Study on the Combined Effects of Bromelain (Ananas comosus) Enzyme Treatment and Bacteria Cultures on the Physicochemical Properties and Oxidative Stability of Horse Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Technology of Meat Processing with Enzyme
2.3. Treatment of Meat with Lactic Acid Bacteria
2.4. Determination of Fat Content
2.5. Determination of Protein Content
2.6. Determination of Water Content
2.7. Determination of Ash Content
2.8. Determination of Organoleptic Properties
2.9. Determination of Cooking Loss
2.10. Determination of Shear Stress
2.11. Determination of Water-Holding Capacity
2.12. Determination of pH
2.13. Determination of Peroxide Number
2.14. Determination of Water Activity (aw)
2.15. Determination of Titratable Acidity, ⁰T
2.16. Clotting Time, h
2.17. Microstructure
2.18. Statistical Analysis
3. Results
3.1. Determination of Chemical Composition
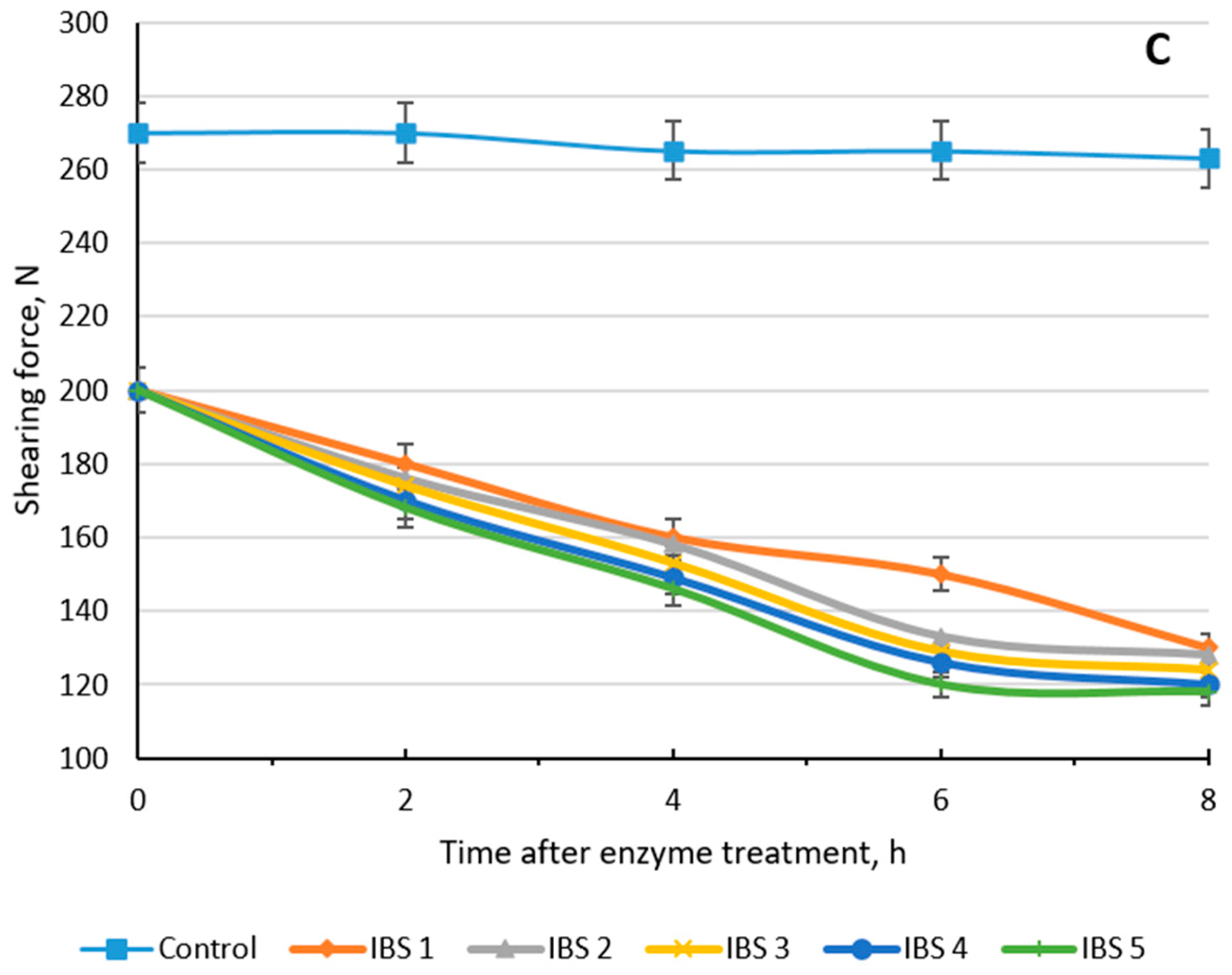
3.2. Shear Force of Meat before and after Enzyme Treatment
3.3. Sensory Analysis
3.4. Study of Water-Holding Capacity
3.5. Cooking Losses
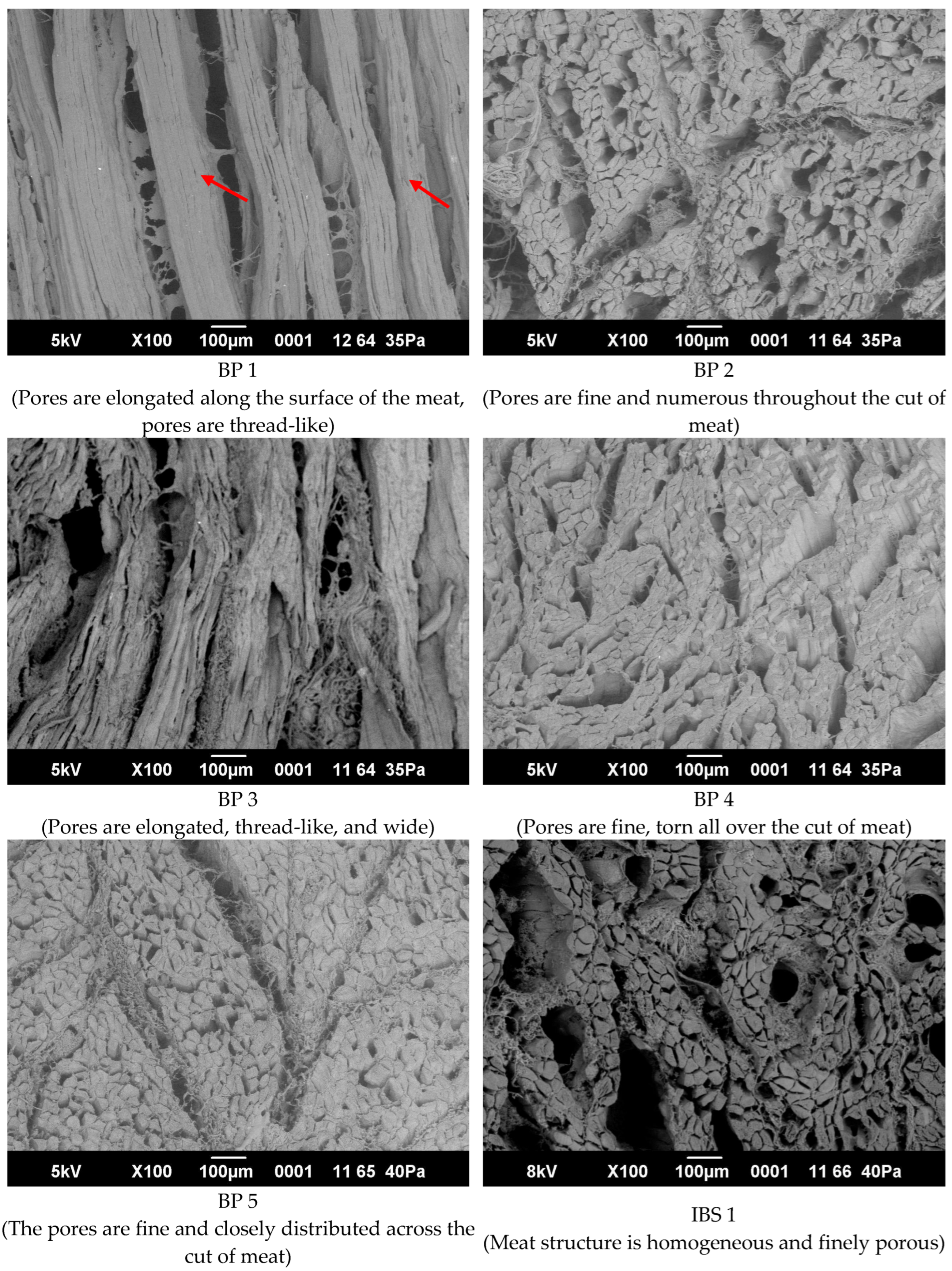
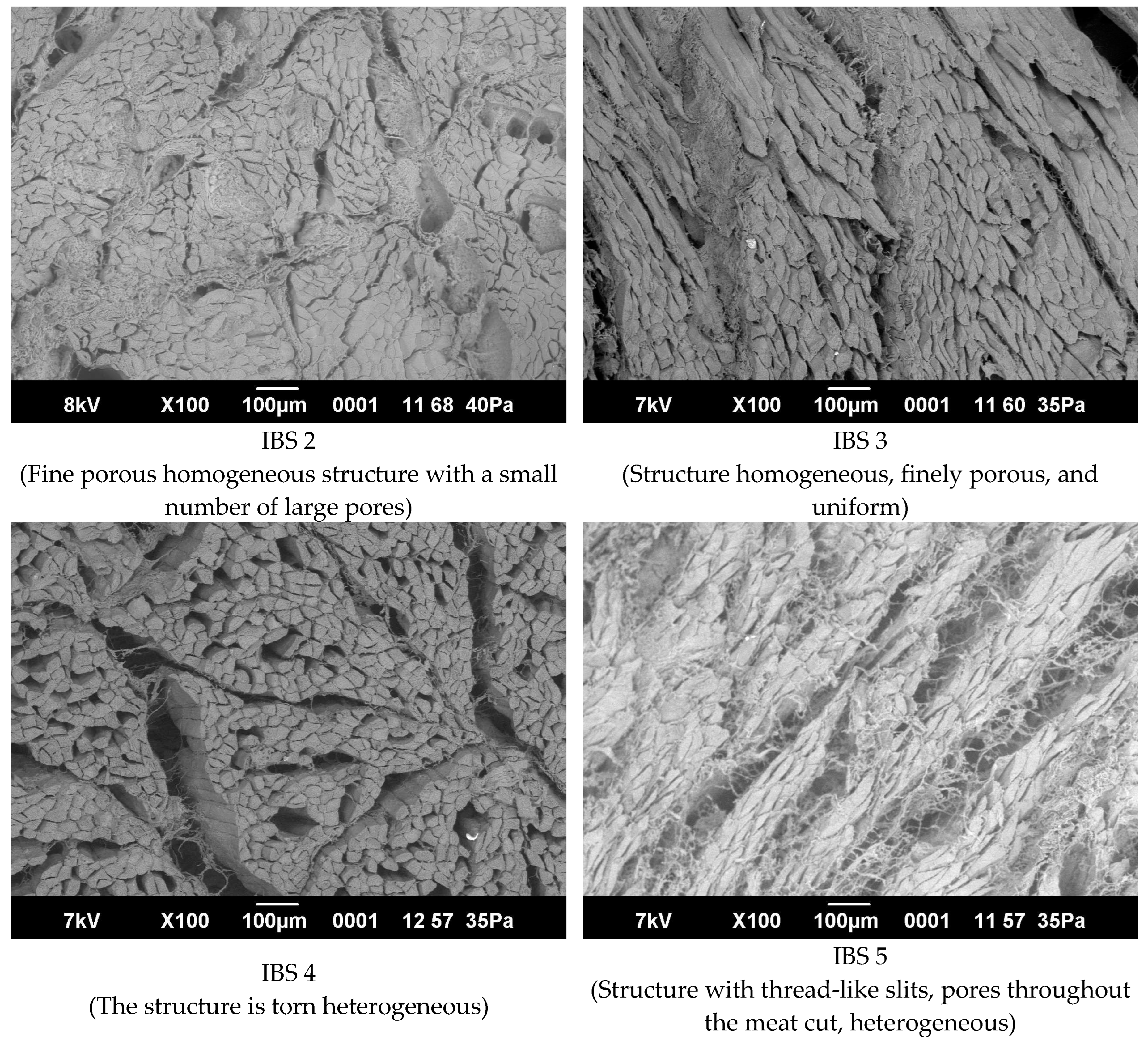
3.6. Microstructure of Meat Muscles before and after Treatment
3.7. Treatment of Fermented Meat with Lactic Acid Bacteria
3.8. pH Changes during Storage of Meat Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Warner, R.D.; Wheeler, T.L.; Ha, M.; Li, X.; Bekhit, A.E.-D.; Morton, J.; Vaskoska, R.; Dunshea, F.R.; Liu, R.; Purslow, P.; et al. Meat tenderness: Advances in biology, biochemistry, molecular mechanisms and new technologies. Meat Sci. 2022, 185, 108657. [Google Scholar] [CrossRef] [PubMed]
- Madhusankha, G.D.M.P.; Thilakarathna, R.C.N. Meat tenderization mechanism and the impact of plant exogenous proteases: A review. Arab. J. Chem. 2021, 14, 102967. [Google Scholar] [CrossRef]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Motta, J.F.G.; de Freitas, B.C.B.; de Almeida, A.F.; de Souza Martins, G.A.; Borges, S.V. Use of enzymes in the food industry: A review. Food Sci. Technol. 2023, 43, e106222. [Google Scholar] [CrossRef]
