Qualitative and Quantitative Potential of Low-Cost Near-Infrared (NIR) Devices for Rapid Analysis of Infant Formulas for Regular and Special Needs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Infant Formulas
2.2. Energy–Nutritional and Chemical Composition of Infant Formulas
2.3. Conductivity and Total Dissolved Solids
2.4. Colorimetry
2.5. NIR Spectroscopy
2.5.1. Benchtop Device
2.5.2. Portable NIR Device
2.5.3. Processing of Spectral Data
2.6. Data Analysis and Chemometrics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fung, F.; Wang, H.S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Jurinjak Tušek, A.J.; Benković, M.; Malešić, E.; Marić, L.; Jurina, T.; Gajdoš Kljusurić, J.G.; Valinger, D. Rapid quantification of dissolved solids and bioactives in dried root vegetable extracts using near infrared spectroscopy. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2021, 261, 120074. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Miniaturized NIR spectroscopy in food analysis and quality control: Promises, challengles and perspectives. Foods 2022, 11, 1465. [Google Scholar] [CrossRef]
- Osborne, B.G. Near-infrared spectroscopy in food analysis. Encycl. Anal. Chem. 2006, 1–14. [Google Scholar] [CrossRef]
- Williams, P.; Antoniszyn, J.; Manley, M. Near Infrared Technology: Getting the Best out of Light; African Sun Media: Stellenbosch, South Africa, 2019. [Google Scholar]
- Pandiselvam, R.; Prithviraj, V.; Manikantan, M.R.; Kothakota, A.; Rusu, A.V.; Trif, M.; Mousavi Khaneghah, A. Recent advancements in NIR spectroscopy for assessing the quality and safety of horticultural products: A comprehensive review. Front. Nutr. 2022, 9, 973457. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, J. Near-infrared spectroscopy for food quality evaluation. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Evaluation Technologies for Food Quality; Woodhead Publishing: Sawston, UK, 2019; pp. 105–118. [Google Scholar] [CrossRef]
- De Mendonça Pereira, B.F.; de Almeida, C.C.; Leandro, K.C.; da Costa, M.P.; Conte-Junior, C.A.; Spisso, B.F. Occurrence, sources, and pathways of chemical contaminants in infant formulas. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1378–1396. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, L.A.; Altazan, A.D.; Flanagan, E.W.; Beyer, A.G.; Olson, K.N.; O’Connell, A.A.; Nguyen, T.H.; Beyl, R.A.; Redman, L.M. Modifications to Infant Formula Instructions Improve the Accuracy of Formula Dispensing. Nutrients 2020, 12, 1150. [Google Scholar] [CrossRef]
- Lemaire, M.; Le Huërou-Luron, I.; Blat, S. Effects of infant formula composition on long-term metabolic health. J. Dev. Orig. Health Dis. 2018, 9, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Kouwenhoven, S.M.P.; Muts, J.; Finken, M.J.J.; Goudoever, J.B.V. Low-Protein Infant Formula and Obesity Risk. Nutrients 2022, 14, 2728. [Google Scholar] [CrossRef]
- Stevens, E.E.; Patrick, T.E.; Pickler, R. A history of infant feeding. J. Perinat. Educ. 2009, 18, 32–39. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Guo, M.R. Infant formula product regulation. In Human Milk Biochemistry and Infant Formula Manufacturing Technology, 2nd ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 281–316. [Google Scholar]
- Mikulec, V.; Adamović, P.; Cvetković, Ž.; Ivešić, M.; Gajdoš Kljusurić, J. Green Techniques for Detecting Microplastics in Marine with Emphasis on FTIR and NIR Spectroscopy—Short Review. Processes 2023, 11, 2360. [Google Scholar] [CrossRef]
- Posom, J.; Sirisomboon, P. Evaluation of the higher heating value, volatile matter, fixed carbon and ash content of ground bamboo using near infrared spectroscopy. J. Near Infrared Spectrosc. 2017, 25, 301–310. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Payne, C.; Schwartz, A.; Jacobs, J.; Kressin, R.W. A Performance Comparison of Low-Cost Near-Infrared (NIR) Spectrometers to a Conventional Laboratory Spectrometer for Rapid Biomass Compositional Analysis. Bioenerg. Res. 2020, 13, 1121–1129. [Google Scholar] [CrossRef]
- Rinnan, Å.; Van Der Berg, F.W.J.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Mata, M.M.D.; Rocha, P.D.; Farias, I.K.T.; Silva, J.L.B.D.; Medeiros, E.P.; Silva, C.S.; Simões, S.D.S. Distinguishing cotton seed genotypes by means of vibrational spectroscopic methods (NIR and Raman) and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120399. [Google Scholar] [CrossRef]
- Kljusurić, J.G.; Boban, A.; Mucalo, A.; Budić-Leto, I. Novel Application of NIR Spectroscopy for Non-Destructive Determina-tion of ‘Maraština’Wine Parameters. Foods 2022, 11, 1172. [Google Scholar] [CrossRef]
- Fearn, T. Assessing Calibrations: SEP, RPD, RER and R2. NIR News 2002, 13, 12–13. [Google Scholar] [CrossRef]
- Mendonça, M.A.; Araújo, W.M.C.; Borgo, L.A.; Alencar, E.R. Lipid profile of different infant formulas for infants. PLoS ONE 2017, 12, e0177812. [Google Scholar] [CrossRef]
- USDA. Guidance for Industry: Labeling of Infant Formula. 2023. Available online: https://www.fda.gov/media/99701/download (accessed on 15 June 2024).
