Study on Ship Exhaust Gas Denitrification Technology Based on Vapor-Phase Oxidation and Liquid-Phase Impingement Absorption
Abstract
:1. Introduction
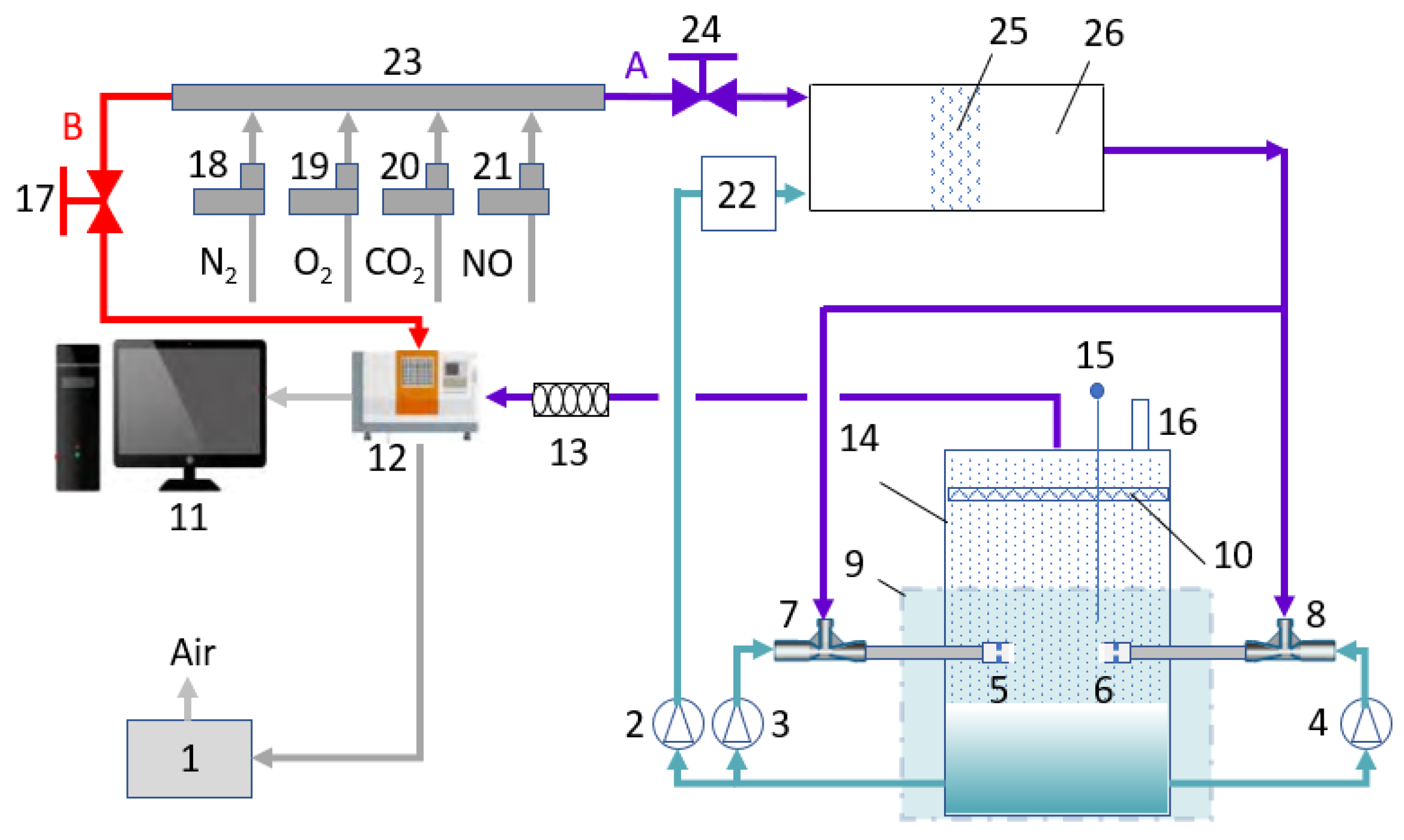
2. Experimental
2.1. Materials
2.2. Experimental Method
3. Results and Discussion
