Abstract
This study aimed to determine the optimal pressure and temperature for the maximum extraction yield and recovery of lipophilic bioactive compounds (BACs) during the supercritical carbon dioxide extraction (SCO2) of fennel seeds. For this purpose, the SCO2 pressure (78.6–361.4 bar) and temperature (35.9–64.1 °C) were varied and optimized, and all of the extracts obtained were analyzed for the volatiles, fatty acids, sterols, tocochromanols and carotenoids. The results showed that the maximum extract yield and content of all of the compounds analyzed favored a higher pressure (320 bar) and lower temperature (40 °C), except for the volatiles, which were the highest at 120 bar and 42 °C. However, the optimal SCO2 conditions for obtaining the highest overall total lipophilic fraction were 320 bar and 40 °C, respectively. The fennel SCO2 extract obtained under these conditions contained 18 volatiles (trans-anethole as the major component), 12 fatty acids (oleic and petroselinic as the major compounds), 12 sterols (β-sitosterol and stigmasterol as the major compounds), two pentacyclic triterpenoids (α-, β-amyrin), one tocopherol (α-tocopherol), two tocotrienols (γ-, δ-tocotrienol) as well as two carotenoids (lutein and β-carotene). The SCO2 proved to be very efficient for the isolation of various lipophilic BACs from fennel, and the results of this study may be of interest to academia and industry.
1. Introduction
Fennel (Foeniculum vulgare Mill.) is a widespread perennial herbaceous plant from the Apiaceae family, characterized by specific small, golden-yellow flowers and ellipsoid, yellowish to greenish seeds [1]. Fennel seeds are known for their characteristic anise-like flavor, which is due to their richness in essential oil, the main component of which is usually anethole [2]. In addition to the essential oil, fennel seeds are also rich in other hydrophilic and lipophilic bioactive compounds (BACs) such as phenols, especially hydroxycinnamic acids and flavonols [3], fatty acids, of which oleic acid and petroselinic acid occur in the largest amounts, phytosterols, such as β-sitosterol and stigmasterol [4], tocochromanols [5] and pigments [6]. Many studies have documented the positive biological effects of F. vulgare, such as the antioxidant [7], anti-inflammatory [8], antimicrobial [9,10], antitumor and cytoprotective [11], chemopreventive [12], hypoglycemic [13], hepatoprotective [14] and estrogenic effects [15], which are due to the presence of various BACs and that make the plant well-suited for pharmaceutical and cosmetic purposes [1,16]. In addition to its wide therapeutic use, fennel, especially its essential oil, is also used in the food industry as a natural additive [17].
To reap the benefits of fennel, the choice of the extraction method is decisive. As fennel volatile oil is the most widely used fennel product, hydrodistillation and steam distillation are the most commonly applied for its extraction [2]. However, these conventional extraction methods require the use of high temperatures, are time and energy consuming and have limited process yields. This has encouraged the development of alternative advanced approaches to overcome these drawbacks, which, in addition to the questionable cost-effectiveness, may also lead to the reduced quality of the final product due to the degradation of the heat-sensitive components [2]. These innovative strategies include, for example, microwave- and ultrasound-assisted extraction, subcritical water extraction and supercritical fluid extraction (SFE), all of which allow for a shorter extraction time and lower energy costs while providing higher yields of products with more stable quality, and which are overall more environmentally friendly. Among the extraction methods listed, SFE is certainly preferred due to its high extraction efficiency, the facile removal of the solvent and good selectivity [18].
The main steps of SFE are extraction and separation. Extraction takes place in a column filled with the sample of interest, through which a stream of supercritical solvent is passed under pressure, separating the extracted solids from the solid sample. The dissolved substances are then separated by diffusion and the supercritical solvent is isolated from the extract by reducing the pressure and/or increasing the temperature [19]. The most common supercritical solvent for the SFE is carbon dioxide due to its desirable properties, such as its chemical inertness, non-toxicity, food-grade status, low cost and availability [20]. It is characterized by its non-polarity, low critical temperature and pressure (31.1 °C and 73.8 bar) at which it behaves simultaneously as a liquid and a gas, and has the potential to extract heat-sensitive components. Moreover, its high density enables the better solubility of compounds, while its low viscosity allows for the penetration into solids and better diffusion due to lower friction [19]. Precisely because of its moderate working conditions and the use of low temperatures, supercritical carbon dioxide extraction (SCO2) provides an energy-efficient, highly soluble, selective and solvent-free extract. All of these characteristics provide SCO2 with the advantage of choice in the extraction of BACs from plants.
However, precisely because of the SCO2 selectivity and the possibility of fractionation, which depend mainly on the temperature and pressure applied during extraction, it is very important to choose these parameters carefully by relying on the choice of the target compounds, as any change can affect the final result. The behavior and mutual interaction and dependence of the pressure and temperature is an unavoidable consideration in terms of achieving the maximum extraction yield of the target compounds. The extraction efficiency is mainly affected by the pressure applied during the process, as, generally, an increase in pressure increases the solubility of the target compounds as a result of the increased density of the solvent. Thus, if a higher pressure is applied, the extraction is carried out with a lower volume of fluid. However, one should be careful, as high pressure is not suitable for the extraction of all desired compounds. On the other hand, the influence of the extraction temperature at a certain pressure manifests oppositely based on the density and the vapor pressure, i.e., an increased temperature reduces the density of the solvent and therefore the solubility of the compounds; at the same time, it enhances their solubility due to the improved vapor pressure of the solutes [21]. So, the solubility of the solutes no longer depends on the density of the solvent, which is known as the crossover phenomena of retrogradation [19,21]. Therefore, a higher temperature would gain a lower extraction yield for non-volatiles, while the volatiles compete between their solubility in the solvent (which decreases with the temperature increase) and volatility (which increases with the temperature increase) [21]. This emphasizes the need to adjust the processing conditions of the pressure and temperature according to the solubility equilibrium of the target compounds. According to Cvjetko Bubalo et al. [21], a pressure of 90–100 bar and a temperature of 40–50 °C are recommended for the extraction of essential oil, while Fornari et al. [22] suggest a slightly wider span, namely, 90–120 bar and 35–50 °C. At higher pressures, the extracted essential oil is not pure, as it is extracted together with cuticular waxes [21]. On the contrary, seed oils prefer SCO2 at higher pressures, as triglycerides are soluble at 40 °C and a pressure > 280 bar [23]. Regarding the polar compounds, their extraction via SCO2 requires the addition of co-solvents due to their low solubility in supercritical carbon dioxide, which is a very non-polar solvent, as mentioned earlier. The general rule is that the solubility of compounds in supercritical carbon dioxide decreases as the number of polar functional groups (hydroxyl, carboxyl, etc.) increases. Therefore, polar co-solvents such as ethanol and methanol are used in the SCO2 of phenolic compounds at concentrations < 10% of carbon dioxide, with ethanol being preferred due to its lower toxicity. Although the efficiency depends on the plant material, the target compounds and the process conditions, the general conditions for the SCO2 of phenolics are a pressure of 200 bar, a temperature of 60 °C and an addition of 5% ethanol as a co-solvent [19].
Although SCO2 offers many advantages, such as the preservation of heat-sensitive components, solvent-free pure extracts, the enhanced recovery of target compounds, the possibility of fractionation and selective extraction, and carbon dioxide is environmentally friendly, generally recognized as safe (GRAS) and of a low cost, its main shortcomings are the high investment and operating costs, the inability to extract insoluble high polar components (sugars, amino acids, proteins, etc.) and the difficult design of the extraction conditions due to the complex phase equilibrium of the solvent/solute system [21]. Still, significant progress in the industrial application of SCO2 has been observed in recent years, where it has been extensively applied in the food, pharmaceutical and cosmetics industries, and SCO2 equipment at varying scales according to its intended use (in the laboratory, pilot studies or industrial use) is now commercially produced [19].
The application of SCO2 for the extraction of BACs from fennel has already been documented in the literature, focusing mainly on the extraction of essential oil and volatiles as well as fatty acids [24,25], but to the best of the authors’ knowledge, no study has provided a comprehensive and detailed analysis of the fennel SCO2 extract with respect to the other lipophilic components present. Therefore, the aim of this study was to determine the best-fitting pressure and temperature which will ensure the maximum recovery of lipophilic BACs, namely, volatiles, fatty acids, sterols and pentacyclic triterpenoids, tocochromanols and carotenoids, during the SCO2 of fennel seeds. In addition, the goal was to provide an overall qualitative and quantitative characterization of these compounds in the obtained fennel SCO2 extract.