- Barido, F.; Lee, S. Changes in proteolytic enzyme activities, tenderness-related traits, and quality properties of spent hen meat affected by adenosine 5′-monophosphate during cold storage. Poult. Sci. 2021, 100, 101056. [Google Scholar] [CrossRef] [PubMed]
- Grau, R.; Verdú, S.; Pérez, A.; Barat, J.; Talens, P. Laser-backscattering imaging for characterizing pork loin tenderness. Effect of pre-treatment with enzyme and cooking. J. Food Eng. 2021, 299, 110508. [Google Scholar] [CrossRef]
- Ahmad, I.Z.; Tabassum, H.; Ahmad, A.; Kuddus, M. Food enzymes in pharmaceutical industry: Perspectives and limitations. In Enzymes in Food Technology; Kuddus, M., Ed.; Springer: Singapore, 2018; pp. 41–62. [Google Scholar] [CrossRef]
- Akimova, D.; Kakimov, A.; Suychinov, A.; Urazbayev, Z.; Zharykbasov, Y.; Ibragimov, N.; Bauyrzhanova, A.; Utegenova, A. Enzymatic hydrolysis in food processing: Biotechnological advancements, applications, and future perspectives. Potravin. Slovak J. Food Sci. 2024, 18, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Shrivastava, N.; Ojha, B. Enzymes in the Meat Industry. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Hopkins, D.L.; Geesink, G.; Bekhit, A.A.; Franks, P. Exogenous proteases for meat tenderization. Crit. Rev. Food Sci. Nutr. 2014, 54, 1012–1031. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Bekhit, A.E.D.A.; Carne, A.; Hopkins, D.L. Characterisation of commercial papain, bromelain, actinidin and zingibain protease preparations and their activities toward meat proteins. Food Chem. 2012, 134, 95–105. [Google Scholar] [CrossRef]
- Dhital, S.; Vangnai, K. Meat Tenderisation Effect of Protease from Mango Peel Crude Extract. Int. Food Res. J. 2019, 26, 991–998. [Google Scholar]
- Antipova, L.V.; Podvigina, Y.N.; Kosenko, I.S. Application of enzyme preparations in the technology of meat products production. Fundam. Res. 2008, 6, 134–135. [Google Scholar]
