attention from researchers [19,20].
This study aimed to investigate the distribution characteristics of ARGs, and key environmental factors affecting the distribution of ARGs under reclaimed water recharge stress in the Wenyu River, a core area and “mother river” of Beijing. The Wenyu River is a major sink that receives reclaimed water (2.81 × 108 kiloton/year) from five wastewater treatment plants (WWTPs) that account for the majority of its flow. Therefore, the Wenyu River is an ideal region for investigating the impact of reclaimed water [21]. With the help of modern molecular biology macro-genome sequencing technology and bioinformatic analysis, the diversity and distribution of antibiotic resistance genes in a river replenished by reclaimed water were identified to provide a theoretical basis for the resourceful use of reclaimed water and the control of ecological risks.
2. Materials and Methods
2.1. Study Area and Sampling
The Wenyu River originates in Beijing and has water flowing year-round [22]. It is the upstream reach of the Beijing-Hangzhou Grand Canal, which is located in the core area of Beijing and is regarded as the “Mother River” of Beijing [22]. The Wenyu River is formed by the confluence of three main tributaries, respectively, the Dongsha River, the Beisha River, and the Nansha River, and receives tributaries such as the Qinghe River, the Beixiahe River, and other tributaries along the way, and then merges into the North Canal at the Beiguan Gate in Tongzhou with a total length of 55 km and a watershed area of 2478 km2 [23,24]. It undertakes the drainage of the Changping District, the Shunyi District, and other parts of the urban area and receives 90% of Beijing’s drainage tasks [25]. In August 2022, surface water samples were collected from the main stream of the Wenyu River and the effluents of the five WWTPs, and the information on the sampling sites is shown in Figure 1.
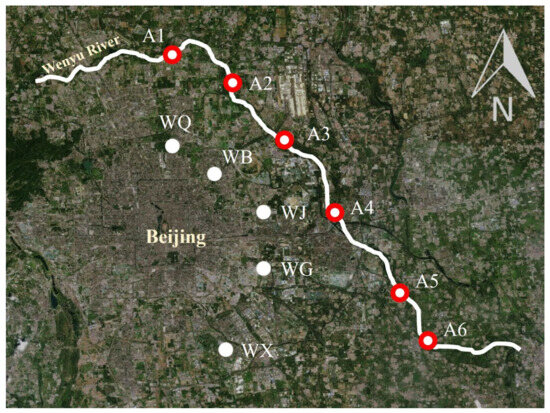
Figure 1.
Schematic diagram of study area and sampling point locations (WQ, WB, WJ, WG and WX, represented with the white dot) and river sites (A1–A6, represented with the red circle).
2.2. Sample Collection and Storage
Surface water samples were collected using a stainless steel water collector, which was cleaned three times with ultrapure water before collecting samples, and then rinsed three times with water samples from the sampling area during sampling. Samples for physical and chemical testing of water quality were contained in brown glass bottles soaked in acid and cleaned, and samples for microbial DNA extraction and sequencing were contained in 1.5 L polyethylene sampling bottles cleaned and aseptically processed in advance.
2.3. Chemical Analysis
Water temperature (T), pH, oxidation-reduction potential (ORP), conductivity, and other conventional water quality indicators were measured on-site using a HACH HQ30D (USA). Chemical oxygen demand (COD), total phosphorus (TP), total nitrogen (TN), and other indicators refer to the Monitoring and analysis methods of previously described protocol [21]. Chlorophyll a was determined by spectrophotometry [26], and ammonia nitrogen (NH3-N), nitrate nitrogen (NO3-N), and nitrite nitrogen (NO2-N) were determined by Nessler’s reagent photometry [27], UV spectrophotometry [28], and spectrophotometry [29], respectively. The detection of pharmaceuticals and personal care products (PPCPs) in water was carried out using solid phase extraction (SPE) followed by ultraperformance liquid chromatography–triple quadrupole mass spectrometry [30].
2.4. Biological Analysis
Total microbial DNA was extracted from the samples, and the total amount and purity of DNA samples were examined. The hypervariable V3–V4 region of the bacterial 16S rRNA gene in each sample was amplified using a universal primer set of 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 909R (5′-CCCCGYCAATTCMTTTRAGT-3′) with a 12 nt unique barcode. PCR amplification was performed according to a previously described method [31]. The PCR products were tested with 1% agarose gel electrophoresis and a NanoDrop Spectrophotometer. The PE150 bipartite sequencing was performed using the Illumina HiSeq Xten platform [32]. Firstly, the quality control of the original sequences was carried out, removing the sequences with a base number greater than 40 bp but a mass fraction lower than 38, removing the sequences with more than 10 divergent nucleotides, and removing the sequences with a 15 bp overlap with the adapter sequence. SOAP-denovo software (version 2.2.4) was used to assemble clean reads [33].