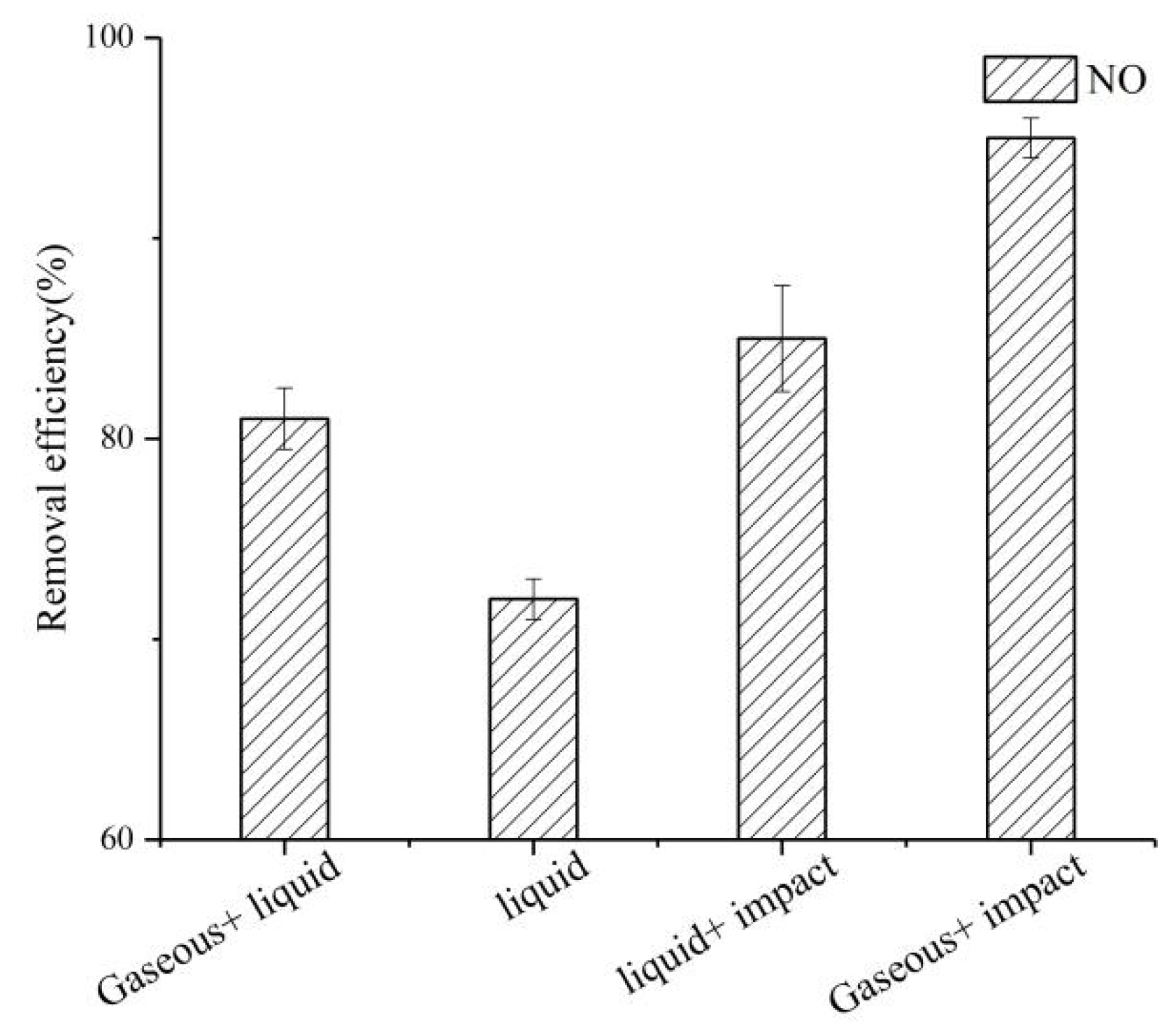
3.1. Comparison of NO Removal Efficiency across Different Systems
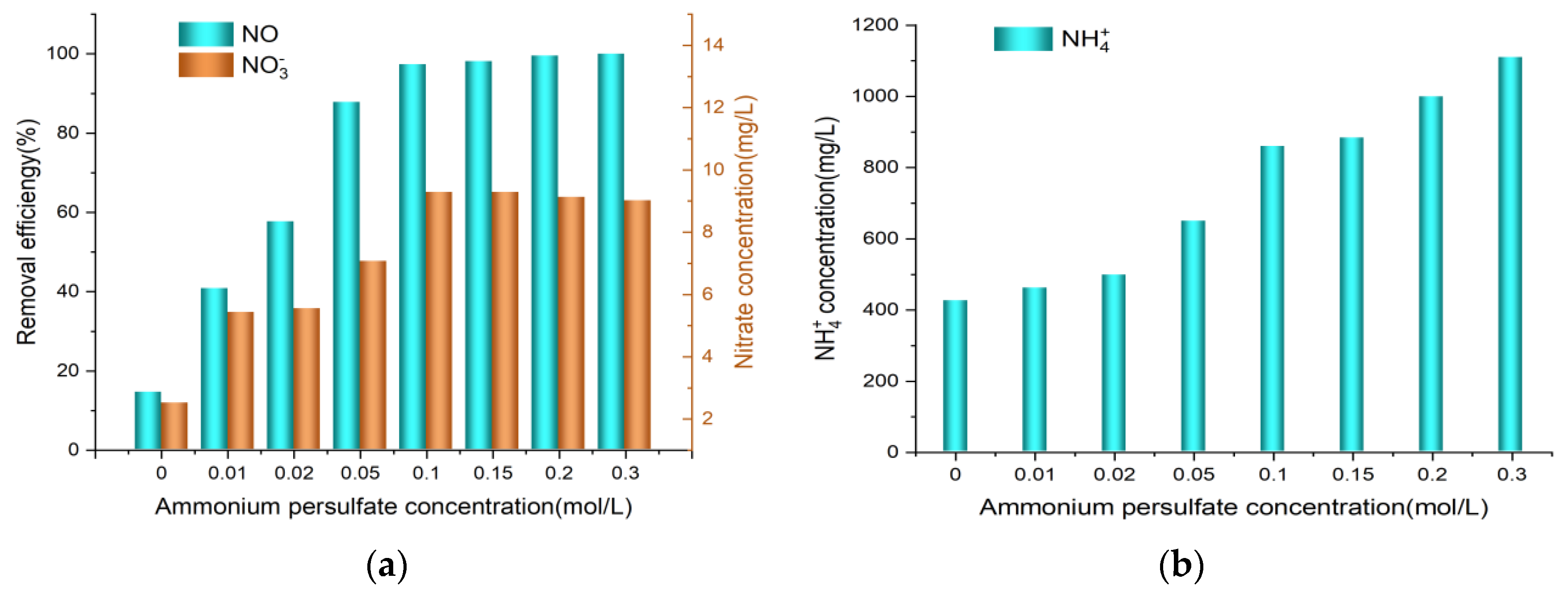
3.2. Effect of (NH4)2S2O8 Concentration
3.3. Effect of Urea Concentration
3.4. Effect of Temperature
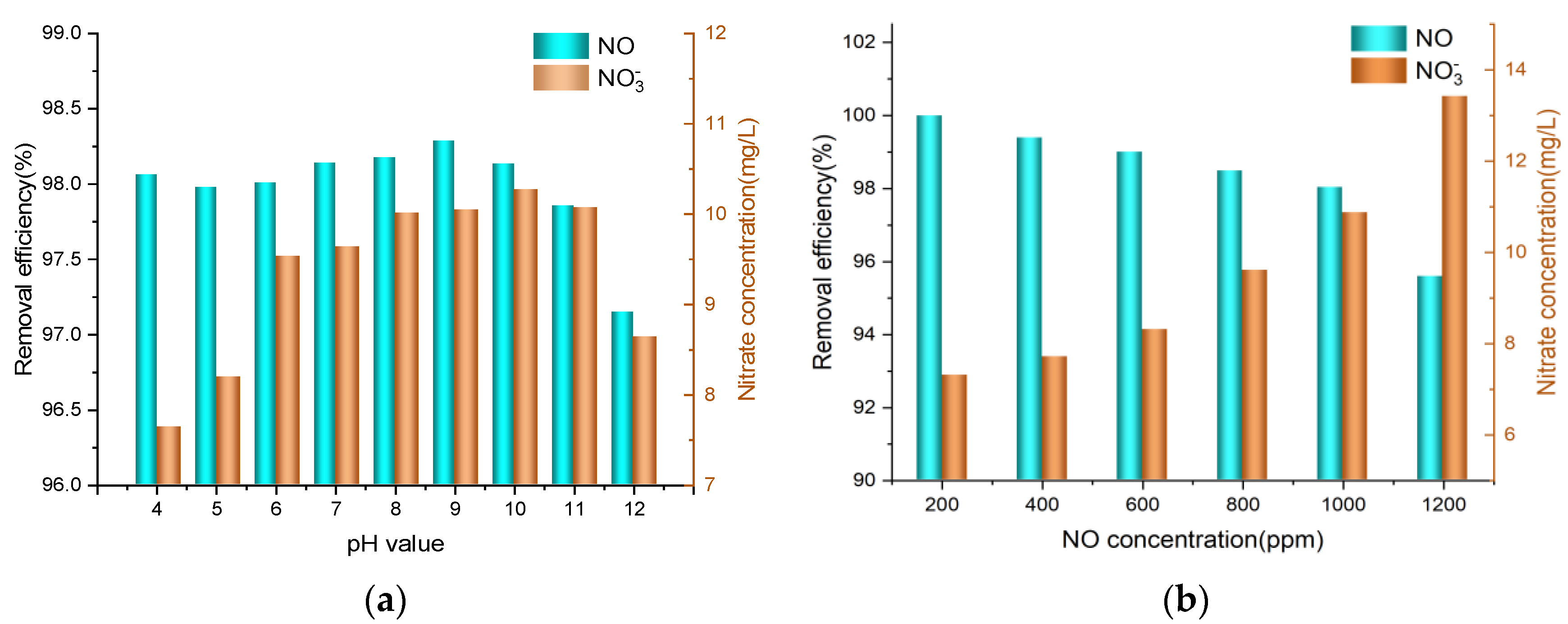