- Mohd Azmi, S.I.; Kumar, P.; Sharma, N.; Sazili, A.Q.; Lee, S.-J.; Ismail-Fitry, M.R. Application of Plant Proteases in Meat Tenderization: Recent Trends and Future Prospects. Foods 2023, 12, 1336. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, R.S.; Sakhare, P.Z.; Bhaskar, N.; Hebbar, H.U. Efficacy of reverse micellar extracted fruit bromelain in meat tenderization. J. Food Sci. Technol. 2015, 52, 3870–3880. [Google Scholar] [CrossRef] [PubMed]
- Nadzirah, K.Z.; Zainal, S.; Noriham, A.; Normah, I. Application of Bromelain Powder Produced from Pineapple Crowns in Tenderising Beef Round Cuts. Int. Food Res. J. 2016, 23, 1590–1599. [Google Scholar]
- Feng, X.; Hang, S.; Zhou, Y.; Liu, Q.; Yang, H. Bromelain Kinetics and Mechanism on Myofibril from Golden Pomfret (Trachinotus blochii). J. Food Sci. 2018, 83, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Taub, I.A. Specific degradation of myosin in meat by bromelain. Food Chem. 1991, 40, 337–343. [Google Scholar] [CrossRef]
- Botinestean, C.; Gomez, C.; Nian, Y.; Auty, M.A.E.; Kerry, J.P.; Hamill, R.M. Possibilities for developing texture-modified beef steaks suitable for older consumers using fruit-derived proteolytic enzymes. J. Texture Study 2018, 49, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kim, H.T.; Choi, M.-J.; Ko, E.-Y. Effects of Bromelain and Double Emulsion on the Physicochemical Properties of Pork Loin. Food Sci. Anim. Resour. 2019, 39, 888–902. [Google Scholar] [CrossRef] [PubMed]
- Jamil, N.H.; Nura Ruhaya, A.H.; Sarbon, N. Optimization of enzymatic hydrolysis condition and functional properties of eel (Monopterus sp.) protein using response surface methodology (RSM). Int. Food Res. J. 2016, 23, 1–9. [Google Scholar]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [PubMed]
- Manohar, J.; Gayathri, R.; Vishnupriya, V. Tenderisation of meat using bromelain from pineapple extract. Int. J. Pharm. Sci. Rev. Res. 2016, 39, 81–85. [Google Scholar]