2.5. Data Analysis
Trend analysis was performed using Microsoft Excel (Version 2019). Statistical analysis was carried out with SPSS software (version 20.0). Redundancy analysis (RDA) and nonmetric multidimensional scaling analysis (NMDS) was processed and plotted with Canoco 5.0. Principal coordinate analysis (PCoA) and constrained principal coordinate analysis (CPCoA) were processed and plotted with R (4.0.3) using its amplicon package. SOAPaligner software (SOAP2, version 2.20) was used to compare the retained clean reads with the gene library to calculate the number of matches. Unigene was compared with the NCBI-NR database using DIAMOND software, setting the BLASTP parameter E-value ≤ 1 × 10−5, and annotated using the LCA algorithm of MEGEN software (version 1.3.4-1). The sequencing depth should have reached at least 10,000×, and the representative sequences with ≥97% similarity were assigned to the same OTUs. The ARGs were annotated using the comprehensive antibiotic resistance database (CARD) [34].
3. Results and Discussion
3.1. Physicochemical Indices
An in-depth analysis of the physicochemical attributes of water samples collected from various points along the Wenyu River and the sewage treatment facility utilizing principal coordinate analysis (PCoA), revealed notable disparities in water quality between river samples and reclaimed water (Figure 2 and Figure 3). Nevertheless, a noteworthy variation emerged in the water quality at the downstream river sampling stations (A4–A6), as compared to the upstream location (A1), potentially attributed to the substantial influx of reclaimed water. Electrical conductivity (EC), a metric that quantifies a solution’s capacity to conduct electricity, serves as a proxy for dissolved mineral content fluctuations [35]. Within this study area, the EC of reclaimed water stood notably higher at 939.40 μs/cm, contrasted with 888.5 μs/cm for river water, reflecting the elevated ionic concentrations inherent in the reclaimed water.
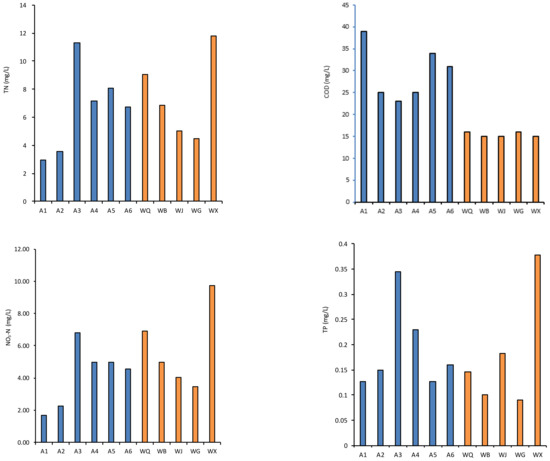
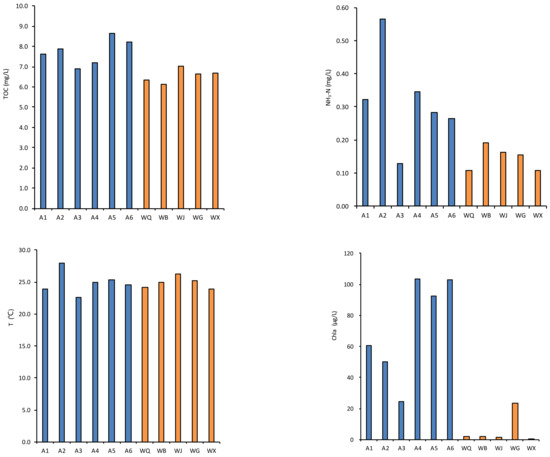
Figure 2.
Variation of water quality parameters of the effluents of WWTP sampling sites (WQ, WB, WJ, WG and WX, represented with the color blue) and river sites (A1–A6, represented with the color yellow) in the Wenyu River basin.
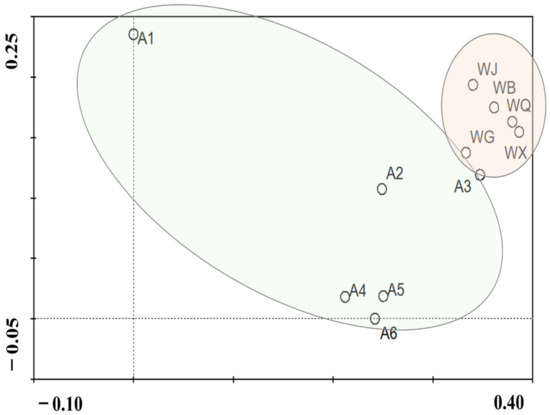
Figure 3.