2. Materials and Methods
2.1. Chemicals
Water was purified in a Milli-Q Plus water system (Millipore, Bedford, MA, USA). CO2 (99.97%, w/w) was procured from Messer Croatia Plin Ltd. (Zaprešić, Croatia); n-hexane was procured from Fisher Scientific (Loughborough, UK); methanol, ethanol (96%), acetone, isooctane, diethyl ether and isopropanol were procured from Lach-Ner Ltd. (Brno, Czech Republic); formic acid (98–100%) was procured from T.T.T. Ltd. (Sveta Nedelja, Croatia); acetonitrile was procured from J.T. Baker Chemicals (Deventer, The Netherlands). Potassium hydroxide, sodium chloride and anhydrous sodium hydrogen sulfate were obtained from Kemika Ltd. (Zagreb, Croatia), and aluminum oxide was obtained from Alfa Aesar Chemical (Thermo Fisher Scientific, Geel, Belgium). Commercial standards of R-(+)-limonene and nerol were purchased from Fluka® Analytical (Munich, Germany); myrcene was purchased from Merck (Darmstadt, Germany); α-terpinene and estragole were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany); (+)-α-pinene, camphene, (−)-β-pinene, (R)-(-)-α-phellandrene, 3-carene, p-cymene, γ-terpinene, (+)-carvone, eucalyptol, (+)-fenchone, (±)-camphor, p-anisaldehyde, trans-anethole, FAME Mix and petroselinic acid, α-tocopherol, γ-tocotrienol, δ-tocotrienol, β-carotene, lutein, methyl tert-butyl ether and alkane standard solution C7–C30 were purchased from Sigma Aldrich (St. Louis, MO, USA). Finally, α-cholestanol and a mixture of pyridine, hexamethyldisilazane and trimethylchlorosilane were obtained from Honeywell Fluka (Seelze, Germany). All of the solvents were of HPLC grade.
2.2. Plant Material
Dry bitter fennel seeds (F. vulgare Mill.) cultivated in Croatia were procured from Agristar Ltd. (Višnjevac, Croatia). The seeds were stored in their original paper bag packaging in a dry and dark place until extraction. Immediately before extraction, the seeds were ground using the IKA Basic A11 laboratory mill (Staufen, Germany). The moisture content of the ground seeds was determined by drying at 105 °C to a constant mass [26], which was <5%.
2.3. SCO2
SCO2 was performed in a laboratory-scale extraction system described in detail by Moslavac et al. [27]. An amount of 100 g of ground dry fennel seeds was put into an extraction vessel, and the extraction was carried out by varying the pressure (78.6–361.4 bar) and temperature (35.9–64.1 °C) according to the experimental design, with a constant CO2 flow rate of 1.4 kg/h. Pressure and temperature are two key parameters in the SCO2 extraction process, as changing them changes the density of the fluid and thus the selectivity, hence the solubility, of the bioactive compounds. To see how the pressure and temperature affected the process, the extraction was carried out at different pressures from 78.6 bar (slightly above the critical pressure of the CO2) up to 361.4 bar (almost the maximum working pressure of the device). The extraction temperature of 35.9 °C that was used is just above the critical temperature of the CO2, and the temperature of around 40 °C was generally used for the plant extraction with SCO2. The selected upper temperature limit (64.1 °C) was low enough to avoid damage to the heat-sensitive compounds. The extraction time was established in a preliminary study and set at 15 min (a longer extraction time was not required), at which a full extraction yield was achieved. The separator conditions were 15 bar and 25 °C. The extracts were collected in previously weighed glass vials and weighed (±0.0001 g). The process yield (%) was calculated as the percentage of the ratio between the mass of the extract obtained (g) and the initial mass of the seeds (g) (w/w). The SCO2 extracts were stored at −18 °C until the analysis.
2.4. Determination of Volatiles
Gas chromatography–mass spectrometry (GC-MS) was used for the determination of the volatiles in the fennel SCO2 extracts. The analysis was performed according to the method of Marčac et al. [2] on an Agilent Technologies 6890N Network GC System connected to an Agilent 5973 inert mass selective detector (Agilent Technologies, Santa Clara, CA, USA). The compounds were separated on a capillary column HP-5MS ((5%-phenyl)-methylpolysiloxane; 30 m × 0.25 mm × 0.25 μm) (Agilent Technologies). Prior to the analysis, the extracts were diluted with n-hexane and an internal standard (nerol, 1.0518 mg/mL) was added to a final dilution ratio of 1:99. A volume of 1.0 µL of the prepared sample was injected automatically (Agilent 7683B autosampler injector (Agilent Technologies, Santa Clara, CA, USA)) in split mode (1:100 ratio) at 250 °C and a constant helium (carrier gas) flow rate of 1 mL/min. The temperature program was set as follows: 60–145 °C (3 °C/min), 145–250 °C (30 °C/min) and holding for 3 min at 250 °C. The temperatures of the transfer line, MS source and quadrupole were 280, 230 and 150 °C, respectively, while the ionization energy of the detector was 70 eV. Mass spectra (m/z) were recorded in a range of 30–550 (1 scan/s), and the single-ion monitoring (SIM) mode was used for the quantification. To determine the retention indices (RI) of the compounds, an alkane solution was analyzed under the same analytical conditions, and the RI were calculated according to Bianchi et al. [28].
Identification of the volatiles was performed by comparing their retention times, RI and mass spectra with those of the authentic standards, and by comparison with the mass spectra in the NIST library (ChemStation Data Analysis), while quantification was based on the calibration curves of α-pinene (1.0–9.0 mg/mL; y = 1.4289x + 0.0277, R2 = 0.999), camphene (0.002–0.2 mg/mL; y = 0.5818x − 0.0023, R2 = 0.998), β-pinene (0.0015–0.15 mg/mL; y = 2.4216x − 0.0104; R2 = 0.998), myrcene (0.005–0.5 mg/mL; y = 0.784x − 0.0169, R2 = 0.996), α-phellandrene (0.02–2.0 mg/mL; y = 3.0566x − 0.0485, R2 = 0.999), α-terpinene (0.0005–0.05 mg/mL; y = 0.436x − 0.0005, R2 = 0.998), p-cymene (0.01–0.1 mg/mL; y = 5.5476x − 0.0472, R2 = 0.999), D-limonene (0.005–0.5 mg/mL; y = 1.2779x − 0.0094, R2 = 1.000), eucalyptol (0.005–0.5 mg/mL; y = 0.5776x − 0.0018, R2 = 0.998), γ-terpinene (0.005–0.5 mg/mL; y = 2.0663x − 0.0247, R2 = 0.998), L-fenchone (0.05–5.0 mg/mL; y = 2.5055x + 0.0883, R2 = 0.999), camphor (0.0015–0.15 mg/mL; y = 1.3539x − 0.0054, R2 = 0.998), estragole (0.2–4.0 mg/mL; y = 2.3108x + 0.0337, R2 = 0.998), carvone (0.005–0.5 mg/mL; y = 1.5493x − 0.0162, R2 = 0.998), p-anisaldehyde (0.015–1.5 mg/mL; y = 1.0737x − 0.0397, R2 = 0.998) and trans-anethole (0.2–20.0 mg/mL; y = 2.4576x + 0.9618, R2 = 0.999). Sabinene and cis-sabinene hydrate were identified based on their mass spectra, RI and agreement with the literature data, while the quantification was based on the calibration curve of 3-carene (0.003–0.3 mg/mL; y = 2.05x − 0.0075, R2 = 0.999). The results were expressed in g/100 g of the SCO2 extract.
2.5. Determination of Fatty Acids
The profile of the fatty acids was determined by the gas chromatography of their methyl esters produced by transmethylation according to the standard method, ISO 12966-2:2017 [29]. In brief, the fennel SCO2 extract (0.1 g) and 2 mL of isooctane were mixed in a 10 mL glass tube with a plastic stopper, and 100 μL of methanolic potassium hydroxide solution was added. The tube was shaken vigorously on a vortexer for 1 min, and then allowed to stand at room temperature for a further 2 min to allow the contents to react. Then, 2 mL of sodium chloride solution was added and the mixture was shaken for a few seconds. The resulting isooctane layer with the methyl esters was removed and 1 g of anhydrous sodium hydrogen sulfate was added to remove the residual water. The mixture was shaken again, and the clear solution was transferred to a glass vial. The prepared sample was then analyzed according to the standard method, ISO 12966-4:2015 [30], using the Agilent Technologies 6890N Network GC System (Agilent Technologies, Santa Clara, CA, USA) gas chromatograph coupled with a flame ionization detector (FID). The analysis was performed on a capillary column TR-FAME (70% cyanopropyl polysylphenylene-siloxane; 30 m × 0.22 mm × 0.25 μm) (Thermo Fisher Scientific, Waltham, MA, USA). A volume of 1.0 µL of the sample was injected at 250 °C with a split ratio of 1:75. Helium was used as the carrier gas at a constant flow rate of 0.7 mL/min, and the detector temperature was 280 °C. The oven temperature program was set from 120 to 160 °C at 4 °C/min, and then from 160 to 190 °C at 10 °C/min, with a hold time of 10 min at the final temperature. The fatty acids were identified by comparing the retention times of the fatty acids with the retention times of the 37 Component FAME Mix. Quantification was based on the area normalization method, and quantitative values were expressed as the % of total fatty acids.