- Mokrzycki, W.S.; Tatol, M. Colour diference ΔE–A survey. Mach. Graph. Vis. 2012, 20, 383–414. [Google Scholar]
- Rusydi, A.F. Correlation between conductivity and total dissolved solid in various type of water: A review. IOP Conf. Ser. Earth Environ. Sci. 2018, 118, 012019. [Google Scholar] [CrossRef]
- Chen, H.; Tan, C.; Lin, Z.; Wu, T. Detection of melamine adulteration in milk by near-infrared spectroscopy and one-class partial least squares. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 832–836. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Esquerre, C.A.; Eilts, K.; Allen, D.P.; McCoy, S.M.; Varela, S.; Singh, V.; Leakey, A.D.B.; Kamruzzaman, M. Rapid and high-throughput determination of sorghum (Sorghum bicolor) biomass composition using near infrared spectroscopy and chemometrics. Biomass Bioeng. 2024, 186, 107276. [Google Scholar] [CrossRef]
- Rukundo, I.R.; Danao, M.-G.; Mitchell, R.B.; Weller, C.L. Evaluation of predictive performance of PLS regression models after being transferred from benchtop to handheld NIR spectrometers. Biosyst. Eng. 2022, 218, 245–255. [Google Scholar] [CrossRef]
- Nie, Z.; Tremblay, G.F.; Bélanger, G.; Berthiaume, R.; Castonguay, Y.; Bertrand, A.; Michaud, R.; Allard, G.; Han, J. Near-infrared reflectance spectroscopy prediction of neutral detergent-soluble carbohydrates in timothy and alfalfa. J. Dairy. Sci. 2009, 92, 1702–1711. [Google Scholar] [CrossRef]
- Duta, K.; Nath, R.; Samanta, A.K. Advances in Colorimetry; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar] [CrossRef]