The diversity of the water quality parameters between river (Green ellipse) and WWTP sample (Red ellipse) sites is demonstrated by Principal coordinate analyses (PCoA).
The pH levels of the water samples ranged from 7.85 to 8.84, indicating a weakly alkaline nature with robust buffering capabilities. Conversely, the effluents from the reclaimed water plants exhibited a pH spectrum of 7.63 to 8.91 across the tributaries. Intriguingly, the pH of the Wenyu River water demonstrated a gradual increase from upstream to downstream, with its average value exceeding that of the reclaimed water plants’ effluents.
The mean chlorophyll a (Chla) content in the river amounted to 72.31 μg/L, peaking at 103.32 μg/L. Parallel to this, the dissolved oxygen (DO) levels followed a comparable pattern, with the river water system maintaining a generally higher DO concentration than the effluent. The total organic carbon (TOC) and chemical oxygen demand (COD) concentrations exhibited an increasing trend from the upper reaches to the lower reaches along the mainstream. The COD concentration in the effluents from the sewage treatment plants within the study area is lower than that in the river, primarily attributed to the adoption of advanced sewage treatment processes. Through physical, chemical, and biological methods, organic matter in sewage can be effectively removed, leading to a marked reduction in COD values. Furthermore, the sewage treatment plants adhere to stringent discharge standards, ensuring that the quality of their effluents meets or exceeds environmental protection requirements. The DO concentration in rivers is influenced by a myriad of natural factors, including water temperature, atmospheric pressure, flow velocity, and the photosynthetic activity of aquatic plants, among others. Conversely, the DO concentration in the effluents of sewage treatment plants typically hinges on the sewage treatment processes employed. Because of the consumption of DO in the secondary sedimentation tank and aerobic tank in the sewage treatment process, the DO concentration in the river may be higher than that in the effluents from the sewage treatment plants.
In terms of nutrient salt concentration in water bodies, the total nitrogen (TN) concentration range in river water was 2.94–11.30 mg/L. The effluents emanating from the reclaimed water facility exhibited an average concentration of 7.45 mg/L, surpassing the river’s average of 6.63 mg/L. Along the course of the Wenyu River, a discernible trend of rising total nitrogen levels is observed, progressing from the upstream reaches towards the downstream end. This augmentation primarily stems from the influx of nitrogen-laden reclaimed water. Nitrate nitrogen (NO3-N) concentration had a similar trend to that of TN, with river NO3-N values (mean 4.21 mg/L) lower than reclaimed water effluents (mean 5.81 mg/L). The concentration of ammonia nitrogen (NH3-N) in the river water (mean 0.32 mg/L) was higher than that in the reclaimed water (mean 0.15 mg/L). Total phosphorus (TP) concentrations in the river ranged from 0.13 to 0.34 mg/L, which was similar to the reclaimed water effluents’ concentration levels (0.09 to 0.38 mg/L).
3.2. Characterization of Antibiotic Distribution in a River Replenished by Reclaimed Water
The main antibiotic concentrations in the water bodies of the Wenyu River and the effluents from the sewage treatment plants were shown in Figure 4. Eleven antibiotics were detected to varying degrees at all sampling points of the Wenyu River and the effluents from the sewage treatment plants. The average antibiotic concentration at the sampling points of the Wenyu River was 64.5 μg/L, with the highest concentration of 146.9 μg/L at A3. The average antibiotic concentration at the sampling points for the effluents from the sewage treatment plants was 84.1 μg/L, with the highest antibiotic concentration of 124.9 μg/L in WQ. Among different kinds of antibiotics, quinolones accounted for the highest proportion, accounting for 59.6%, followed by macrolide (34.5%), chloramphenicol (3.5%) and tetracycline (2.3%); at the reclaimed water sampling points, quinolones accounted for 84.7%, followed by macrolides (10.2%), tetracyclines (2.8%) and chloramphenicol (2.3%). This result is consistent with the conclusions of relevant research on recycled water in China, such as Li et al., who studied quinolones and found they were the dominant antibiotics detected in 45 wastewater treatment plants in 23 cities in China, followed by macrolides, and sulfonamides. High concentrations of quinolones in sewage sludge indicated that antibiotics are widely used and extensive pollutants in China [36]. In this study, the detected concentration of quinolone antibiotics in the Wenyu River water ranged from 21.4 to 76.5 μg/L, with ofloxacin and norfloxacin exhibiting higher average concentrations and 100% detection rates, reaching 7.9 μg/L and 21.9 μg/L, respectively. The detected concentration of macrolide antibiotics ranged from 10.2 to 60.4 μg/L, with clindamycin having a higher average concentration (9.2 μg/L) and a 100% detection rate. In the effluents of sewage treatment plants, the detected concentration of quinolone antibiotics ranged from 30.6 to 108.7 μg/L, with ofloxacin and norfloxacin exhibiting higher average concentrations and 100% detection rates, reaching 27.5 μg/L and 35.6 μg/L, respectively. The detected concentration of macrolide antibiotics ranged from 0.99 to 14.0 μg/L, with clindamycin having a higher average concentration (5.9 μg/L) and a 100% detection rate.