- Settanni, L.; Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Zdolec, N.; Mikuš, T.; Kiš, M. Lactic acid bacteria in meat fermentation: Dry sausage safety and quality. In Lactic Acid Bacteria in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Castellano, P.; Belfiore, C.; Fadda, S.; Vignolo, G. A review of bacteriocinogenic lactic acid bacteria used as bioprotective cultures in fresh meat produced in Argentina. Meat Sci. 2008, 79, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Kudryashov, V.L.; Alekseev, V.V.; Fursova, N.A. Nisin and natamycin-effective food microbial preservatives. Food Ind. 2020, 2, 67–71. [Google Scholar] [CrossRef]
- Li, Q.; Montalban-Lopez, M.; Kuipers, O.P. Increasing the Antimicrobial Activity of Nisin-Based Lantibiotics against Gram-Negative Pathogens. Appl. Environ. Microbiol. 2018, 84, e00052-18. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Monfort, J.M. Bacterial starter cultures for meat fermentation. Food Chem. 1997, 59, 547–554. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Fusieger, A.; Martins, M.C.F.; de Freitas, R.; Nero, L.A.; de Carvalho, A.F. Technological properties of Lactococcus lactis subsp. lactis bv. diacetylactis obtained from dairy and non-dairy niches. Braz. J. Microbiol. 2020, 51, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [PubMed]
- Hutt, P.; Shchepetova, J.; Loivukene, K.; Kullisaar, T.; Mikelsaar, M. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J. Appl. Microbiol. 2006, 100, 1324–1332. [Google Scholar] [CrossRef]
- GOST 23042–2015; Meat and Meat Products. Methods of Fat Determination. Standartinform: Moscow, Russia, 2019.
- GOST 25011–2017; Meat and Meat Products. Protein Determination Methods. Standartinform: Moscow, Russia, 2018.
- GOST 33319-2015; Meat and Meat Products. Method for Determining the Mass Fraction of Moisture. Standardinform: Moscow, Russia, 2016; p. 6.