- Qasim, W.; Mane, A.V. Characterization and treatment of selected food industrial effluents by coagulation and adsorption techniques. Water Resour. Ind. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Cai, L.; Fang, L. Characteristics on the oxidation stability of infant formula powder with different ingredients during storage. Food Sci. Nutr. 2020, 8, 6392–6400. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Han, W.; Li, C.; Liu, W.; Qiu, Z.; Chen, H. Detection of Adulteration in Infant Formula Based on Ensemble Convolutional Neural Network and Near-Infrared Spectroscopy. Foods 2021, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Casiraghi, E. Advances in NIR Spectroscopy analytical technology in food industries. Foods 2022, 11, 1250. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Rupert, M.; Koczon, P.; Derewiaka, D.; Gajdoš Kljusurić, J.; Petravić Tominac, V.; Mrvčić, J.; Stanzer, D. Characterisation of flavour compounds in Biska—A herbal spirit produced with mistletoe. J. Inst. Brew. 2019, 125, 143–154. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Gajdoš Kljusurić, J.; Putnik, P.; Vukušić, T.; Herceg, Z.; Dragović-Uzelac, V. Stability of polyphenols in chokeberry juice treated with gas phase plasma. Food Chem. 2016, 212, 323–331. [Google Scholar] [CrossRef]