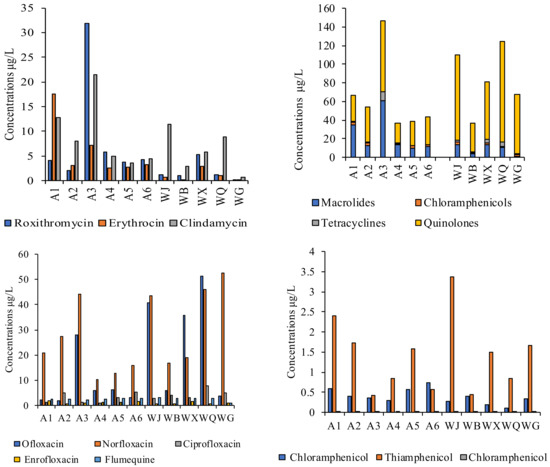
Figure 4.
Concentrations of antibiotics in different sampling sites.
3.3. Diversity of Antibiotic Resistance Genes in a River Replenished by Reclaimed Water
A total of 1217 ARGs were detected in the reclaimed water river system, which were classified into 21 categories according to antibiotic resistance categories, including multidrug, macrolide-lincosamide-streptogramin (MLS), tetracycline, glycopeptide, peptide, aminocoumarin, and others (Figure 5). The abundance of different types of resistance genes was as follows: multidrug (643,362 reads/L) > MLS (175,918 reads/L) > tetracycline (148,575 reads/L) > glycopeptide (126,129 reads/L) > peptide (94,859 reads/L) > aminocoumarin (68,281 reads/L).
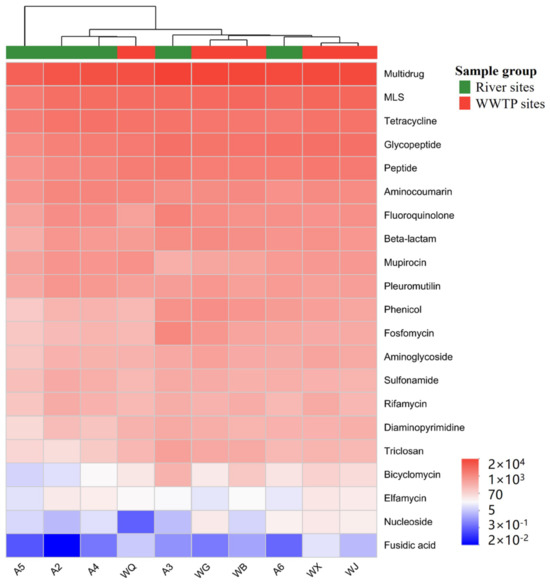
Figure 5.
Heatmap of ARGs’ abundance at different sampling points.
The abundance of ARGs was also different at different sampling points. The average ARGs’ gene abundance in river water (73,950 reads/L) was lower than that in reclaimed water (75,289 reads/L). Meanwhile, numerous previous studies have found that the abundance of ARGs in the effluents of WWTPs was much higher than that in river water. Hang et al., for example, studied the effects of reclaimed water recharge on bacterial communities and ARGs in urban landscape water bodies [37], and found that the amount of specific ARGs in urban landscape water bodies replenished by reclaimed water was relatively increased. This may be explained by the effects of horizontal gene transfer (HGT) and wastewater treatment processes on ARGs. The effluent is prepared from sewage through the advanced treatment process. During the treatment process, a considerable number of ARGs may be greatly removed. It also had been suggested that most of the microorganisms and ARGs in the reclaimed water came from the pipe network and may exist in dark conditions during transportation and migration, making their response to sunlight irradiation more drastic, leading to gradual attenuation after entering the receiving river. However, it is worth noting that domestic wastewater containing a large amount of residual antibiotics and ARGs may create an ideal environment for the physical transportation, acquisition and spread of ARGs from wastewater treatment systems into rivers.