- GOST 31727-2012; Meat and Meat Products. Method for Determining the Mass Fraction of Total Ash. Standardinform: Moscow, Russia, 2013; p. 8.
- GOST 9959-2015; Meat and Meat Products. General Conditions of Organoleptic Evaluation. Standardinform: Moscow, Russia, 2016; p. 19.
- Vujadinović, D.P.; Grujić, R.D.; Tomović, V.M.; Vukić, M.S.; Jokanović, M.R. Cook Loss as a Function of Meat Heat Treatment and Regime. Qual. Life-APEIRON 2014, 10, 3–4. [Google Scholar] [CrossRef][Green Version]
- Bekeshova, G.; Ibragimov, N.; Kakimov, A.; Suychinov, A.; Yessimbekov, Z.; Kabdylzhar, B.; Tokhtarov, Z.; Zhumadilova, G.; Abdilova, G. Effect of Rotational Speed and Gap between Rotating Knives of the Grinder on the Yield Stress and Water-Binding Capacity of Fine Ground Chicken Bone. Appl. Sci. 2022, 12, 3533. [Google Scholar] [CrossRef]
- Kudryashov, L.S.; Kudryashova, O.A. Water-holding and water-holding capacity of meat and methods of its determination. Theory Pract. Meat Process. 2023, 8, 62–70. [Google Scholar] [CrossRef]
- ST RK ISO 2917-2009; Meat and Meat Products. Determination of pH. Control Method. Gosstandart: Astana, Kazakhstan, 2010; p. 16.
- GOST 8285-91; Animal Fats Ghee. Acceptance Rules and Test Methods. Izd vo Standards: Moscow, Russia, 2005; p. 2.
- GOST 26593-85; Vegetable Oils Method of Peroxide Number Measurement. Ministry of Food Industry of the USSR: Moscow, Russia, 1985.
- Kamerbaev, A.Y. The Role of Water in Food Products and Its Functions: Mοnοgraphy; Tengri Publisher: Almaty, Kazakhstan, 2001; p. 203. [Google Scholar]
- GOST 3624-92; Milk and Milk Products. Titrimetric Methods for Determination of Acidity. Standardinform: Moscow, Russia, 2009; p. 9. Available online: https://files.stroyinf.ru/Data/100/10071.pdf (accessed on 3 June 2024).
- GOST 34372-2017; Bacterial Leaven for Dairy Production. General Technical Conditions. Standardinform: Moscow, Russia, 2018; p. 17. Available online: https://files.stroyinf.ru/Index2/1/4293740/4293740391.htm (accessed on 3 June 2024).
- Yessimbekov, Z.; Kakimov, A.; Kabdylzhar, B.; Suychinov, A.; Baikadamova, A.; Akimova, D.; Abdilova, G. Chemical, physical properties, microstructure and granulometric composition of ultra-finely ground chicken bone paste. Appl. Food Res. 2023, 3, 100318. [Google Scholar] [CrossRef]
- Lorenzen, C.L.; Calkins, C.R.; Green, M.D.; Miller, R.K.; Morgan, J.B.; Wasser, B.E. Efficacy of performing Warner–Bratzler and slice shear force on the same beef steak following rapid cooking. Meat Sci. 2010, 85, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Martinez, H.A.; Miller, R.K.; Kerth, C.; Wasser, B.E. Prediction of beef tenderness and juiciness using consumer and descriptive sensory attributes. Meat Sci. 2023, 205, 109292. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Rawdkuen, S. Application of bromelain extract for muscle foods tenderization. Food Nutr. Sci. 2012, 2, 393–401. [Google Scholar] [CrossRef]
- Naveena, B.M.; Mendiratta, S.K.; Anjaneyulu, A.S.R. Tenderization of Buffalo Meat Using Plant Proteases from Cucumis trigonus Roxb (Kachri) and Zingiber Officinale roscoe (Ginger Rhizome). Meat Sci. 2004, 68, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Saengsuk, N.; Laohakunjit, N.; Sanporkha, P.; Kaisangsri, N.; Selamassakul, O.; Ratanakhanokchai, K.; Uthairatanakij, A. Physicochemical characteristics and textural parameters of restructured pork steaks hydrolysed with bromelain. Food Chem. 2021, 361, 130079. [Google Scholar] [CrossRef] [PubMed]
- Jun-hui, X.; Hui-juan, C.; Bin, Z.; Hui, Y. The mechanistic effect of bromelain and papain on tenderization in jumbo squid (Dosidicus gigas) muscle. Food Res. Int. 2020, 131, 108991. [Google Scholar] [CrossRef] [PubMed]