3.5. Effect of NU Solution Initial pH Value
3.6. Effect of NO Concentration
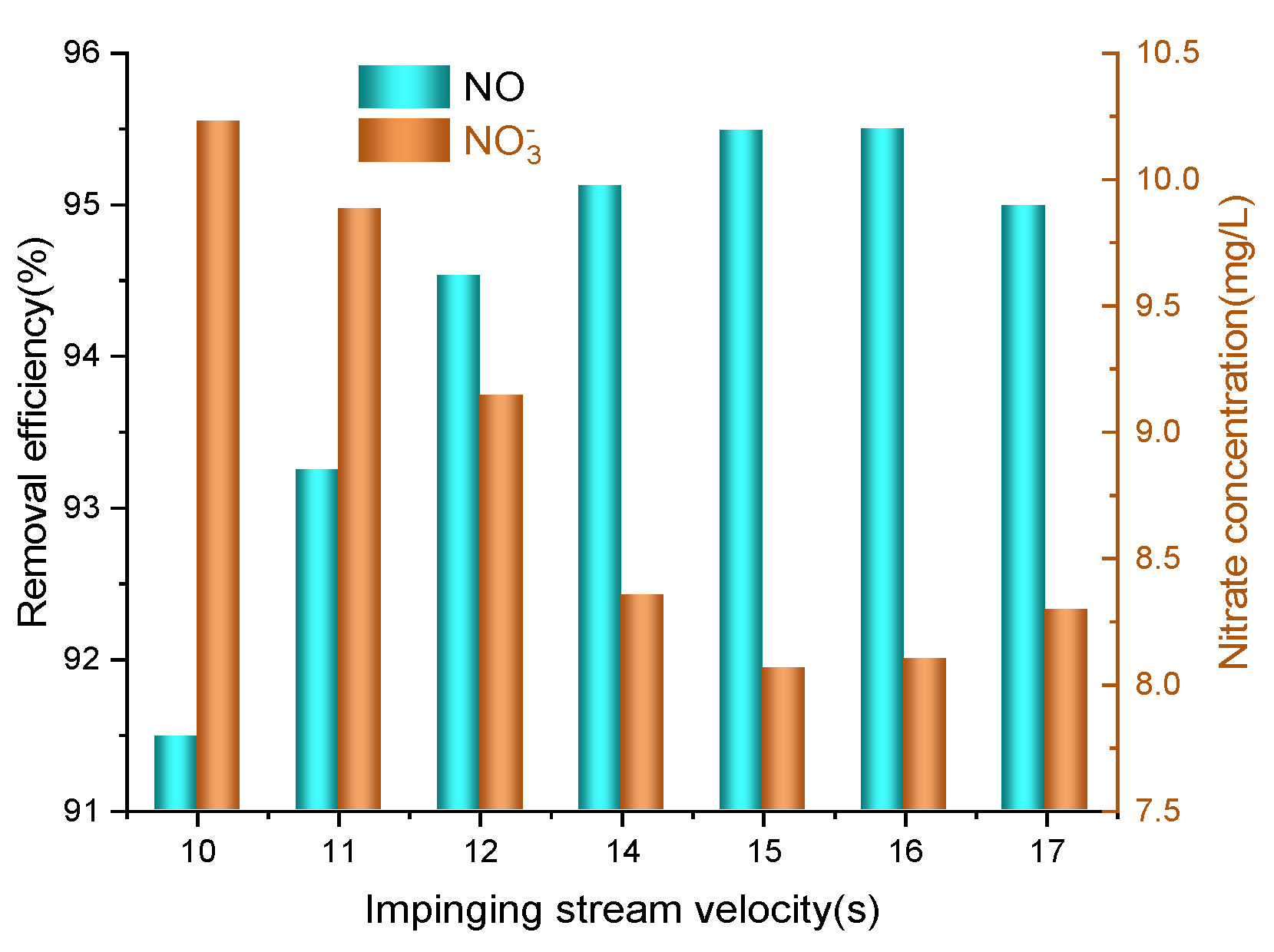
3.7. Effects of Impinging Stream Velocity
3.8. Analysis of Products in Solution
4. Conclusions
- (a)
- The NO removal efficiency improved with higher reaction temperatures and increased liquid–gas ratios. However, when the impinging stream velocity and pH value became too high, it instead reduced the removal efficiency of nitric oxide.
- (b)
- The addition of urea to the solutions effectively reduced nitrate levels and significantly improved NO removal efficiency under certain conditions.
- (c)
- The NO removal efficiency improved with increasing (NH4)2S2O8 concentration, provided that the urea concentration was fixed and the (NH4)2S2O8 concentration was below 0.05 mol/L.