Among the detected resistance gene classes, multidrug and MLS were the two most abundant classes at all sites, and all had lower average abundance in river water than in reclaimed water. Combined with the main antibiotic classes detected in river surface water, the degree of antibiotic residues can affect the production, migration, and spread of some ARGs to a certain extent. The ARGs detected in the river and reclaimed water bodies mainly contained 442 β-lactamase resistance genes, 188 multidrug resistance genes, 132 aminoglycoside resistance genes, 98 MLS resistance genes, 70 glycopeptide resistance genes, 62 tetracycline resistance genes, etc. Among them, β-lactamase accounted for the highest proportion (36.3%), followed by multidrug resistance genes (15.4%), aminoglycoside antibiotics (10.8%), MLS (8.1%), glycopeptides (5.8%), and tetracyclines (5.1%). The number of β-lactamase species is 36%, but its abundance is only 2.9%, and the number of aminoglycoside antibiotics reached 10.8%, but their abundance was only 0.8%. Resistance genes not only had a high abundance (40.5%) but also a high number of detected species (36.3%). This showed that under the long-term selection of multiple antibiotics, microorganisms in rivers and reclaimed water bodies had screened and evolved ARGs that were resistant to multiple antibiotics and more adaptable to complex and changeable environments, thus promoting the survival and reproduction of multi-resistant ARGs and multi-antibiotic resistant microorganisms.
3.4. Diversity of Multi-Resistant ARGs in a River Replenished by Reclaimed Water
As a category with a relatively high number of species and abundance, multi-antibiotic resistance genes (MARGs) were detected with an average abundance of 643,362 reads/L in the surface water of the Wenyu River and reclaimed water (Figure 6 and Supplementary Table S1). Specifically, the average abundance in the surface water of the Wenyu River was 620,105 reads/L, while that in reclaimed water was 666,618 reads/L. A total of 188 MARGs were identified in both water bodies, with 185 species detected in the surface water of the Wenyu River and an equal number found in reclaimed water. Notably, the surface water of the Wenyu River harbors unique MARGs such as H-NS, LAP-2, and mdtO, whereas AAC(6′)-Ib-cr and Klebsiella pneumoniae KpnF are exclusive to reclaimed water. Regarding the abundance ranking of MARGs in the Wenyu River, the most prevalent is msbA (42,972 reads/L), followed by evgS (254,984 reads/L), smeS (33,809 reads/L), mtrA (31,880 reads/L), and rpoB2 (22,505 reads/L). The abundance of multi-antibiotic resistance genes (MARGs) in reclaimed water was msbA (44,956 reads/L) > evgS (30,578 reads/L) > adeL (29,028 reads/L) > smeS (21,804 reads/L) > arlS (21,427 reads/L). Based on the clustering analysis of MARGs, significant differences were observed in the microbial community structures between river water and reclaimed water. Specifically, the compositions of MARGs in samples A2 and A4 from the Wenyu River are similar to those in the reclaimed water WQ, whereas the downstream site A6 in the Wenyu River shares similar MARG compositions with reclaimed water samples WX and WJ. This indicates that the presence of wastewater treatment plants exerts an influence on the microbial functional structure of local river segments.
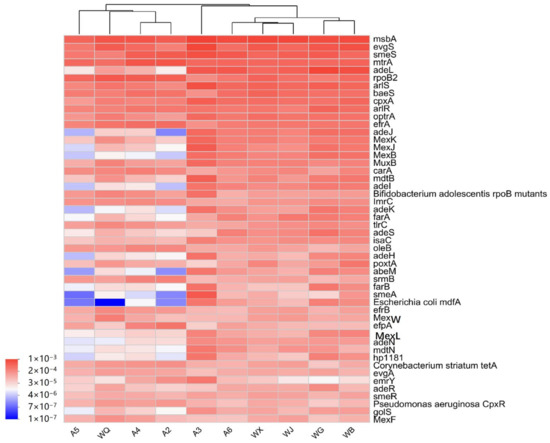
Figure 6.
Heatmap of multi-resistant ARGs’ abundance at different sampling points.
The resistance mechanisms of multi-antibiotic resistance genes (MARGs) in the surface water samples collected from the Wenyu River and reclaimed water sites are statistically annotated and illustrated in Figure 7. The major resistance mechanisms of MARGs can be categorized into eight types, including antibiotic efflux (140 species, 78%), antibiotic target protection (25 species, 14%), antibiotic target alteration (7 species, 4%), antibiotic target alteration/antibiotic target replacement (2 species, 1%). Across different water bodies, the abundance of these eight resistance mechanisms consistently follows antibiotic efflux > antibiotic target protection > antibiotic target alteration/antibiotic target replacement > antibiotic target alteration. The top five most abundant MARGs in reclaimed water all belong to the antibiotic efflux mechanism, while the most abundant five MARGs in the Wenyu River comprise both antibiotic efflux and antibiotic target alteration/antibiotic target replacement. There is a significant difference in abundance between antibiotic efflux and other resistance mechanisms (p < 0.01).