- Štreimikytė, P.; Viškelis, P.; Viškelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Zinina, O.; Rebezov, M.; Khayrullin, M.; Neverova, O.; Bychkova, T. Functional and technological indicators of fermented minced meat. In Proceedings of the III International Scientific Conference: AGRITECH-III-2020: Agribusiness, Environmental Engineering and Biotechnologies, Krasnoyarsk, Russia, 18–20 June 2020; Volume 548. [Google Scholar] [CrossRef]
- Ma, Y.; Yuan, Y.; Bi, X.; Zhang, L.; Xing, Y.; Che, Z. Tenderization of Yak Meat by the Combination of Papain and High-Pressure Processing Treatments. Food Bioprocess Technol. 2019, 12, 681–693. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Y.; Wu, R.; Liu, D.; Hu, F.; Wang, C.; Wu, D. A method to improve water-holding capacity of beef during freezing-thawing process using ultrasound treatment. J. Food Process. Preserv. 2020, 45, e15004. [Google Scholar] [CrossRef]
- Palka, K.; Daun, H. Changes in texture, cooking losses, and myofibrillar structure of bovine M. semitendinosus during heating. Meat Sci. 1999, 51, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, D.; Pasquini, M. Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT-Food Sci. Technol. 2005, 38, 895–901. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Zhang, S.-S.; Sun, B.-Z.; Xie, P.; Wen, K.-X.; Xu, C.-C. Changes in Physical Meat Traits, Protein Solubility, and the Microstructure of Different Beef Muscles during Post-Mortem Aging. Foods 2020, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Beldarrain, L.R.; Sentandreu, E.; Aldai, N.; Sentandreu, M.A. Horse meat tenderization in relation to post-mortem evolution of the myofibrillar sub-proteome. Meat Sci. 2022, 188, 108804. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.I.; Fraqueza, M.J.; Pissarra, H.; Saraiva, J.A.; Vicente, A.A.; Moldão-Martins, M. Optimization of the Effect of Pineapple By-Products Enhanced in Bromelain by Hydrostatic Pressure on the Texture and Overall Quality of Silverside Beef Cut. Foods 2020, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, S.; Hashemi, S.M.B.; Abedi, E.; Amiri, M.J.; Conte, F.L. Bio-Preservation of Meat and Fermented Meat Products by Lactic Acid Bacteria Strains and Their Antibacterial Metabolites. Sustainability 2023, 15, 10154. [Google Scholar] [CrossRef]
- Toldrá, F.; Hui, Y.H. Dry-Fermented Sausages and Ripened Meats: An Overview. In Handbook of Fermented Meat and Poultry; Toldrá, F., Hui, Y.H., Astiasarán, I., Sebranek, J.G., Talon, R., Eds.; Wiley Online Library: New York, NY, USA, 2014; pp. 1–6. [Google Scholar]
- Ammor, S.; Dufour, E.; Zagorec, M.; Chaillou, S.; Chevallier, I. Characterization and Selection of Lactobacillus sakei Strains Isolated from Traditional Dry Sausage for Their Potential Use as Starter Cultures. Food Microbiol. 2005, 22, 529–538. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant Activity and Probiotic Properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, P.; Lou, L.; Zhan, J.; Fan, M.; Li, D.; Liao, Q. Antioxidant activities of lactic acid bacteria for quality improvement of fermented sausage. J. Food Sci. 2017, 82, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, C.; Dučić, M.; López, M.; Prieto, M.; Álvarez-Ordoñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2021, 183, 108661. [Google Scholar] [CrossRef] [PubMed]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cherroud, S.; Cachaldora, A.; Fonseca, S.; Laglaoui, A.; Carballo, J.; Franco, I. Microbiological and physicochemical characterization of dry-cured Halal goat meat. Effect of salting time and addition of olive oil and paprika covering. Meat Sci. 2014, 98, 129–134. [Google Scholar] [CrossRef]