2.6. Determination of Sterols
The composition and content of the sterols in the fennel SCO2 extracts were determined following the standard method, ISO 12228-1:2014 [31]. An amount of 0.25 g of the sample, 1 mL of internal standard solution (α-cholestanol, 1 mg/mL) and 5 mL of ethanolic solution of potassium hydroxide (0.5 mol/L) were heated under reflux for 15 min, after which 5 mL of 96% ethanol was immediately added. The unsaponifiable fraction was then eluted on a column containing 10 g of aluminum oxide with 5 mL of ethanol and 3 aliquots of 10 mL of diethyl ether at a solvent flow rate of about 2 mL/min. The resulting eluate was evaporated to dryness at 45 °C using a Büchi R-205 Rotavapor (Büchi Labortechnik AG, Flawil, Switzerland). The residue was then mixed with 0.5 mL diethyl ether and transferred to a silica chromatographic plate, where it was allowed to develop in a 100 mL hexane/diethyl ether solution (1:1, v/v). After development, the dry plate was sprayed evenly with methanol to localize the zone of the sterols. The detected sterol zone was removed with a spatula and filtered through a Büchner funnel with 3 aliquots of 5 mL of diethyl ether. The solvent was then reduced to a volume of 1 mL on a rotary evaporator at 45 °C, transferred to a 10 mL tube and purged to dryness under a stream of nitrogen. Silylation reagent (pyridine, hexamethyldisilazane and trimethylchlorosilane; 5:2:1, v/v/v) was added to the tube containing the sterol fraction (50 µL per 1 mg unsaponifiable fraction), and the sealed tube was heated at 105 °C for 15 min, cooled and centrifuged at 5000 rpm for 10 min. The resulting supernatant was analyzed by gas chromatography using an Agilent Technologies 6890N Network GC System equipped with an FID and DB-17MS ((50%-phenyl)-methylpolysiloxane; 30 m × 0.32 mm × 0.25 µm) capillary column (Agilent Technologies, Santa Clara, CA, USA)). The sample (1.0 µL) was injected at a split ratio of 13.3:1 at 290 °C, and the detector temperature was set to 280 °C. Helium was used as the carrier gas at a constant flow of 1.5 mL/min. The oven temperature was initially 180 °C, heated to 270 °C at 6 °C/min and held at the final temperature for a further 30 min. To confirm the identification of the sterols, the gas chromatograph was coupled to the 5973 inert mass selective detector (Agilent Technologies) with the transfer line temperature set at 280 °C, the MS source at 230 °C and the quadrupole at 150 °C. Mass spectra were scanned in the range 30–550 at 1 scan/s, and sterols were identified using the NIST database. In addition to the sterols, two pentacyclic triterpenoids, namely, α- and β-amyrin, were also identified. The quantification was based on an internal standard and the results were expressed as mg/100 g of the SCO2 extract.
2.7. Determination of Tocochromanols
The standard method, ISO 9936:2006 [32], was used for the determination of the tocochromanols in the fennel SCO2 extracts. Prior to the analysis, the extract (0.001 g) was diluted in 10 mL of n-hexane. The prepared sample (20 μL) was injected into an Agilent 1260 Infinity HPLC system equipped with a 1260 fluorescence detector (FLD) (Agilent Technologies). The analysis was performed on a LiChroCART Silica 60 column (250 mm × 4.6 mm × 5 μm) (Merck) at room temperature by isocratic elution with a 0.7% isopropanol/n-hexane solution (v/v) as the mobile phase at a flow rate of 0.9 mL/min. Tocochromanols were detected at 295 and 330 nm excitation and emission wavelengths, respectively, and quantified using the calibration curves (0.01–0.2 mg/mL) of α-tocopherol (y = 40.137x − 2.815, R2 = 0.995), γ-tocotrienol (y = 49.602x − 5.2343, R2 = 0.965) and δ-tocotrienol (y = 55.528x − 5.0058, R2 = 0.997). The results were expressed as mg/100 g of the SCO2 extract.
2.8. Determination of Carotenoids
The determination of the carotenoids was performed according to the method of Castro-Puyana et al. [33] by high-performance liquid chromatography (HPLC) using the Agilent Infinity 1260 system equipped with an Agilent 1260 photodiode array detector (PDA) (Agilent Technologies). Prior to the analysis, the fennel SCO2 extract (0.5 g) was dissolved in acetone (5 mL). Reverse-phase separation of the compounds was carried out on a Develosil RP-Aqueus C30 column (250 mm × 4.6 mm i.d. × 3 µm) (Phenomenex, Torrance, CA, USA) using the mixture of MeOH/MTBE/water (90:7:3, v/v/v) (A) and MeOH/MTBE (10:90, v/v) (B) as the mobile phase at a flow rate of 0.8 mL/min. The injection volume was 10 µL and the carotenoids were detected at 450 nm. Identification was performed by comparing the retention times and spectral data with those of the authentic standards (lutein and β-carotene; 6.67–100 µg/mL), while the quantification was based on their calibration curves (lutein y = 11.86x, R2= 0.949; β-carotene y = 374.11x, R2 = 0.994). All of the results were expressed as mg/100 g of the SCO2 extract.
2.9. Experimental Design and Statistical Analysis
The experimental design and statistical analysis were performed using Statsoft STATISTICA v.13 Experimental Design Software (Statsoft Inc., Tulsa, OK, USA). The response surface methodology (RSM) and the central composite experimental design, comprising 13 trials with five replicates of the central point, were chosen to analyze the combined effect of two independent variables, pressure and temperature, referred to as X1 and X2, respectively, during the SCO2 of the lipophilic fraction of fennel seeds on the extraction yield and content of the total volatiles, unsaturated fatty acids, sterols and pentacyclic triterpenoids, tocochromanols and carotenoids, and to optimize the extraction process. The independent variables were considered at three levels, namely, low (−1), central (0) and high (1), as follows: for pressure, 120 (−1), 220 (0) and 320 bar (1); for temperature, 40 (−1), 50 (0) and 60 °C (1). The applied α-value for the rotatability of the experimental design was 1.414. The experiments were performed in randomized order according to the experiment number as determined by the software. The experimental data were fitted to a second-order polynomial model to obtain the regression coefficients for the intercept, linear, quadratic and cross-product terms. Analysis of variance (ANOVA) was used to determine the significance between the treatments applied and their effects on the observed responses. The models were fitted by multiple linear regressions, and their validity was tested by the ANOVA and a lack-of-fit test. The confidence level used was 95%. A prediction and profiling tool was used to optimize the SCO2 process. The preference for all of the observed responses was set to high (1.0). The factors were set to the optimal value and observed in 10 steps.
3. Results and Discussion
3.1. SCO2 of Fennel Seeds
The results of the extract yield and contents of the lipophilic BACs in the SCO2 extracts of the fennel seeds, including the total volatiles, unsaturated fatty acids, sterols and pentacyclic triterpenoids, tocochromanols and carotenoids, obtained at different pressures (78.6–361.4 bar) and temperatures (35.9–64.1 °C) during the SCO2 are shown in Table 1.

Table 1.
Yield and composition of the lipophilic fennel SCO2 extracts according to the central composite design.