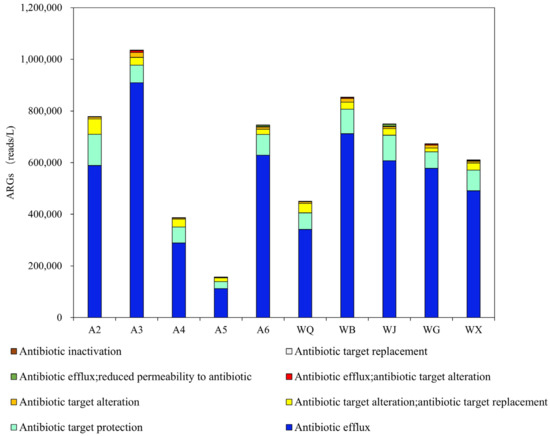
Figure 7.
Mechanism of multi-drug resistance genes in the surface water of the Wenyu River and reclaimed water sampling sites.
3.5. Correlation Analysis of Antibiotic Resistance Gene Distribution and Environmental Factors
Correlation analysis between the distribution of ARGs and multiple environmental factors (including temperature, COD, pH, DO, electrical conductivity (EC), and antibiotics) was conducted using the RDA method. The results revealed that ARGs explained 94.65% and 3.05% of the variations along Axes I and II, respectively, suggesting a close relationship between ARGs’ abundance and environmental factors to a certain extent. As depicted in Figure 8, the physicochemical factor EC and tetracycline antibiotics (TCs) were intimately associated with the distribution of resistance genes in the surface water of the study area, aligning with previous research findings. Notably, DO emerged as a crucial parameter influencing ARGs’ distribution, as it reflects the self-purification capacity of water. This observation concurs with previous reports of a strong negative correlation between DO and various ARGs, such as sul2, blaTEM, and four tetracycline resistance genes. In this study, DO also showed a negative correlation with multiple ARGs, including rifamycin resistance genes. The figure further indicated a positive correlation between TCs and multi-resistance genes (r = 0.64848, p < 0.05), while the relationship with tetracycline resistance genes was not significant. EC displayed a positive correlation with both multi-resistance and aminocoumarin resistance genes. Overall, the combined effects of antibiotics and physicochemical factors significantly influence the distribution of ARGs.
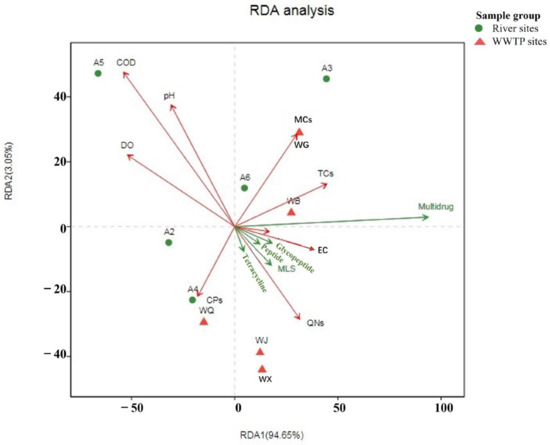
Figure 8.
RDA analysis of total ARGs and environmental factors in the surface water of sampling sites in the Wenyu River and reclaimed water.
4. Conclusions
- (1)
- As a result of significant quantities of recycled water being introduced, the water quality downstream of the river noticeably varies from that of the upstream. With the input of reclaimed water, the total organic carbon (TOC), chemical oxygen demand (COD), chlorophyll (Chla), and total nitrogen (TN) in the surface water of the Wenyu River gradually increased.
- (2)
- Eleven types of antibiotics were detected in the river water and sewage treatment plants’ effluents. Among them, quinolones and macrolides were the main types of antibiotics. The distribution of ARGs in river water bodies was significantly different from that of reclaimed water, and the abundance of ARGs in river water bodies was lower than that of reclaimed water, with multi-resistance genes the most widespread class, which may gradually become the main trend of evolutionary variation of ARGs.
- (3)
- Through the correlation analysis between the distribution of ARGs and environmental variables, the environmental factors EC and DO, as well as tetracycline antibiotics (TCs) among antibiotics, may be important environmental factors affecting the distribution of ARGs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr12081717/s1, Table S1: The ARGs conferring resistance to different antibiotic classes, multiple drugs, and MGEs detected in the effluents of WWTPs and river sites.
Author Contributions
Conceptualization and Writing, X.Z.; data curation, H.L.; funding acquisition, J.N.; methodology and project administration, X.W. and W.W.; supervision, P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China, grant number [2022YFB2602000].