- Sun, D.; Cruz, J.; Alcalá, M.; Romero del Castillo, R.; Sans, S.; Casals, J. Near infrared spectroscopy determination of chemical and sensory properties in tomato. J. Near Infrared Spectrosc. 2021, 29, 289–300. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Liu, X.; Jiang, H.; Zhao, Z.; Niu, X.; Li, C.; Pang, B.; Li, Y. Single- and Multiple-Adulterants Determinations of Goat Milk Powder by NIR Spectroscopy Combined with Chemometric Algorithms. Agriculture 2022, 12, 434. [Google Scholar] [CrossRef]
- Mishra, P.; Rutledge, D.N.; Roger, J.M.; Wali, K.; Khan, H.A. Chemometric pre-processing can negatively affect the performance of near-infrared spectroscopy models for fruit quality prediction. Talanta 2021, 229, 122303. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Fanning, E.; Sim, J.; Eyres, G.T.; Frew, R.; Kebede, B. The Potential of NIR Spectroscopy and Chemometrics to Discriminate Roast Degrees and Predict Volatiles in Coffee. Molecules 2024, 29, 318. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Miao, Y.; Gong, H.; Tao, S.; Chen, Z.; Wang, J.; Chen, Y.; Chen, Y. NIR spectroscopy coupled with chemometric algorithms for the prediction of cadmium content in rice samples. Spectrochim. Acta. Part. A Mol. Biomol. Spectrosc. 2021, 257, 119700. [Google Scholar] [CrossRef]
- Heil, K.; Schmidhalter, U. An Evaluation of Different NIR-Spectral Pre-Treatments to Derive the Soil Parameters C and N of a Humus-Clay-Rich Soil. Sensors 2021, 21, 1423. [Google Scholar] [CrossRef] [PubMed]
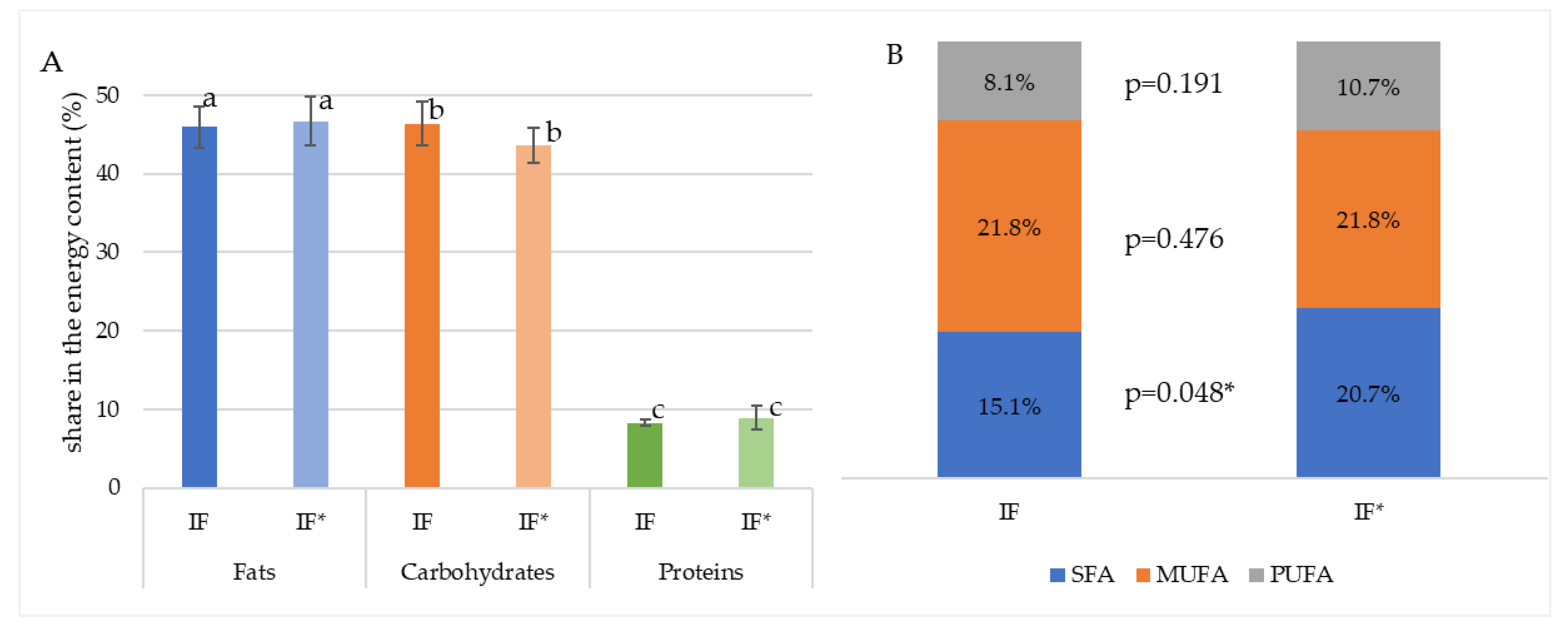

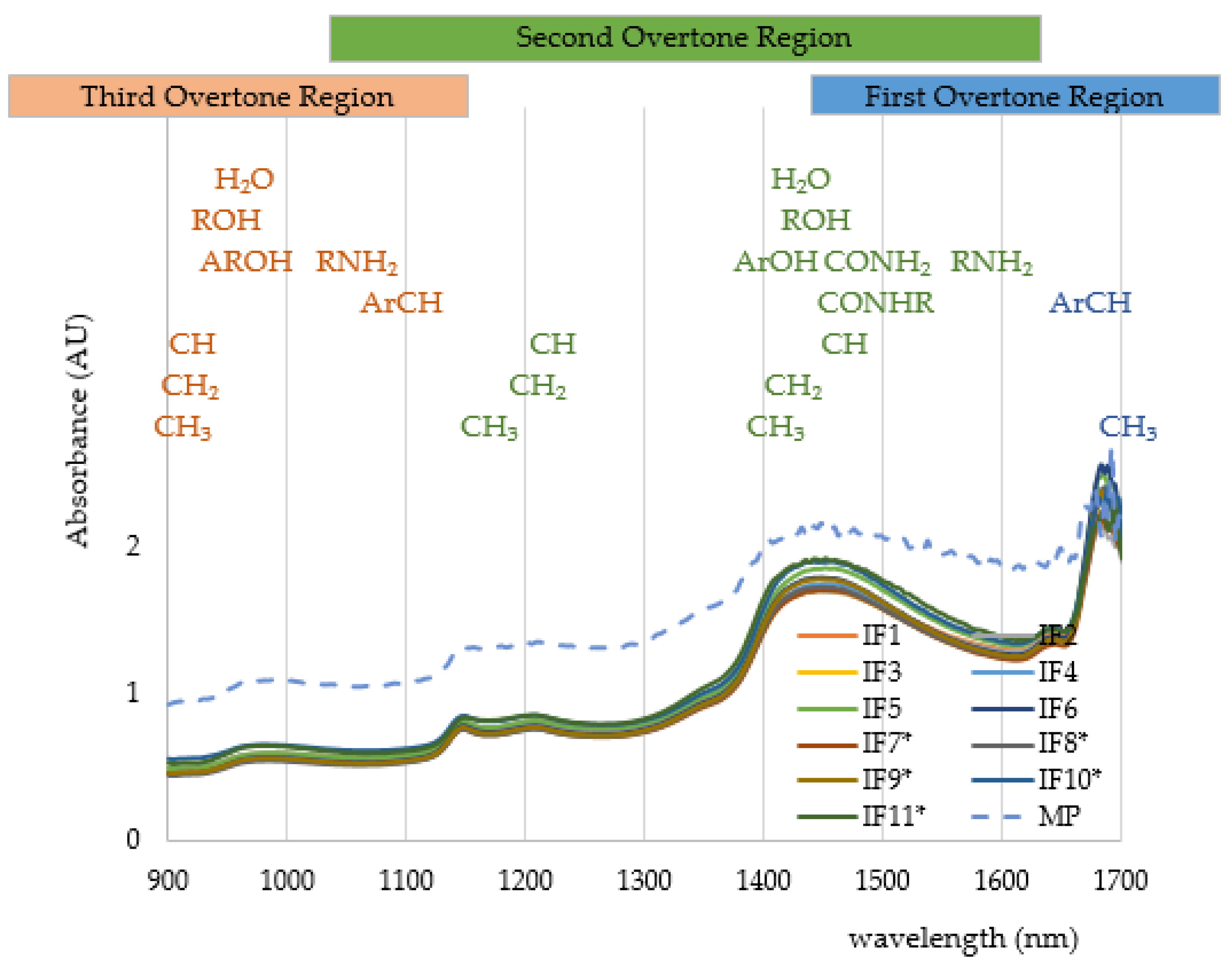
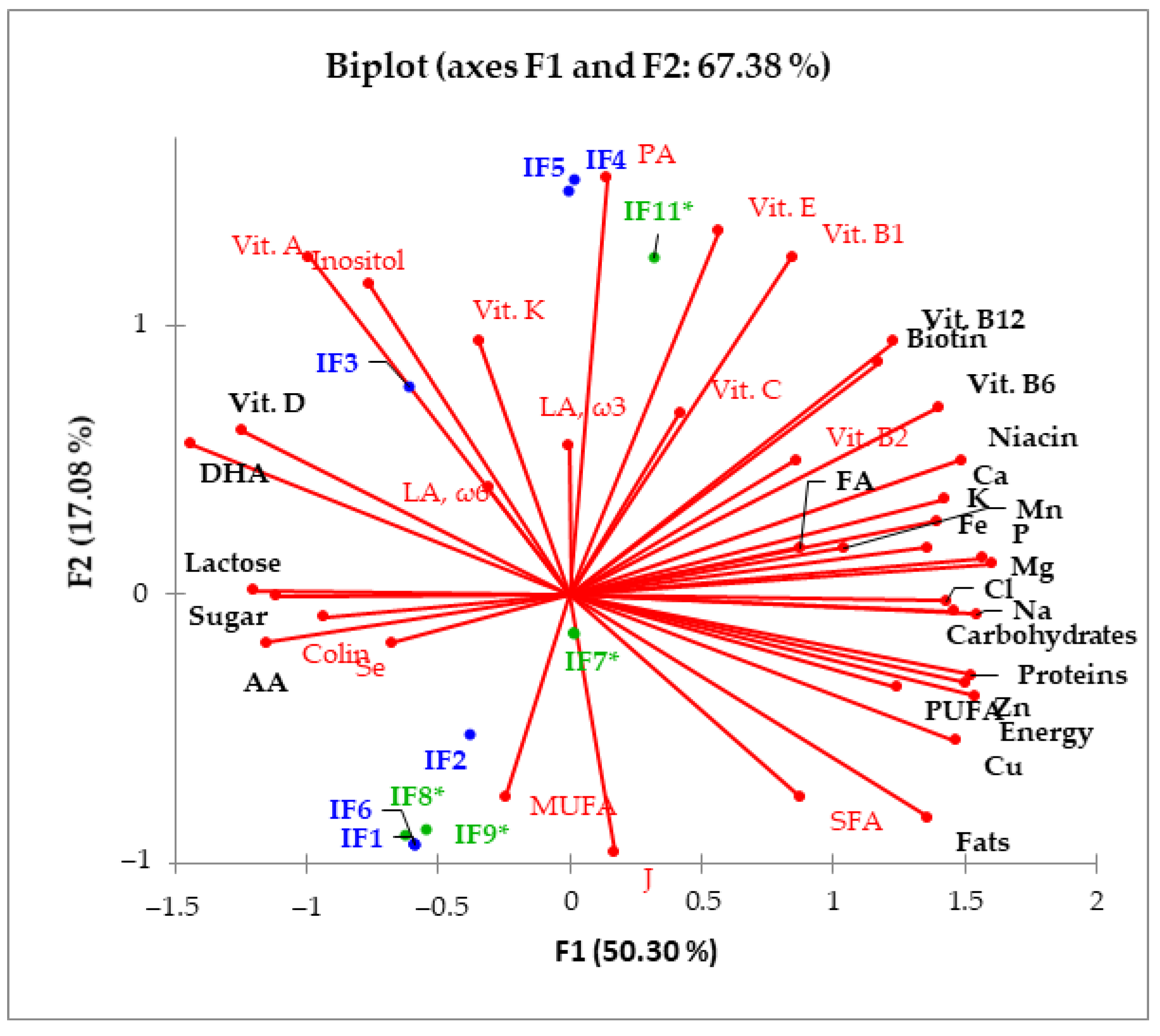
| Scheme | Number of Prepared Samples | Volume Range per Serving (mL) |