The yield ranged from 0.44 to 8.77%, with the highest value obtained at 361.4 bar and 50 °C, while the lowest value was observed at 78.6 bar and 50 °C, indicating the determining effect of the applied pressure. Similarly, numerous studies have reported the application of relatively high-pressure values to achieve high extract yields. For example, Hatami et al. [34] reported 9.8% of the SCO2 extract from fennel seeds at 200 bar and 40 °C, while Maitusong et al. [25] documented 3.8% of aromatic oil extract from fennel seeds obtained under the optimal SCO2 conditions (177 bar and 40 °C). Furthermore, Simandi et al. [35] obtained a total of 9.98% of the fennel SCO2 extract at 270 bar and 50 °C using two separators connected in a series, with the conditions of the first separator being 80 bar and 40 °C. In the study by Moura et al. [36], the effects of the harvest time and maturity of the fennel seeds on the SCO2 yield were investigated. The highest yield (12.5%) was obtained with dried fennel seeds harvested in spring and extracted at 300 bar and 40 °C. Interestingly, the extract yield decreased by 42% when the same seeds were harvested in summer, showing the importance of carefully picking an adequate harvest time. Shams et al. [37] even reported a 13.72% yield from the SCO2 extraction of fennel seeds at 200 bar and 50 °C, while Piras et al. [38] reported only a 0.4% yield when the SCO2 was performed at 90 bar and 40 °C on Sardinian wild fennel umbels with seeds, confirming our observations on the pressure effect. However, Coelho et al. [39] reported that mild conditions (90 bar and 40 °C) were sufficient to extract 94% of the fennel seed volatile oil, which could be related to the compounds of interest, namely, the volatiles, as opposed to the total lipophilic fraction, as is the case in this study.
The content of total volatiles ranged from 53.14 to 96.61 g/100 g (Table 1). The highest amount of total volatiles was obtained at 120 bar and 60 °C, while the conditions of 361.4 bar and 50 °C yielded the lowest content of total volatiles, indicating the preference for moderate extraction conditions when the yield of volatiles is in focus. It is important to mention that it was difficult to compare the results obtained in this study with the literature data in terms of the content of total volatiles, as other studies usually provide results expressed as the relative amount of individual volatiles in the total aroma, while the content of total and individual volatiles in this study is expressed as a concentration. However, Maitusong et al. [25] predicted 95.5 mg/g of trans-anethole at 177 bar and 40 °C, also demonstrating moderate SCO2 conditions to be effective for the recovery of volatiles. Hatami et al. [34] documented that a slightly higher pressure, namely, 200 bar, along with 40 °C, is optimal for the extraction of 6.19 mg of anethole/g seeds and 0.29 mg of fenchone/g seeds. On the other hand, Rodríguez-Solana et al. [40] used 3% methanol as a co-solvent and much higher SCO2 conditions (240 bar and 60 °C) to obtain the maximum estragole content (0.132 g/100 g) from dry fennel seeds.
Regarding the fatty acids, only the unsaturated fatty acids were included in the process optimization due to their beneficial properties, as the aim was to optimize the process to obtain an extract rich in these components, while the saturated fatty acids were not considered. The share of total unsaturated fatty acids was found to range from 91.86 to 95.64% (Table 1). The highest yield of total unsaturated fatty acids was observed at 320 bar and 60 °C, and the lowest was observed at 78.6 bar and 50 °C, following the same trend as the total extract yield with respect to the applied pressure. The results obtained are in agreement with the findings of Bettaieb Rebey et al. [41], who reported 94.71% of total unsaturated fatty acids obtained at 200 bar and 40 °C during the SCO2 of fennel seeds.
Furthermore, the results of the sterol analysis revealed that pentacyclic triterpenoids are present in addition to the sterols, so that the content of both chemical groups is given as the content of the total sterols and pentacyclic triterpenoids, which ranged from 19.26 to 478.49 mg/100 g (Table 1). The highest content was found in the extract obtained at 361.4 bar and 50 °C, while the extract obtained at 78.6 bar and 50 °C was characterized with the lowest value, following the previously observed pattern of preference for higher pressure. These results are in agreement with those of Bettaieb Rebey et al. [41], who reported the maximum sterol content of 4.74 mg/g of fennel seed oil extracted with the SCO2 (200 bar and 40 °C).
The total tocochromanols were determined in a range from 25.36 to 37.00 mg/100 g, depending on the applied pressure and temperature (Table 1). The highest content of total tocochromanols was obtained at 220 bar and 50 °C, while the lowest content was determined in an extract produced at 78.6 bar and 50 °C. Total tocopherol contents of 85.57 and 88.20 mg/100 g of kenaf seed oil were reported when extracted with the SCO2 at 600 bar and 80 °C and 600 bar and 40 °C, respectively [42], which is consistent with our findings regarding the use of higher pressure to obtain the maximum yield. In addition, Peng et al. [43] obtained the highest value (5.6 mg/100 g) of γ-tocopherol with the SCO2 of Roselle seed oil at 200 bar and 40 °C, which is similar to our results.
With respect to the total carotenoids, their content ranged from 0.31 to 17.92 mg/100 g (Table 1), with the highest value found in the extract obtained at 361.4 bar and 50 °C, while the extract with the lowest level of total carotenoids was that obtained at 120 bar and 60 °C. Interestingly, no carotenoids were determined in the extract produced at 78.6 bar and 50 °C. As far as the authors are aware, there is no information in the literature on the content of carotenoids in the SCO2 extracts from fennel seeds. However, in the absence of reliable literature sources, the results of this study can be partially compared with similar studies on other matrices. For example, the maximum recovery of total carotenoids from rosehip fruits extracted with the SCO2 was 20.88 mg/g of fruit, achieved at 450 bar and 80 °C [44].
3.2. Process Optimization
Considering that the maximum yield of the extract as well as the recovery of the desired BACs are of great importance, the SCO2 of fennel seeds was optimized using RSM. First, the influence of the examined SCO2 parameters on the extract yield and the content of total volatiles, unsaturated fatty acids, sterols and pentacyclic triterpenoids, tocochromanols and carotenoids was tested using ANOVA, and the results are given in Table 2. The pressure (X1) had a statistically significant influence on the extract yield (Table 2). Figure 1a shows the extract yield in the dependence of the pressure and temperature, where it can be observed that the yield increased with the rise of pressure and reached the highest levels at the maximum applied values, while the influence of the temperature was moderate, as shown by the results of the ANOVA, where only the quadratic term of the temperature effect (X2) showed a significant influence. This indicates that the pressure has a stronger influence on the yield than the temperature. Similar to these results, Machmudah et al. [44] described a significant influence of the pressure and temperature and their interactions on the extract yield, where a low temperature (about 40 °C) and moderate pressure (about 300 bar) contributed to the maximum SCO2 yield of rosehip fruit oil.

Table 2.
Analysis of variance for the effect of pressure and temperature on the yield and composition of the lipophilic fennel SCO2 extract and polynomial fitting models for their prediction.
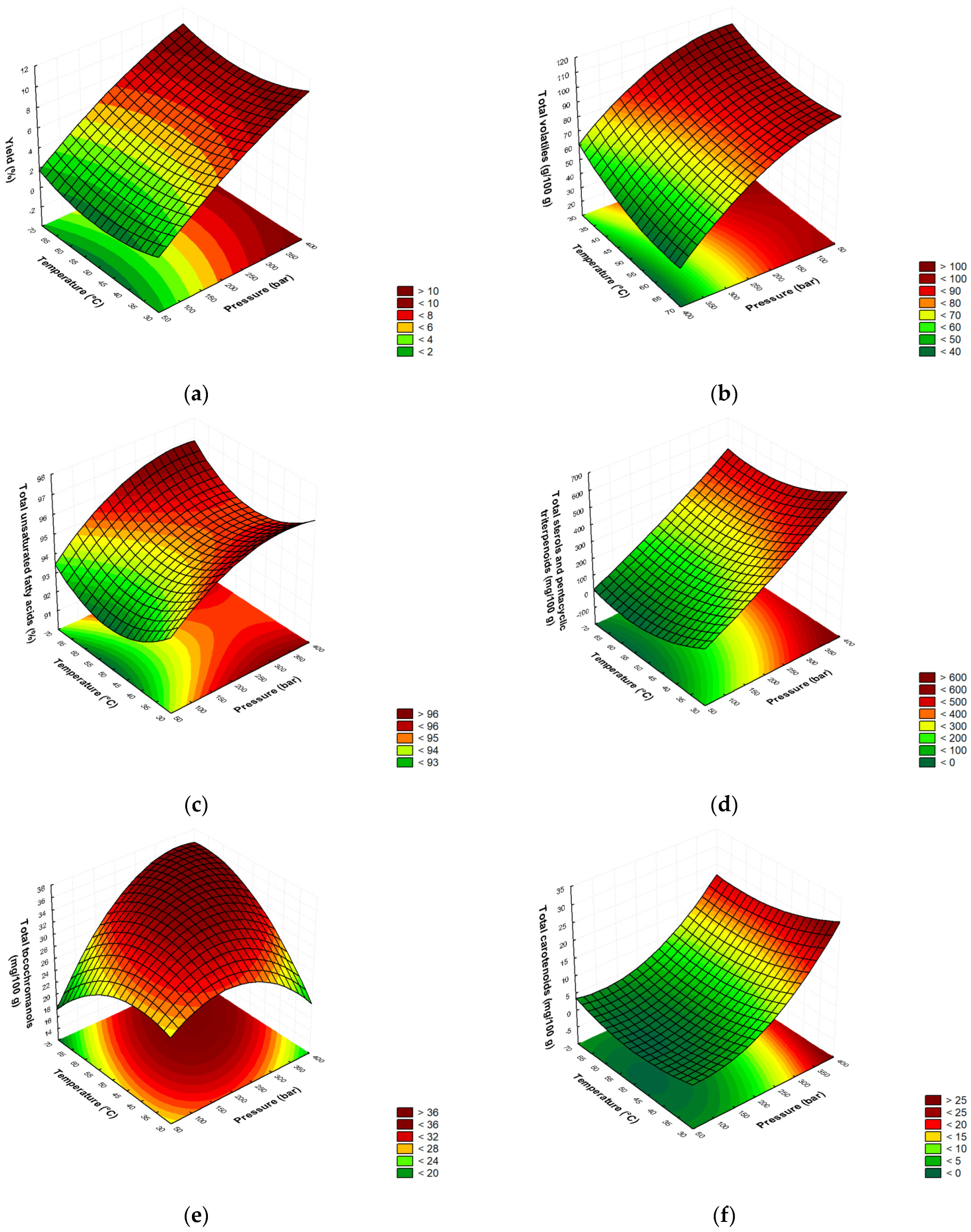
Figure 1.