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Geng, Y.; Wang, M.; Sarkis, J.; Xue, B.; Zhang, L.; Fujita, T.; Yu, X.; Ren, W.; Zhang, L.; Dong, H. Spatial-temporal patterns and driving factors for industrial wastewater emission in China. J. Clean. Prod. 2014, 76, 116–124. [Google Scholar] [CrossRef]
- Barker-Reid, F.; Fox, E.M.; Faggian, R. Occurrence of antibiotic resistance genes in reclaimed water and river water in the Werribee Basin, Australia. J. Water Health 2010, 8, 521–531. [Google Scholar] [CrossRef][Green Version]
- Cai, Y.; Bi, Y.; Tian, B.; Cheng, L.; Zhou, S.; Qi, Q. Water quality characteristics and ecological risk evaluation of a landscaped river replenished by three reclaimed water sources in Qingdao, China. Environ. Sci. Pollut. Res. 2024, 31, 35609–35618. [Google Scholar] [CrossRef]
- White, S.A. Pharmaceuticals and Persistent Organic Micropollutants in Reclaimed Irrigation Water. In Proceedings of the 2013 ASHS Annual Conference, Palm Desert, CA, USA, 24 July 2013. [Google Scholar]
- Du, C.; Guo, W.; Li, G.; Bai, M.; Zhu, Q.; Tian, Z.; Li, M.; Zhao, C.; Zhang, L. Biomanipulation as a strategy for minimizing ecological risks in river supplied with reclaimed water. Environ. Res. Sect. A 2023, 228, 115801. [Google Scholar] [CrossRef] [PubMed]
- Zhong-Hong, C.; Yong-Min, M.A.; Wei, H.U. Endocrine Disrupting Compounds in Reclaimed Water Produced by a Certain Water Plant: Some Preliminary Results. J. Environ. Health 2005, 22, 171–173. [Google Scholar]
- Zhu, G.; Xue, C.; Fan, H.; Wu, N. Research progress of antibiotic resistance genes in environment. Prev. Med. 2020, 32, 1121–1125. [Google Scholar]
- He, R.; Yuan, K.; Lin, L.; Yang, Y.; Zou, S.; Luan, T.; Chen, B. Functional metagenomics: One of the most robust tools for discovering new antibiotics resistance genes. Environ. Chem. 2019, 7, 1548–1556. [Google Scholar]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hao, F.; Wang, W.; Lu, Y.; He, L.; Wang, G.; Chen, W. Multicenter cross-sectional observational study of antibiotic resistance and the genotypes of Propionibacterium acnes isolated from Chinese patients with acne vulgaris. J. Dermatol. 2016, 43, 406–413. [Google Scholar] [CrossRef]
- Mahaney, A.; Franklin, R. Persistence of wastewater-associated antibiotic resistant bacteria in river microcosms. Sci. Total Environ. 2022, 819, 153099. [Google Scholar] [CrossRef]
- Caroline, P.; Orosco, W.G.; Deon, K.J.; Marisa, C. Genotyping and antimicrobial resistance in Escherichia coli from pig carcasses. Pesqui. Veterinária Bras. 2017, 37, 1253–1260. [Google Scholar]
- Lian, C.; Piksa, M.; Yoshida, K.; Persheyev, S.; Pawlik, K.J.; Matczyszyn, K.; Samuel, I.D.W. Flexible organic light-emitting diodes for antimicrobial photodynamic therapy. Npj Flex. Electron. 2019, 3, 18. [Google Scholar] [CrossRef]
- Nan, W.U. Tetracycline Residues and Tetracycline Resistance Gene Pollution in Soil:A Review. Asian J. Ecotoxicol. 2010, 5, 618–627. [Google Scholar]
- Matarese, V. Kinds of Replicability: Different Terms and Different Functions. Axiomathes 2022, 32, 647–670. [Google Scholar] [CrossRef]
- Dafale, N.A.; Srivastava, S.; Purohit, H.J. Zoonosis: An Emerging Link to Antibiotic Resistance Under “One Health Approach”. Indian J. Microbiol. 2020, 60, 139–152. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Y.; Wang, H.; Guo, C.; Qiu, H.; He, Y.; Zhang, Y.; Li, X.; Meng, W. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 2015, 119, 1379–1385. [Google Scholar] [CrossRef]
- Szmolka, A.; Wami, H.; Dobrindt, U. Comparative Genomics of Emerging Lineages and Mobile Resistomes of Contemporary Broiler Strains of Salmonella Infantis and E. coli. Front. Microbiol. 2021, 12, 642125. [Google Scholar] [CrossRef]
- Guan, X.; Guo, Z.; Wang, X.; Xiang, S.; Sun, T.; Zhao, R.; He, J.; Liu, F. Transfer route and driving forces of antibiotic resistance genes from reclaimed water to groundwater. Environ. Pollut. 2023, 330, 121800. [Google Scholar] [CrossRef]
- Wang, C.; Hong, P.Y. Genome-Resolved Metagenomics and Antibiotic Resistance Genes Analysis in Reclaimed Water Distribution Systems. Water 2020, 12, 3477. [Google Scholar] [CrossRef]