- Parks, A.; Brashears, M.; Woerner, W.; Martin, J.; Thompson, L.; Brooks, J. Spoilage characteristics of ground beef with added lactic acid bacteria and rosemary oleoresin packaged in a modified-atmosphere package and displayed at abusive temperatures. J. Anim. Sci. 2012, 90, 2054–2060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doulgeraki, A.I.; Ercolini, D.; Villani, F.; Nychas, G.J.E. Spoilage microbiota associated to the storage of raw meat in different conditions. Int. J. Food Microbiol. 2012, 157, 130–141. [Google Scholar] [CrossRef]
- Segli, F.; Melian, C.; Muñoz, V.; Vignolo, G.; Castellano, P. Bioprotective extracts from Lactobacillus acidophilus CRL641 and Latilactobacillus curvatus CRL705 inhibit a spoilage exopolysaccharide producer in a refrigerated meat system. Food Microbiol. 2021, 97, 103739. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.; Cunha, R. Gelation property and water holding capacity of heat-treated collagen at different temperature and pH values. Food Res. Int. 2013, 50, 213–223. [Google Scholar] [CrossRef]
- Wu, G.; Farouk, M.; Clerens, S.; Rosenvold, K. Effect of beef ultimate pH and large structural protein changes with aging on meat tenderness. Meat Sci. 2014, 98, 637–645. [Google Scholar] [CrossRef] [PubMed]






| # | Sample | Treatment |
|---|---|---|
| 1 | Control | Horse meat without treatment |
| 2 | BS 0.5 | Horse meat in 0.5% bromelain solution |
| 3 | BS 1.0 | Horse meat in 1.0% bromelain solution |
| 4 | BS 1.5 | Horse meat in 1.5% bromelain solution |
| 5 | BS 2.0 | Horse meat in 2.0% bromelain solution |
| 6 | BS 2.5 | Horse meat in 2.5% bromelain solution |
| 7 | BP 1 | Horse meat treated with bromelain powder (1% by weight of raw material) |
| 8 | BP 2 | Horse meat treated with bromelain powder (2% by weight of raw material) |
| 9 | BP 3 | Horse meat treated with bromelain powder (3% by weight of raw material) |
| 10 | BP 4 | Horse meat treated with bromelain powder (4% by weight of raw material) |
| 11 | BP 5 | Horse meat treated with bromelain powder (5% by weight of raw material) |
| 12 | BI 1 | Horsemeat treated by injection with 1% bromelain solution |
| 13 | BI 2 | Horse meat treated by injection with 2% bromelain solution |
| 14 | BI 3 | Horse meat treated by injection with 3% bromelain solution |
| 15 | BI 4 | Horse meat treated by injection with 4% bromelain solution |
| 16 | BI 5 | Horse meat treated by injection with 5% bromelain solution |
| Type of Bacteria | Clotting Time, h | Titratable Acidity, ⁰T | Total Viable Count CFU/g × 109 | Welling Temperature, ⁰T |
|---|---|---|---|---|
| Lc. lactis | 10–12 | 80 | 2.8 | 30 |
| L. lactis ssp. lactis bv. diacetylactis | 7–8 | 80 | 2.4 | 28 |
| B. longum | 8–10 | 85 | 2.4 | 37 |
| L. acidophilus | 9–12 | 100 | 2.4 | 42 |
| Storage Time, Days | Different LAB Combinations and Ratios | Control Sample (Horse Meat) | |||||
|---|---|---|---|---|---|---|---|
| Lc. lactis, L. lactis ssp. lactis bv. diacetylactis, B. longum (1.5:1.5:2) | Lc. lactis, L. lactis ssp. lactis bv. diacetylactis, B. longum (1.5:2:1.5) | Lc. lactis, L. lactis ssp. lactis bv. diacetylactis, B. longum (2:1.5:1.5) | L. acidophilus, Lc. lactis, B. longum (1.5:1.5:2) | L. acidophilus, Lc. lactis, B. longum (1.5:2:1.5) | L. acidophilus, Lc. lactis, B. longum (2:1.5:1.5) | ||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | ||
| 1 day | 0.015 ± 0.000 e | 0.013 ± 0.000 c | 0.012 ± 0.000 b | 0.011 ± 0.000 a | 0.014 ± 0.000 d | 0.014 ± 0.000 d | 0.016 ± 0.000 f |
| 2 days | 0.040 ± 0.001 b | 0.037 ± 0.001 a | 0.036 ± 0.001 a | 0.035 ± 0.001 a | 0.035 ± 0.001 a | 0.036 ± 0.001 a | 0.049 ± 0.001 c |
| 3 days | 0.044 ± 0.001 a | 0.044 ± 0.001 ab | 0.043 ± 0.001 a | 0.042 ± 0.001 a | 0.045 ± 0.001 b | 0.044 ± 0.001 ab | 0.051 ± 0.001 c |
| 4 days | 0.053 ± 0.001 b | 0.051 ± 0.001 a | 0.051 ± 0.001 a | 0.050 ± 0.001 a | 0.055 ± 0.001 b | 0.053 ± 0.001 b | 0.063 ± 0.001 c |
| 5 days | 0.069 ± 0.001 c | 0.062 ± 0.001 a | 0.065 ± 0.001 b | 0.061 ± 0.001 a | 0.070 ± 0.001 c | 0.070 ± 0.001 c | 0.076 ± 0.001 d |