Three-dimensional graphs of the evolution of the extraction of fennel non-polar bioactives during the SCO2: (a) yield; (b) total volatiles; (c) total unsaturated fatty acids; (d) total sterols and pentacyclic triterpenoids; (e) total tocochromanols; (f) total carotenoids (SCO2 = supercritical carbon dioxide extraction).
Maitusong et al. [25] investigated the influence of the extraction time (30, 60 and 90 min), pressure (100, 150 and 200 bar) and temperature (35, 40 and 45 °C) on the yield of fennel oil and the content of trans-anethole, and found that the extraction time had the greatest influence, while the influence of the temperature and pressure was less significant. However, the interaction between the pressure and temperature on the oil yield was significant. They reported that the oil yield increased with the increasing pressure (about 150 bar) at a stable temperature of 35 °C. In contrast, Moura et al. [36] reported no significant effect of the temperature on the yield of fennel oil during the SCO2, and found that a pressure of 250 bar maximized the yield.
The same statistical methodology was applied to the content of all of the analyzed fennel lipophilic BACs. The results showed that the content of all of the compounds was significantly affected only by the pressure (X1), with the exception of the total sterols and pentacyclic triterpenoids, which were the only ones significantly affected by both the pressure (X1) and temperature (X2) (Table 2). These observations can also be seen in Figure 1b–f, where the main influence of the pressure is clearly visible and the highest amounts of the analyzed compounds were obtained at the highest applied pressure. The only exception is the total volatiles, which reached their highest yield at lower pressure values (Figure 1b). At a constant temperature, increasing the pressure from 50 to 150 bar leads to a dominant increase in the density, i.e., the solubility, and thus to an increase in the total volatile content. Thereafter, the content of the volatiles decreases with further pressure, which leads to a decrease in diffusivity and the mass transfer coefficient becomes more important [24]. Damjanović et al. [45] further explained the dominant effect of the pressure on the yield of volatiles, where increasing the temperature from 40 to 57 °C increased the yield of SFEs from fennel by 9.4%, while increasing the pressure from 100 to 150 bar improved the yield by 38.9%. Additionally, the lower recovery of volatiles at a higher pressure seems to be due to the extraction of compounds with a higher molecular weight, e.g., cuticular waxes, which makes it difficult to analyze the volatile content [21,22]. Damjanović et al. [45] indicated the range of 100–110 bar as the crossover pressure for cuticular waxes and highlighted the pressure of 150 bar as the one at which a significant increase in the yield of cuticular waxes occurs. Mokhtari et al. [24] also confirmed the mentioned extraction trend for the recovery of trans-anethole. They reported that pressure has a dual effect on the extraction, as the volume of supercritical CO2 decreases and its density increases with an increasing pressure, resulting in an increased solubility and higher recovery rate. The opposite effect occurs with a further increase in the pressure, which reduces the diffusivity and mass transfer coefficient, leading to a decrease in the recovery rate [24]. However, increasing the temperature up to a condition called retrograde solubility improves the evaporation of the solvent and thus the recovery of the volatiles. Thereafter, a further increase leads to a lower recovery of the volatiles, which is due to the lower density of the supercritical CO2 and the lower solubility of the solute [24], previously referred to as the “crossover phenomenon”.
Figure 1c shows that the proportion of total unsaturated fatty acids increases overall when the pressure rises above 250 bar. This is in agreement with the statement of Cvjetko Bubalo et al. [21], according to which triglycerides, which form the seed oil, are easily soluble in supercritical CO2 at pressures greater than 280 bar and a temperature of 40 °C. Maheshwari et al. [46] reported that the solubility of fatty acids (lauric, linoleic, myristic, oleic, palmitic and stearic acids) depends on the temperature and pressure of the SCO2. The solubility of oleic acid in CO2 increased from 57 g/g CO2 to 150 g/g of CO2 at 40 °C when the pressure was increased from 138 to 207 bar, while in the same pressure range at 60 °C, the solubility increased from 4.8 to 76 g/g of CO2. A further pressure increase to 276 bar significantly increased the solubility at 40 °C to 210 g/g of CO2 and at 60 °C to 200 g/g of CO2. In contrast, Simándi et al. [35] reported that the different pressures (220, 270 and 320 bar) had no significant effect on the fatty acid composition of the extracts in the second separator during the SCO2 of fennel seeds, while they kept the temperature of the extractor (50 °C), the temperature of the separator (30 °C) and the pressure (80 bar) constant.
The response surface for the recovery of total sterols and pentacyclic triterpenoids as a function of the pressure and temperature is shown in Figure 1d. It can be seen that, at constant temperature, with the further increase in the pressure above 300 bar, the content of total sterols and pentacyclic triterpenoids increased rapidly due to the increased density, resulting in the better solubility of the sterols and pentacyclic triterpenoids and a higher yield. At a temperature of 40–50 °C, the vapor pressure of the solutes increases, which increases the extraction yield. Thereafter, the extraction efficiency decreases due to reaching a transition point called the retrograde solubility, where the density shrinks and the solubility decreases [47]. The positive effect of the pressure and temperature on the extraction of total sterols and pentacyclic triterpenoids could be due to the additional destruction of the cell wall at a high pressure and the increased diffusivity of the oil within the cell at a higher temperature. A temperature of 50 °C in combination with a high pressure improved the recovery of sterols and pentacyclic triterpenoids, but at temperatures above 50 °C, the density of the solvents decreased and exceeded the vapor pressure of the solutes, leading to a decrease in recovery [41,48].
Figure 1e shows the effect of the pressure and temperature on the extraction of total chromanols. It can be seen that the content of total tocochromanols increases with the increase in both the pressure and temperature. It can be observed that increasing the pressure at a constant temperature of 50 °C has no positive effect on the recovery of the tocochromanols in the extract. A similar behavior of the pressure effect on the recovery of tocopherols was observed in the SCO2 extract from quinoa seeds [49].
The behavior of total carotenoids in the dependence of the pressure and temperature is shown in Figure 1f. At constant temperature and pressure above 300 bar, the content of total carotenoids increased. This was to be expected, as the density of supercritical CO2 increases at higher pressure values, which leads to an increase in the dissolution of the carotenoids in the solvent. Machmudah et al. [44] also reported a significantly increased extraction of β-carotene and lutein from rosehip fruits when the pressure was increased, with the highest yield achieved at the maximum pressure. Due to the very low polarity of carotenoids and their high molecular weight, their vapor pressure is low, so the dominant effect of high pressure is understandable. Therefore, a high yield can be expected above 300 bar, as the disruption of the cell wall structures by high pressure leads to a stronger detachment of the carotenoids from the previous structures and allows them to be expelled rapidly from the extraction bed. As for the temperature, lower values favor extraction, but a rapid increase above a moderate value decreases the content of carotenoids, as their stability and bioactivity are affected under these conditions due to their degradation and isomerization [50].