- Zhao, X.; Xie, E. Reclaimed water influences bacterioplankton and bacteriobenthos communities differently in river networks. Water Res. 2023, 243, 120389. [Google Scholar] [CrossRef]
- Linjin, L.; Baohui, M.; Rui, P. Water Quality Evaluation of Wenyu River Based on Single Factor Evaluation and Comprehensive Pollution Index Method. Nat. Environ. Pollut. Technol. 2021, 20, 1041–1046. [Google Scholar] [CrossRef]
- Mengyuan, Z.; Li, Y.; Fan, S.; Dong, L.; Hao, P. Study on Greenway Plant Landscape Based on Bird Habitat Conservation—A Case Study of Wenyu River—North Canal Greenway in Beijing. In Proceedings of the Fábos Conference on Landscape and Greenway Planning, Amherst, MA, USA, 28–30 March 2019. [Google Scholar]
- Qi, W.; Liu, H.; Pernet-Coudrier, B. Polycyclic aromatic hydrocarbons in wastewater, WWTPs effluents and in the recipient waters of Beijing, China. Environ. Sci. Pollut. Res. 2013, 20, 4254–4260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Liu, Y.; Wang, J.L.; Zhao, J.Z. Factor Analysis of Heavy Metals in the Sediment in Changping Section of the Wenyu River and Their Pollution Assessment. Res. Environ. Sci. 2013, 26, 838–843. [Google Scholar]
- Ergun, E.; Demirata, B.; Gumus, G.; Apak, R. Simultaneous determination of chlorophyll a and chlorophyll b by derivative spectrophotometry. Anal Bioanal Chem. 2004, 379, 803–811. [Google Scholar] [CrossRef]
- Xian, G.; Fen, C.; Lan, Q. Application Research of Environmental Monitoring on Ammonia Nitrogen Determination. J. Green Sci. Technol. 2017, 18, 117–119. [Google Scholar]
- Ji, X. Comparison on nitrate nitrogen by detections of RP-HPLC and thymol UV-Vis spectrophotometry. J. Chang. Inst. Technol. (Nat. Sci. Ed.) 2014, 15, 1–4. [Google Scholar]
- Gou, Y. Determination of total nitrogen in water samples by spectrophotometry using phenol after alkaline peroxodisulfate digestion. Bunseki Kagaku 2001, 50, 481–486. [Google Scholar] [CrossRef][Green Version]
- Lara-Martín, P.A.; González-Mazo, E.; Pintado-Herrera, M.G.; Baena-Nogueras, R.M. Determination of Pharmaceuticals in Coastal Systems Using Solid Phase Extraction (SPE) Followed by Ultra Performance Liquid Chromatography—Tandem Mass Spectrometry (UPLC-MS/MS). Curr. Anal. Chem. 2016, 12, 183–201. [Google Scholar]
- Xie, E.; Zhao, X.; Li, K.; Zhang, P.; Zhou, X.; Zhao, X. Microbial community structure in the river sediments from upstream of Guanting Reservoir: Potential impacts of reclaimed water recharge. Sci. Total Environ. 2021, 766, 142609. [Google Scholar] [CrossRef]
- Shahbazi, Z.; Rostami, G.; Hamid, M. New heritableATRXmutation identified by whole exome sequencing and review. Egyptian J. Med. Hum. Genet. 2022, 23, 19. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Liu, W.; Luo, Z.; Wang, P.; Zhang, Y.; Zheng, R.; Shi, J. Transcriptome Characteristics and Six Alternative Expressed Genes Positively Correlated with the Phase Transition of Annual Cambial Activities in Chinese Fir (Cunninghamia lanceolata (Lamb.) Hook). PLoS ONE 2013, 8, e71562. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shen, P.; Zheng, B.; Yu, W.; Xiao, Y. Comparative Genomic Analysis of 19 Clinical Isolates of Tigecycline-Resistant Acinetobacter baumannii. Front. Microbiol. 2020, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Yevatikar, R.; Raxamwar, T.S. Comparative study of physico-chemical parameters and water quality index of river. Mater. Today Proc. 2022, 60, 859–867. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Gao, L.; Liu, J.; Cai, Y. Occurrence, Distribution and Potential Affecting Factors of Antibiotics in Sewage Sludge of Wastewater Treatment Plants in China. Sci. Total Environ. 2013, 445–446, 306–313. [Google Scholar] [CrossRef]
- Zhang, C.M.; Liang, J.; Liu, W.Y. Comparative study on the bacterial diversity and antibiotic resistance genes of urban landscape waters replenished by reclaimed water and surface water in Xi’an, China. Environ. Sci. Pollut. Res. 2021, 28, 41396–41406. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).