- (d)
- NO removal efficiencies were nearly 97% at 1000 ppm, and the nitrate concentration was 7.53 mg/L at 343 K with 0.05 mol/L (NH4)2S2O8, 1.5 mol/L urea, and 15 m/s impinging stream velocity, which were the optimal results in this work.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ağbulut, Ü.; Sarıdemir, S. Synergistic effects of hybrid nanoparticles along with conventional fuel on engine performance, combustion, and environmental characteristics. Energy 2024, 292, 130267. [Google Scholar] [CrossRef]
- Charbouillot, T.; Janet, D.C.; Schaal, P.; Beynier, I.; Boulat, J.-M.; Grandchamp, A.; Biesse, F. Methodology for the direct measurement of tire emission factors. Sci. Total Environ. 2023, 863, 160853. [Google Scholar] [CrossRef]
- Kawakami, K.; Watatani, K.; Yamasaki, H.; Kuroki, T.; Okubo, M. Performance evaluation of PM, NOx, and hydrocarbon removal in diesel engine exhaust by surface discharge-induced plasma. J. Hazard. Mater. 2024, 462, 132685. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, X.; Wei, Z.; Wang, L.; Wang, C.; Chen, Z. A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fuelled vessels. Sci. Total Environ. 2021, 766, 144319. [Google Scholar] [CrossRef]
- Repka, S.; Erkkilä-Välimäki, A.; Jonson, J.E.; Posch, M.; Törrönen, J.; Jalkanen, J.P. Assessing the costs and environmental benefits of IMO regulations of ship-originated SOx and NOx emissions in the Baltic Sea. Ambio 2021, 50, 1718–1730. [Google Scholar] [CrossRef]
- Ni, P.; Wang, X.; Li, H. A review on regulations, current status, effects and reduction strategies of emissions for marine diesel engines. Fuel 2020, 279, 118477. [Google Scholar] [CrossRef]
- Masera, K.; Hossain, A.K. Advancement of biodiesel fuel quality and NOx emission control techniques. Renew. Sustain. Energy Rev. 2023, 178, 113235. [Google Scholar] [CrossRef]
- Di Natale, F.; Carotenuto, C.; Parisi, A.; Flagiello, D.; Lancia, A. Wet electrostatic scrubbing for flue gas treatment. Fuel 2022, 325, 124888. [Google Scholar] [CrossRef]
- Li, S.; Huang, W.; Xu, H.; Liu, K.; Wang, J.-N.; Sun, Y.; Qu, Z.; Yan, N. Enhanced simultaneous absorption of NOx and SO2 in oxidation-reduction-absorption process with a compounded system based on Na2SO3. J. Environ. Sci. 2022, 111, 1–10. [Google Scholar] [CrossRef]
- Enache, F.; Cursaru, D.; Danulescu, D. Integration of wet scrubbing system and SOx additive technologies to reduce the SO2 emissions generated in FCCU. Chem. Pap. 2022, 76, 6537–6549. [Google Scholar] [CrossRef]
- Premkumar, S.; Balaji, G. Study on efficient removal of NO from CI engine exhaust gas by wet scrubbing method using NaOH solution. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1130, p. 012077. [Google Scholar]
- Ren, Y.; Li, Y.; Wu, X.; Wang, J.; Zhang, G. S-scheme Sb2WO6/g-C3N4 photocatalysts with enhanced visible-light-induced photocatalytic NO oxidation performance. Chin. J. Catal. 2021, 42, 69–77. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Zhang, Z.; He, Y.; Zhu, Y.; Shao, J.; Yuan, D.; Chen, G.; Cen, K. Flue gas treatment with ozone oxidation: An overview on NOx, organic pollutants, and mercury. Chem. Eng. J. 2020, 382, 123030. [Google Scholar] [CrossRef]