Further, a quadratic regression model was found to be the best-fitted model for all of the variables analyzed in this study (Table 2). However, only the models for the extract yield, total volatiles, total sterols and pentacyclic triterpenoids and total carotenoids were described with adequate R2, R2adj and “lack-of-fit” values, indicating the good fit of the models, while the models for the total unsaturated fatty acids and total tocochromanols showed a poor fit due to the low R2 and R2adj values.
Since the models obtained for the extract yield and content of total volatiles, total sterols and pentacyclic triterpenoids and total carotenoids met the criteria required for a good fit to predict their content under the SCO2, these variables were included in a further optimization step, and the optimal pressure and temperature values for each variable were calculated using the desirability function (Table 3). The optimal pressure and temperature were the same for the extract yield, total volatiles, total sterols and pentacyclic triterpenoids and total carotenoids, namely, 320 bar and 40 °C, while 120 bar and 42 °C were optimal to obtain the highest amount of total volatiles. Based on the models, these conditions ensured an extract yield of 8.18%, 97.51 g/100 g of total volatiles, 431.57 mg/100 g of total sterols and pentacyclic triterpenoids and 13.12 mg/100 g of total carotenoids. These predicted values were confirmed experimentally, with the values obtained being similar to those predicted (Table 3), again showing the good fit of the models. As the optimal conditions for the total volatiles differed compared to the other variables included, the optimal SCO2 conditions for the total lipophilic fraction covering all of the variables in total were also calculated, and the results showed that using 320 bar and 40 °C, 8.18% of the extract yield, 70.22 g/100 g of the total volatiles, 431.57 mg/100 g of the total sterols and pentacyclic triterpenoids and 14.29 mg/100 g of the total carotenoids can be obtained in the total lipophilic fraction. The experimental values were very similar and differed only slightly from the predicted values. It can be noted that only the content of the total volatiles was notably lower when extracted at 320 bar and 40 °C compared to the extract produced at 120 bar and 42 °C, which is understandable given the properties of these compounds and the SCO2 principles, but still sufficient, especially considering that fennel volatiles have a strong flavor. This was also confirmed by the authors, who noted a pleasant and distinctive flavor of the extract produced.

Table 3.
Optimal conditions and corresponding predicted and experimental values of each observed response for the fennel SCO2 extract.
3.3. Characterization of Fennel SCO2 Extract Obtained at Optimal Conditions
After establishing the optimal SCO2 conditions to maximize the recovery of the lipophilic fennel BACs, namely, a pressure of 320 bar and a temperature of 40 °C, the extract obtained under these conditions was further analyzed to determine its chemical profile. The characterization of the extract was carried out by determining the individual volatile compounds, fatty acids, sterols and pentacyclic triterpenoids, tocochromanols and carotenoids and their amounts. The chemical profile obtained is shown in Table 4.

Table 4.
Lipophilic profile of the fennel SCO2 extract obtained at optimal conditions.
As far as the volatiles are concerned, a total of 18 compounds belonging to monoterpene hydrocarbons, oxygenated monoterpenes, phenylpropanoids and aromatic aldehydes (others) were detected in the fennel SCO2 extract tested. The most numerous group was monoterpene hydrocarbons, which included α-pinene, camphene, sabinene, β-pinene, myrcene, α-phellandrene, α-terpinene, p-cymene, D-limonene, γ-terpinene and cis-sabinene hydrate, totaling 7.66 g/100 g. The α-pinene (2.91 g/100 g) accounted for almost 40% of the monoterpene hydrocarbons, followed by myrcene (1.69 g/100 g) and D-limonene (1.17 g/100 g), while the other monoterpene hydrocarbons were present at concentrations < 1 g/100 g. The following oxygenated monoterpenes were also identified: eucalyptol, L-fenchone, camphor and carvone, which accounted for a total of 11.90 g/100 g. Among them, L-fenchone was the most abundant (11.45 g/100 g) and accounted for 96% of the oxygenated monoterpenes. Phenylpropanoids were the most represented group in terms of quantity, accounting for 70% of the volatiles, with trans-anethole being the major volatile compound (45.32 g/100 g), while estragole was found in a low concentration (1.50 g/100 g). The last compound identified was p-anisaldehyde (0.40 g/100 g), an aromatic aldehyde classified as ‘others’. The results obtained are consistent with the chemical composition of the fennel essential oil [2,51,52]. Regarding the volatiles in the fennel SCO2 extract, Damjanović et al. [45] also confirmed a higher content of phenylpropanoids and oxygenated monoterpenes in the fennel SCO2 extract than monoterpene hydrocarbons. However, they identified 21 volatiles, but with the same dominant compounds, trans-anethole (68.6–70.0%) and fenchone (8.40–14.7%), as in this report. Another study [38] also showed similar results, but in an SCO2 extract of fennel aerial parts, which contained 13 volatiles, with trans-anethole (43.4%), fenchone (8.8%) and estragole (42.6%) being predominant, as did Coelho et al. [39], who identified 17 volatiles, with trans-anethole (43%), fenchone (17%) and estragole (21%) being the major compounds.
Using the standard GC method, ISO 12966-4:2015, 12 fatty acids were detected (Table 4). However, the peaks of oleic acid and petroselinic acid were co-eluted and could not be separated due to the structural similarities with the method used. Therefore, the results were reported as the sum of these two fatty acids. The same occurrence has already been reported [4]. Nevertheless, these two fatty acids were the most represented (81.16%) and accounted for almost the entire amount of monounsaturated fatty acids detected (81.63%). Other studies also confirmed that oleic and petroselinic fatty acids are the major and characteristic fatty acids of fennel seeds [4,41]. In addition to oleic and petroselinic fatty acids, other monounsaturated fatty acids found were myristoleic acid, palmitoleic acid, gadoleic acid and erucic acid. Linoleic acid was the second most abundant fatty acid (13.74%), which belongs to polyunsaturated fatty acids, together with α-linolenic acid (0.05%). It should be noted that unsaturated fatty acids are the most represented fatty acids in the fennel SCO2 extract, considering that monounsaturated and polyunsaturated fatty acids together make up 95.42% of the fatty acids, which makes this extract very valuable. The proportion of saturated fatty acids was 4.58%, with palmitic acid dominating at 4.40%. Myristic acid, stearic acid and arachidic acid were also detected. Bettaieb Rebey et al. [41] reported a slightly different composition of fatty acids in the fennel SCO2 extract. Indeed, they confirmed the identification of the same eight fatty acids as in this study, with the exception of myristic acid, myristoleic acid, gadoleic acid and erucic acid. However, as mentioned above, monounsaturated and polyunsaturated fatty acids also accounted for 94.71% in their study.
The next group of lipophilic compounds detected belongs to the sterols, together with the pentacyclic triterpenoids α- and β-amyrin, which were also identified in the analysis of the sterols (Table 4). Balbino et al. [4] also previously reported the presence of amyrins in fennel seeds. The total amount of sterols in the fennel SCO2 extract was 373.20 mg/100 g, with β-sitosterol (100.65 mg/100 g) and stigmasterol (100.49 mg/100 g) accounting for two-thirds of the sterol fraction. In addition to these sterols, Δ7-stigmastenol and stigmastadienol, campesterol, Δ7-avenasterol, spinasterol, 24-methylenecholesterol, Δ5,23-stigmasterol, campestanol, Δ5-avenasterol and Δ7-campesterol were also detected (in the following trend). Bettaieb Rebey et al. [41] confirmed that stigmasterol (2.23 mg/g oil) dominated in the fennel SCO2 extract, although β-sitosterol, the second most abundant sterol, was present at much lower levels (1.61 mg/g oil). They also did not report the presence of campestanol, Δ5,23-stigmasterol, spinasterol, Δ7-stigmastenol, and 24-methylenecholesterol and stigmastadienol in the fennel SCO2 extract. A possible reason for this could be the different pressure values, as a much higher pressure was used in this study, which probably contributed to the better extraction of the sterols. Other studies have also reported stigmasterol as the dominant sterol in fennel seeds and β-sitosterol as the second most important [4,41,53]. To the best of the authors’ knowledge, and based on the available literature, this is the first study to detect 24-methylenecholesterol and stigmastadienol in the fennel SCO2 extract. Pentacyclic triterpenoids accounted for 23.48 mg/100 g, with β-amyrin being predominant (19.74 mg/100 g), followed by α-amyrin (3.74 mg/100 g). These qualitative results are in agreement with the findings of Balbino et al. [4], who found the same distribution of amyrins in fennel seed oil. A further literature comparison was not possible, as there are no available papers on the presence of amyrins in fennel SCO2 extracts.