| 6 days | 0.075 ± 0.001 c | 0.067 ± 0.001 b | 0.069 ± 0.001 b | 0.062 ± 0.001 a | 0.077 ± 0.001 c | 0.077 ± 0.001 c | 0.089 ± 0.001 d |
| Storage Time, Hours | Lc. lactis, L. lactis ssp. lactis bv. diacetylactis, B. longum (1.5:1.5:2) | Lc. lactis, L. lactis ssp. lactis bv. diacetylactis, B. longum (1.5:2:1.5) | Lc. lactis, L. lactis ssp. lactis bv. diacetylactis, B. longum (2:1.5:1.5) | L. acidophilus, Lc. lactis, B. longum (1.5:1.5:2) | L. acidophilus, Lc. lactis, B. longum (1.5:2:1.5) | L. acidophilus, Lc. lactis, B. longum (2:1.5:1.5) | Control Sample (Horse Meat) |
|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | ||
| 0 | 6.10 ± 0.11 a | 6.10 ± 0.07 a | 6.10 ± 0.09 a | 6.10 ± 0.08 a | 6.10 ± 0.06 a | 6.10 ± 0.07 a | 6.33 ± 0.10 a |
| 2 | 6.0 ± 0.05 a | 6.0 ± 0.06 a | 6.0 ± 0.05 a | 6.0 ± 0.07 a | 6.03 ± 0.08 a | 6.00 ± 0.05 a | 6.27 ± 0.10 a |
| 4 | 5.82 ± 0.08 ab | 5.86 ± 0.09 ab | 5.87 ± 0.08 ab | 5.80 ± 0.05 a | 5.81 ± 0.07 a | 5.85 ± 0.08 ab | 6.11 ± 0.09 b |
| 6 | 5.70 ± 0.06 a | 5.85 ± 0.09 ab | 5.85 ± 0.07 ab | 5.60 ± 0.05 a | 5.76 ± 0.06 ab | 5.75 ± 0.07 ab | 6.01 ± 0.08 b |
| 8 | 5.70 ± 0.08 b | 5.70 ± 0.06 b | 5.70 ± 0.07 b | 5.30 ± 0.06 a | 5.63 ± 0.05 b | 5.60 ± 0.06 b | 5.98 ± 0.08 c |
| 10 | 5.67 ± 0.07 b | 5.65 ± 0.06 b | 5.65 ± 0.07 b | 5.27 ± 0.09 a | 5.59 ± 0.05 a | 5.50 ± 0.05 a | 5.92 ± 0.10 b |
| 12 | 5.54 ± 0.08 b | 5.57 ± 0.09 b | 5.58 ± 0.06 b | 5.21 ± 0.05 a | 5.51 ± 0.07 b | 5.30 ± 0.06 a | 5.81 ± 0.08 b |
| 14 | 5.53 ± 0.08 b | 5.55 ± 0.09 b | 5.56 ± 0.08 b | 5.20 ± 0.07 a | 5.45 ± 0.06 ab | 5.28 ± 0.05 a | 5.79 ± 0.09 b |
| 16 | 5.50 ± 0.07 b | 5.51 ± 0.08 b | 5.50 ± 0.08 b | 5.19 ± 0.06 a | 5.31 ± 0.07 ab | 5.26 ± 0.06 a | 5.71 ± 0.08 b |
| 18 | 5.30 ± 0.06 ab | 5.44 ± 0.09 b | 5.45 ± 0.08 b | 5.17 ± 0.07 a | 5.29 ± 0.07 a | 5.24 ± 0.06 a | 5.63 ± 0.09 b |
| 20 | 5.29 ± 0.08 ab | 5.30 ± 0.09 ab | 5.40 ± 0.08 b | 5.14 ± 0.06 a | 5.26 ± 0.07 a | 5.20 ± 0.08 a | 5.60 ± 0.10 b |
| 22 | 5.27 ± 0.09 a | 5.30 ± 0.10 ab | 5.30 ± 0.09 ab | 5.05 ± 0.05 a | 5.21 ± 0.07 a | 5.15 ± 0.06 a | 5.58 ± 0.08 b |
| 24 | 5.21 ± 0.05 a | 5.30 ± 0.09 ab | 5.34 ± 0.05 ab | 5.04 ± 0.07 a | 5.17 ± 0.08 a | 5.10 ± 0.06 a | 5.55 ± 0.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orynbekov, D.; Amirkhanov, K.; Kalibekkyzy, Z.; Smolnikova, F.; Assenova, B.; Nurgazezova, A.; Nurymkhan, G.; Kassenov, A.; Baytukenova, S.; Yessimbekov, Z. Study on the Combined Effects of Bromelain (Ananas comosus) Enzyme Treatment and Bacteria Cultures on the Physicochemical Properties and Oxidative Stability of Horse Meat. Processes 2024, 12, 1766. https://doi.org/10.3390/pr12081766
Orynbekov D, Amirkhanov K, Kalibekkyzy Z, Smolnikova F, Assenova B, Nurgazezova A, Nurymkhan G, Kassenov A, Baytukenova S, Yessimbekov Z. Study on the Combined Effects of Bromelain (Ananas comosus) Enzyme Treatment and Bacteria Cultures on the Physicochemical Properties and Oxidative Stability of Horse Meat. Processes. 2024; 12(8):1766. https://doi.org/10.3390/pr12081766
Chicago/Turabian StyleOrynbekov, Duman, Kumarbek Amirkhanov, Zhanar Kalibekkyzy, Farida Smolnikova, Bakhytkul Assenova, Almagul Nurgazezova, Gulnur Nurymkhan, Amirzhan Kassenov, Sholpan Baytukenova, and Zhanibek Yessimbekov. 2024. "Study on the Combined Effects of Bromelain (Ananas comosus) Enzyme Treatment and Bacteria Cultures on the Physicochemical Properties and Oxidative Stability of Horse Meat" Processes 12, no. 8: 1766. https://doi.org/10.3390/pr12081766
APA StyleOrynbekov, D., Amirkhanov, K., Kalibekkyzy, Z., Smolnikova, F., Assenova, B., Nurgazezova, A., Nurymkhan, G., Kassenov, A., Baytukenova, S., & Yessimbekov, Z. (2024). Study on the Combined Effects of Bromelain (Ananas comosus) Enzyme Treatment and Bacteria Cultures on the Physicochemical Properties and Oxidative Stability of Horse Meat. Processes, 12(8), 1766. https://doi.org/10.3390/pr12081766