- Li, B.; Wu, H.; Liu, X.; Zhu, T.; Liu, F.; Zhao, X. Simultaneous removal of SO2 and NO using a novel method with red mud as absorbent combined with O3 oxidation. J. Hazard. Mater. 2020, 392, 122270. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, X.; Xue, Y.; Liu, L.; Yang, F. Simultaneous desulfurization and denitrification from flue gas by catalytic ozonation combined with NH3/(NH4) 2S2O8 absorption: Mechanisms and recovery of compound fertilizer. Sci. Total Environ. 2020, 706, 136027. [Google Scholar] [CrossRef] [PubMed]
- Jessieleena, A.A.; Priyanka, M.; Saravanakumar, M.P. Comparative study of Fenton, Fe2+/NaOCl and Fe2+/(NH4) 2S2O8 on tannery sludge dewaterability, degradability of organics and leachability of chromium. J. Hazard. Mater. 2021, 402, 123495. [Google Scholar] [CrossRef]
- Dinesh, V.; Nagarajan, R. (NH4) 2S2O8-Mediated Metal-Free Decarboxylative Formylation/Acylation of α-Oxo/Ketoacids and Its Application to the Synthesis of Indole Alkaloids. J. Org. Chem. 2022, 87, 10359–10365. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Z.; Gu, J.; Yuan, H.; Wu, Y.; Chen, Y. A facile new process for the efficient conversion of spent LiFePO4 batteries via (NH4) 2S2O8-assisted mechanochemical activation coupled with water leaching. Chem. Eng. J. 2023, 471, 144265. [Google Scholar] [CrossRef]
- Miao, J.; Su, Q.; Li, H.; Chen, J.; Wang, J. The promotional effect of MnOx on (NH4) 2S2O8-modified activated coke for selective catalytic reduction of NO with NH3 at low temperature. J. Energy Inst. 2020, 93, 2017–2024. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Wang, P.; Mo, Y.; Lai, W.-D.; Li, Z.-J.; Yu, R.-J.; Du, Y.-T.; Zhang, X.-R.; Chen, Y. Effect of the oxygen functional groups of activated carbon on its electrochemical performance for supercapacitors. New Carbon Mater. 2020, 35, 232–243. [Google Scholar] [CrossRef]
- Malekzadeh, M.; Swihart, M.T. Vapor-phase production of nanomaterials. Chem. Soc. Rev. 2021, 50, 7132–7249. [Google Scholar] [CrossRef]
- Stapelmann, K.; Myers, B.; Quesada, M.H.; Lenker, E.; Ranieri, P.J. Following O and OH in He/O2 and He/H2O gas mixtures—From the gas phase through the liquid phase to modifications on a biological sample. J. Phys. D Appl. Phys. 2021, 54, 434003. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, J.; Sheng, C.; Zhong, H. Theoretical and experimental studies of NO removal in alkaline H2O2 system. Sep. Purif. Technol. 2024, 351, 127992. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, T.; Chen, M.; Feng, H.; Yuan, R.; Zhong, C.; Yan, W.; Tian, Y.; Wu, X.; Chu, W.; et al. High-purity pyrrole-type FeN 4 sites as a superior oxygen reduction electrocatalyst. Energy Environ. Sci. 2020, 13, 111–118. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, C.; Yu, Y.; Lu, S.; Zhang, B. Recent advances in non-noble metal electrocatalysts for nitrate reduction. Chem. Eng. J. 2021, 403, 126269. [Google Scholar] [CrossRef]
- Long, J.; Chen, S.; Zhang, Y.; Guo, C.; Fu, X.; Deng, D.; Xiao, J. Direct electrochemical ammonia synthesis from nitric oxide. Angew. Chem. Int. Ed. 2020, 59, 9711–9718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Liu, Y. Removal of gaseous hydrogen sulfide using ultraviolet/Oxone-induced oxidation scrubbing system. Chem. Eng. J. 2020, 393, 124740. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Dong, X.; Feng, Y. Flow regime transition and bubble behavior in gas-liquid two-phase impinging jet reactor. Chem. Eng. Res. Des. 2024, 201, 275–285. [Google Scholar] [CrossRef]