Using the standard HPLC method according to ISO 9936:2006, one tocopherol (α-tocopherol) and two tocotrienols (γ- and δ-tocotrienol) were detected in the fennel SCO2 extract, which together accounted for 33.80 mg/100 g (Table 4). Moreover, α-tocopherol and γ-tocotrienol were present in similar amounts (12.70 and 11.59 mg/100 g, respectively), while the concentration of δ-tocotrienol was slightly lower (9.50 mg/100 g). It can be seen that the tocotrienols are more abundant in the fennel SCO2 extract (21.10 mg/100 g) than the tocopherols. Again, the literature search did not reveal any studies dealing with the composition of tocochromanols in fennel SCO2 extracts. Nevertheless, Moser et al. [5] also documented the greater presence of tocotrienols in the lipid fraction of steam-distilled fennel seeds than tocopherols, although they also detected γ-tocopherol and α-tocotrienol in addition to the tocochromanols detected in this study. El-Assri et al. [54] confirmed the abundance of tocotrienols over tocopherols in fennel seed oil, with α-tocotrienol having the highest individual content. Matthaus and Ozcan [55] reported that γ-tocotrienol is the most abundant individual component in fennel seed oil, besides α-tocotrienol and γ-tocopherol, which was not detected in this study. Pencheva et al. [56] also reported γ-tocotrienol as the major tocochromanol in fennel seed oil. Qualitative differences in the composition of tocochromanols could be due to the origin of the fennel seeds.
Finally, the carotenoid content was 13.42 mg/100 g, with lutein being the most important carotenoid (12.20 mg/100 g) in the fennel SCO2 extract. In addition to lutein, β-carotene was also detected, although at a much lower level (1.22 mg/100 g). Abdesslem et al. [6] reported the opposite results in fennel seeds, i.e., a high content of β-carotene but no occurrence of lutein.
4. Conclusions
Based on the results of this study, the optimal SCO2 conditions to achieve the maximum process yield and extraction of lipophilic BACs from fennel seeds were established, namely, a pressure of 320 bar and a temperature of 40 °C. These conditions resulted in a yield of 8.28% of the fennel SCO2 extract, which was rich in volatiles (66.78 g/100 g), fatty acids (especially unsaturated; 95.42%), sterols and pentacyclic triterpenoids (396.68 mg/100 g), tocochromanols (33.80 mg/100 g) and carotenoids (13.42 mg/100 g). Although the best conditions to obtain the highest content of volatiles alone (96.58 g/100 g) were 120 bar and 42 °C, the use of 320 bar and 40 °C also ensured their sufficient content in the total lipophilic fraction. The chemical composition of the lipophilic fennel SCO2 extract obtained under optimal SCO2 conditions showed the presence and large number of different lipophilic BACs, proving its richness and high value for further use in various industrial fields. The analyses revealed the presence of 18 volatiles, with phenylpropanoids being the most dominant group (46.82 g/100 g) and trans-anethole being the main compound (45.32 g/100 g). Among the non-volatile compounds, a total of 12 fatty acids were detected, with the prevalence of monounsaturated fatty acids (81.63%) and oleic and petroselinic acids being the major fatty acids (81.16%), followed by polyunsaturated fatty acids (13.79%) and saturated fatty acids (4.58%). The sterol analysis confirmed the presence of 12 sterols along with two pentacyclic triterpenoids, α-(3.74 mg/100 g) and β-amyrin (19.74 mg/100 g), with β-sitosterol (100.65 mg/100 g) and stigmasterol (100.49 mg/100 g) being predominant. In addition, the tocochromanols α-tocopherol (12.70 mg/100 g), γ-(11.59 mg/100 g) and δ-tocotrienol (9.50 mg/100 g), as well as the carotenoids lutein (12.20 mg/100 g) and β-carotene (1.22 mg/100 g), were present. Overall, the SCO2 certainly proved to be a very efficient method for obtaining a rich and highly valuable lipophilic extract from fennel seeds containing various lipophilic BACs, which makes it interesting for further scientific and industrial purposes.
Author Contributions
Conceptualization, M.R.; methodology, S.B., S.J. and M.R.; formal analysis, N.M.D., E.C., S.P., S.J. and M.R.; investigation, M.R.; data curation, N.M.D., E.C., I.E.G., S.B., S.P. and M.R.; writing—original draft preparation, N.M.D. and M.R.; writing—review and editing, I.E.G., S.P. and M.R.; visualization, I.E.G. and M.R.; supervision, M.R.; project administration, V.D.-U.; funding acquisition, V.D.-U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation, grant number IP-01-2018-4924.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Anka, Z.M.; Gimba, S.; Nanda, A.; Salisu, L. Phytochemistry and pharmacological activities of Foeniculum vulgare. IOSR J. Pharm. 2020, 10, 1–10. [Google Scholar]
- Marčac, N.; Balbino, S.; Tonković, P.; Medved, A.M.; Cegledi, E.; Dragović, S.; Dragović-Uzelac, V.; Repajić, M. Hydrodistillation and Steam Distillation of Fennel Seeds Essential Oil: Parameter Optimization and Application of Cryomilling Pretreatment. Processes 2023, 11, 2354. [Google Scholar] [CrossRef]
- Malin, V.; Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic characterization and bioactivity of fennel seed (Foeniculum vulgare Mill.) extracts isolated by microwave-assisted and conventional extraction. Processes 2022, 10, 510. [Google Scholar] [CrossRef]
- Balbino, S.; Repajić, M.; Obranović, M.; Medved, A.M.; Tonković, P.; Dragović-Uzelac, V. Characterization of lipid fraction of Apiaceae family seed spices: Impact of species and extraction method. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100326. [Google Scholar] [CrossRef]
- Moser, B.R.; Zheljazkov, V.D.; Bakota, E.L.; Evangelista, R.L.; Gawde, A.; Cantrell, C.L.; Winkler-Moser, J.K.; Hristov, A.N.; Astatkie, T.; Jeliazkova, E. Method for obtaining three products with different properties from fennel (Foeniculum vulgare) seed. Ind. Crops Prod. 2014, 60, 335–342. [Google Scholar] [CrossRef]
- Abdesslem, S.B.; Elbaz, M.; Boulares, M.; Ben Moussa, O.; Timoumi, M.; Hassouna, M. Value adding search among a selection of Tunisian fennel (Foeniculum vulgare Mill.) cultivars: Nutritional composition, chlorophyll and β-carotene contents of fennel seeds. J. Oasis Agric. Sustain. Dev. 2022, 4, 110–116. [Google Scholar] [CrossRef]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Choi, E.-M.; Hwang, J.-K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef]
- Ghasemian, A.; Al-Marzoqi, A.-H.; Mostafavi, S.K.S.; Alghanimi, Y.K.; Teimouri, M. Chemical composition and antimicrobial and cytotoxic activities of Foeniculum vulgare Mill essential oils. J. Gastrointest. Cancer 2020, 51, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Gulfraz, M.; Mehmood, S.; Minhas, N.; Jabeen, N.; Kausar, R.; Jabeen, K.; Arshad, G. Composition and antimicrobial properties of essential oil of Foeniculum vulgare. Afr. J. Biotechnol. 2008, 7, 4364–4368. [Google Scholar]
- Pradhan, M.; Sribhuwaneswari, S.; Karthikeyan, D.; Minz, S.; Sure, P.; Chandu, A.N.; Mishra, U.; Kamalakannan, K.; Saravanankumar, A.; Sivakumar, T. In-Vitro cytoprotection activity of Foeniculum vulgare and Helicteres isora in cultured human blood lymphocytes and antitumour activity against B16F10 melanoma cell line. Res. J. Pharm. Technol. 2008, 1, 450–452. [Google Scholar]
- Singh, B.; Kale, R.K. Chemomodulatory action of Foeniculum vulgare (Fennel) on skin and forestomach papillomagenesis, enzymes associated with xenobiotic metabolism and antioxidant status in murine model system. Food Chem. Toxicol. 2008, 46, 3842–3850. [Google Scholar] [CrossRef]
- El-Soud, N.A.; El-Laithy, N.; El-Saeed, G.; Wahby, M.S.; Khalil, M.; Morsy, F.; Shaffie, N. Antidiabetic activities of Foeniculum vulgare Mill. essential oil in streptozotocin-induced diabetic rats. Maced. J. Med. Sci. 2011, 4, 139–146. [Google Scholar] [CrossRef]
- Özbek, H.; Uğraş, S.; Dülger, H.; Bayram, I.; Tuncer, I.; Öztürk, G.; Öztürk, A. Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia 2003, 74, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Mallni, T.; Vanithakumari, G.; Devi, N.; Fiango, V. Effect of Foeniculuai vulgare Mill seed extract on the genital organs of male and female rats. Indian J. Physiol. Pharmacol. 1985, 29, 22–26. [Google Scholar]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Mita, G. Supercritical carbon dioxide extraction of carotenoids from pumpkin (Cucurbita spp.): A review. Int. J. Mol. Sci. 2014, 15, 6725–6740. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- Mokhtari, L.; Ghoreishi, S.M. Supercritical carbon dioxide extraction of trans-anethole from Foeniculum vulgare (fennel) seeds: Optimization of operating conditions through response surface methodology and genetic algorithm. J. CO2 Util. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Maitusong, J.; Aimila, A.; Zhang, J.; Mahinur, B.; Maiwulanjiang, M.; Aisa, H.A. Process optimization for the supercritical carbon dioxide extraction of Foeniculum vulgare Mill. seeds aromatic extract with respect to yield and trans-anethole contents using Box-Behnken design. Flavour Fragr. J. 2021, 36, 280–291. [Google Scholar] [CrossRef]
- AOAC International. AOAC Official Method 920.151; Solids (Total) in Fruits and Fruit Products; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Moslavac, T.; Jokić, S.; Šubarić, D.; Aladić, K.; Vukoja, J.; Prce, N. Pressing and supercritical CO2 extraction of Camelina sativa oil. Ind. Crops Prod. 2014, 54, 122–129. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007, 30, 563–572. [Google Scholar] [CrossRef]
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils: Gas Chromatography of Fatty acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 12966-4:2015; Animal and Vegetable Fats and Oils: Gas Chromatography of Fatty Acid Methyl Esters—Part 4: Determination by Capillary Gas Chromatography. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 12228-1:2014; Animal and Vegetable Fats and Oils: Determination of Individual and Total Sterols Contents—Part 1: Gas Chromatographic Method. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 9936:2006; Animal and Vegetable Fats and Oils: Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. International Organization for Standardization: Geneva, Switzerland, 2006.