|---|---|---|
| IF1 | 6 | 60–210 |
| IF2 | 5 | 90–210 |
| IF3 | 4 | 90–210 |
| IF4 | 4 | 90–180 |
| IF5 | 4 | 90–180 |
| IF6 | 6 | 60–210 |
| IF7* | 6 | 60–210 |
| IF8* | 6 | 60–210 |
| IF9* | 3 | 30–150 |
| IF10* | 4 | 90–240 |
| IF11* | 4 | 90–180 |
| MP | 3 | 170 |
| Precision Parameters | NIR Spectra Wavelengths (nm) | ||
|---|---|---|---|
| ≈1150 | ≈1450 | ≈1680 | |
| Repeatability | |||
| Benchtop device | 2.1 × 10−2 | 5.5 × 10−3 | 6.9 × 10−3 |
| Micro device | 2.7 × 10−2 | 7.9 × 10−3 | 1.5 × 10−2 |
| Reproducibility | |||
| Benchtop device | 6.0 × 10−3 | 9.3 × 10−4 | 4.8 × 10−3 |
| Micro device | 8.7 × 10−3 | 6.5 × 10−3 | 2.1 × 10−2 |
| Observed Parameters | Benchtop NIR | Micro NIR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2c | R2P | SEP | RMSEP | RPD | RER | R2c | R2P | SEP | RMSEP | RPD | RER | |
| Color parameters | ||||||||||||
| L* | 0.899 | 0.537 | 2.078 | 4.316 | 1.654 | 6.118 | 0.977 | 0.699 | 1.342 | 3.216 | 2.133 | 7.891 |
| a* | 0.798 | 0.367 | 0.039 | 0.197 | 1.151 | 4.258 | 0.988 | 0.538 | 0.041 | 0.203 | 1.657 | 6.129 |
| b* | 0.889 | 0.456 | 0.353 | 0.594 | 1.414 | 5.232 | 0.973 | 0.732 | 0.928 | 0.963 | 2.230 | 8.252 |
| Physical parameters | ||||||||||||
| TDS | 0.999 | 0.816 | 0.11 | 0.106 | 2.479 | 9.171 | 0.995 | 0.741 | 0.021 | 0.144 | 2.257 | 8.350 |
| Conductivity | 0.998 | 0.814 | 0.045 | 0.213 | 2.473 | 9.149 | 0.995 | 0.755 | 0.088 | 0.296 | 2.298 | 8.504 |
| Chemical composition | ||||||||||||