- Ping, H.; Zhou, S.; Zhou, J.; Zhang, Z. A novel combined system using Na2S2O8/urea to simultaneously remove SO2 and NO in marine diesel engine exhaust. J. Hazard. Mater. 2020, 399, 123069. [Google Scholar]
- Wang, Y.; Wang, Y.; Liu, Y. Fe2+/heat-coactivated PMS oxidation-absorption system for H2S removal from gas phase. Sep. Purif. Technol. 2022, 286, 120458. [Google Scholar] [CrossRef]
- Yang, J.; Tang, T.; Jiang, Y.; Karavalakis, G.; Durbin, T.D.; Miller, J.W.; Cocker, D.R.; Johnson, K.C. Controlling emissions from an ocean-going container vessel with a wet scrubber system. Fuel 2021, 304, 121323. [Google Scholar] [CrossRef]
- Li, D.; Xu, N.; Zhao, Y.; Zhou, C.; Zhang, L.; Wu, L.; Zhang, T. A reliable and precise protocol for urea quantification in photo/electrocatalysis. Small Methods 2022, 6, 2200561. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Zhou, S.; Zhou, J. New experimental results of NO removal from simulated marine engine exhaust gases by Na2S2O8/urea solutions. Chem. Eng. J. 2019, 362, 12–20. [Google Scholar] [CrossRef]
- Xu, H.; Wang, T.; Koppala, S.; Hu, J.; Ma, S.; Miao, W.; Le, T.; Zhang, L. Improving the quality of ammonium sulfate produced from the flue gas desulfurization process by using ammonium persulfate. Sep. Purif. Technol. 2023, 308, 122879. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, J.; Wang, Z. Experimental study on removal performance of SO2 and NOx in marine exhaust gas using seawater/urea peroxide solution and analysis of ions concentration change. Fuel Process. Technol. 2022, 227, 107133. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Sakyi, N.Y. Simultaneous absorption and oxidation of nitric oxide and sulfur dioxide by aqueous solutions of sodium persulfate activated by temperature. Ind. Eng. Chem. Res. 2013, 52, 11702–11711. [Google Scholar] [CrossRef]
- Liu, T.; Abbatt, J.P.D. Oxidation of sulfur dioxide by nitrogen dioxide accelerated at the interface of deliquesced aerosol particles. Nature Chemistry 2021, 13, 1173–1177. [Google Scholar] [CrossRef]
- Xie, R.; Ma, L.; Li, Z.; Qu, Z.; Yan, N.; Li, J. Review of sulfur promotion effects on metal oxide catalysts for NOx emission control. ACS Catal. 2021, 11, 13119–13139. [Google Scholar] [CrossRef]


| Number | (NH4)2S2O8 (mol/L) | Urea (mol/L) | NH4+ (mg/L) | NO2− (mg/L) | NO3− (mg/L) |
|---|---|---|---|---|---|
| 1 | 0.05 | 0 | 129.39 | 0.87 | 139.42 |
| 2 | 0 | 1.5 | 447.47 | 0 | 1.74 |
| 3 | 0.05 | 1.5 | 597.58 | 0 + 0.39 | 7.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Ma, W. Study on Ship Exhaust Gas Denitrification Technology Based on Vapor-Phase Oxidation and Liquid-Phase Impingement Absorption. Processes 2024, 12, 1798. https://doi.org/10.3390/pr12091798
Wang Y, Ma W. Study on Ship Exhaust Gas Denitrification Technology Based on Vapor-Phase Oxidation and Liquid-Phase Impingement Absorption. Processes. 2024; 12(9):1798. https://doi.org/10.3390/pr12091798
Chicago/Turabian StyleWang, Yuanqing, and Wenyao Ma. 2024. "Study on Ship Exhaust Gas Denitrification Technology Based on Vapor-Phase Oxidation and Liquid-Phase Impingement Absorption" Processes 12, no. 9: 1798. https://doi.org/10.3390/pr12091798