- Castro-Puyana, M.; Pérez-Sánchez, A.; Valdés, A.; Ibrahim, O.H.M.; Suarez-Álvarez, S.; Ferragut, J.A.; Micol, V.; Cifuentes, A.; Ibáñez, E.; García-Cañas, V. Pressurized liquid extraction of Neochloris oleoabundans for the recovery of bioactive carotenoids with anti-proliferative activity against human colon cancer cells. Food Res. Int. 2017, 99, 1048–1055. [Google Scholar] [CrossRef]
- Hatami, T.; Johner, J.C.F.; Meireles, M.A.A. Extraction and fractionation of fennel using supercritical fluid extraction assisted by cold pressing. Ind. Crops Prod. 2018, 123, 661–666. [Google Scholar] [CrossRef]
- Simándi, B.; Deák, A.; Rónyai, E.; Yanxiang, G.; Veress, T.; Lemberkovics, É.; Then, M.; Sass-Kiss, Á.; Vámos-Falusi, Z. Supercritical carbon dioxide extraction and fractionation of fennel oil. J. Agric. Food Chem. 1999, 47, 1635–1640. [Google Scholar] [CrossRef]
- Moura, L.S.; Carvalho Jr, R.N.; Stefanini, M.B.; Ming, L.C.; Meireles, M.A.A. Supercritical fluid extraction from fennel (Foeniculum vulgare): Global yield, composition and kinetic data. J. Supercrit. Fluids 2005, 35, 212–219. [Google Scholar] [CrossRef]
- Shams, K.A.; Abdel-Azim, N.S.; Tawfik, W.A.; Hassanein, H.D.; Saleh, M.A.; Hammouda, F.M. Green extraction techniques: Effect of extraction method on lipid contents of three medicinal plants of Apiaceae. J. Chem. Pharm. Res. 2015, 7, 1080–1088. [Google Scholar]
- Piras, A.; Falconieri, D.; Porcedda, S.; Marongiu, B.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L. Supercritical CO2 extraction of volatile oils from Sardinian Foeniculum vulgare ssp. vulgare (Apiaceae): Chemical composition and biological activity. Nat. Prod. Res. 2014, 28, 1819–1825. [Google Scholar] [CrossRef]
- Coelho, J.A.P.; Pereira, A.P.; Mendes, R.L.; Palavra, A.M.F. Supercritical carbon dioxide extraction of Foeniculum vulgare volatile oil. Flavour Fragr. J. 2003, 18, 316–319. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Estragole quantity optimization from fennel seeds by supercritical fluid extraction (carbon dioxide–methanol) using a Box–Behnken design. Characterization of fennel extracts. Ind. Crops Prod. 2014, 60, 186–192. [Google Scholar] [CrossRef]
- Rebey, I.B.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, I.H.; Fauconnier, M.-L. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Mariod, A.A.; Matthäus, B.; Ismail, M. Comparison of supercritical fluid and hexane extraction methods in extracting kenaf (Hibiscus cannabinus) seed oil lipids. J. Am. Oil Chem. Soc. 2011, 88, 931–935. [Google Scholar] [CrossRef]
- Peng, W.L.; Mohd-Nasir, H.; Setapar, S.H.M.; Ahmad, A.; Lokhat, D. Optimization of process variables using response surface methodology for tocopherol extraction from Roselle seed oil by supercritical carbon dioxide. Ind. Crops Prod. 2020, 143, 111886. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Process optimization and extraction rate analysis of carotenoids extraction from rosehip fruit using supercritical CO2. J. Supercrit. Fluids 2008, 44, 308–314. [Google Scholar] [CrossRef]
- Damjanović, B.; Lepojević, Ž.; Živković, V.; Tolić, A. Extraction of fennel (Foeniculum vulgare Mill.) seeds with supercritical CO2: Comparison with hydrodistillation. Food Chem. 2005, 92, 143–149. [Google Scholar] [CrossRef]
- Maheshwari, P.; Nikolov, Z.L.; White, T.M.; Hartel, R. Solubility of fatty acids in supercritical carbon dioxide. J. Am. Oil Chem. Soc. 1992, 69, 1069–1076. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef]
- Santos, K.A.; da Silva, E.A.; da Silva, C. Supercritical CO2 extraction of favela (Cnidoscolus quercifolius) seed oil: Yield, composition, antioxidant activity, and mathematical modeling. J. Supercrit. Fluids 2020, 165, 104981. [Google Scholar] [CrossRef]
- Przygoda, K.; Wejnerowska, G. Extraction of tocopherol-enriched oils from Quinoa seeds by supercritical fluid extraction. Ind. Crops Prod. 2015, 63, 41–47. [Google Scholar] [CrossRef]
- De Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and modelling of supercritical CO2 extraction process of carotenoids from carrot peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Repajić, M.; Elez Garofulić, I.; Marčac Duraković, N.; Balun, M.; Cegledi, K.; Cegledi, E.; Dobroslavić, E.; Dragović-Uzelac, V. Physico-Chemical Characterization of Encapsulated Fennel Essential Oil under the Influence of Spray-Drying Conditions. Processes 2024, 12, 577. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Cegledi, E.; Robić, K.; Elez Garofulić, I.; Dragović-Uzelac, V.; Repajić, M. Encapsulation of Fennel Essential Oil in Calcium Alginate Microbeads via Electrostatic Extrusion. Appl. Sci. 2024, 14, 3522. [Google Scholar] [CrossRef]
- Islam, M.A.; Jeong, B.-G.; Jung, J.; Shin, E.-C.; Choi, S.-G.; Chun, J. Phytosterol determination and method validation for selected nuts and seeds. Food Anal. Methods 2017, 10, 3225–3234. [Google Scholar] [CrossRef]
- El-Assri, E.M.; Hajib, A.; Choukri, H.; Gharby, S.; Lahkimi, A.; Eloutassi, N.; Bouia, A. Nutritional quality, lipid, and mineral profiling of seven Moroccan Apiaceae family seeds. S. Afr. J. Bot. 2023, 160, 23–35. [Google Scholar] [CrossRef]
- Matthäus, B.; Musazcan Özcan, M. Oil content, fatty acid composition and distributions of vitamin-E-active compounds of some fruit seed oils. Antioxidants 2015, 4, 124–133. [Google Scholar] [CrossRef]
- Pencheva, M.; Petkova, Z.; Dincheva, I.; Kostova, I.; Damyanova, S.; Stoyanova, A.; Gaceu, L. Phytochemical and biological profiles of fennel fruits (Foeniculum vulgare Mill. Var. dulce Mill.). Carpathian J. Food Sci. Technol. 2022, 14, 28–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).