| Proteins | 0.899 | 0.637 | 0.642 | 0.802 | 1.949 | 7.213 | 0.993 | 0.672 | 1.321 | 1.149 | 2.053 | 7.596 |
| Carbohydrates | 0.910 | 0.700 | 3.112 | 5.214 | 2.136 | 7.902 | 0.985 | 0.588 | 3.708 | 3.676 | 1.804 | 6.676 |
| Fats | 0.922 | 0.623 | 1.883 | 1.697 | 1.908 | 7.059 | 0.990 | 0.544 | 0.357 | 0.598 | 1.674 | 6.195 |
| PUFA | 0.993 | 0.673 | 0.145 | 0.381 | 7.606 | 7.606 | 0.989 | 0.616 | 0.119 | 0.345 | 1.887 | 6.983 |
| DHA | 0.994 | 0.728 | 3.713 | 5.806 | 8.208 | 8.208 | 0.993 | 0.670 | 4.591 | 5.347 | 2.047 | 7.574 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majić, I.; Zajec, M.; Benković, M.; Jurina, T.; Jurinjak Tušek, A.; Valinger, D.; Gajdoš Kljusurić, J. Qualitative and Quantitative Potential of Low-Cost Near-Infrared (NIR) Devices for Rapid Analysis of Infant Formulas for Regular and Special Needs. Processes 2024, 12, 1771. https://doi.org/10.3390/pr12081771
Majić I, Zajec M, Benković M, Jurina T, Jurinjak Tušek A, Valinger D, Gajdoš Kljusurić J. Qualitative and Quantitative Potential of Low-Cost Near-Infrared (NIR) Devices for Rapid Analysis of Infant Formulas for Regular and Special Needs. Processes. 2024; 12(8):1771. https://doi.org/10.3390/pr12081771
Chicago/Turabian StyleMajić, Iva, Marta Zajec, Maja Benković, Tamara Jurina, Ana Jurinjak Tušek, Davor Valinger, and Jasenka Gajdoš Kljusurić. 2024. "Qualitative and Quantitative Potential of Low-Cost Near-Infrared (NIR) Devices for Rapid Analysis of Infant Formulas for Regular and Special Needs" Processes 12, no. 8: 1771. https://doi.org/10.3390/pr12081771





