Ohmic Heating in Food Processing: An Overview of Plant-Based Protein Modification
Abstract
:1. Introduction
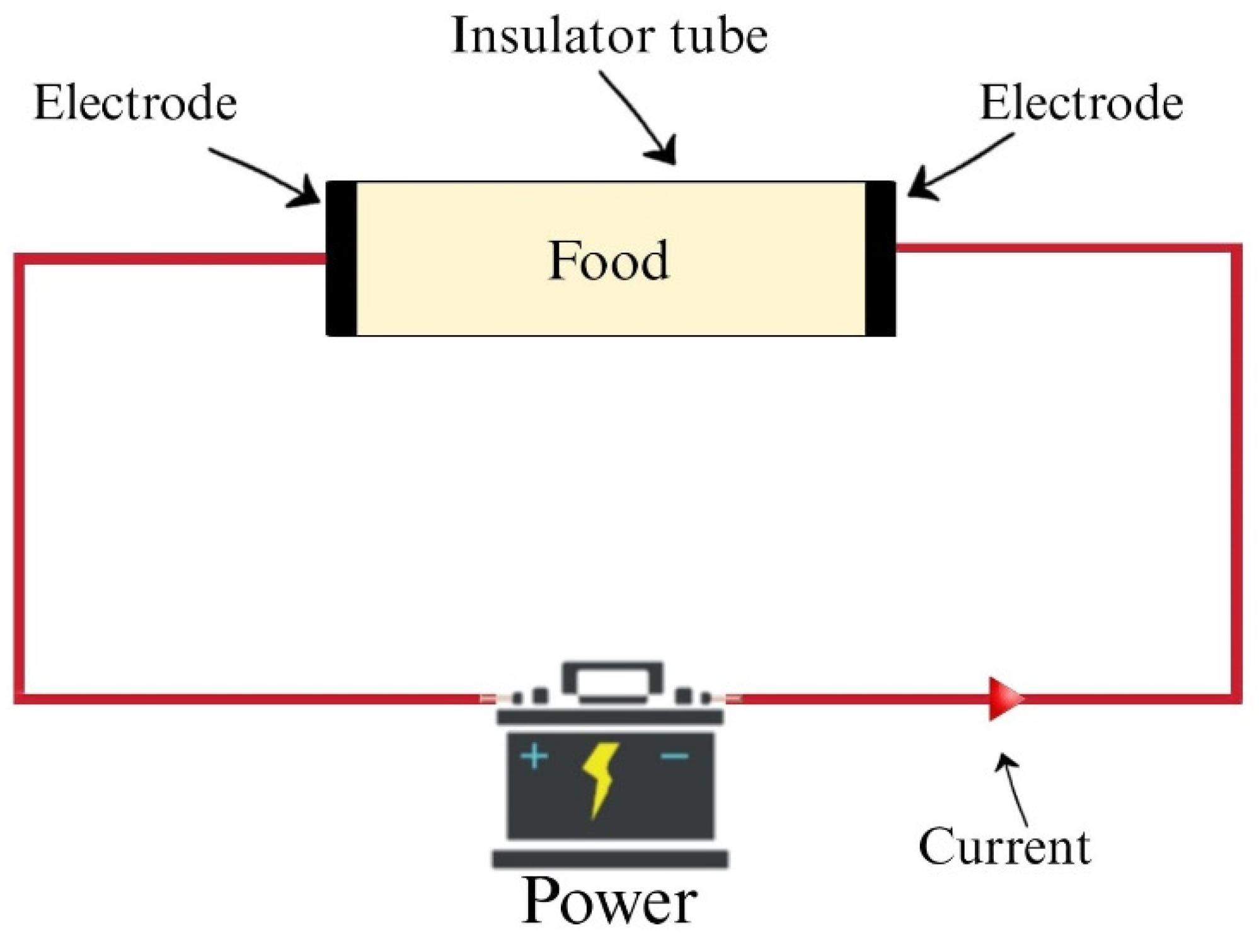
2. Ohmic Heating
3. Ohmic Heating Applied to Food Proteins
| Protein | Experimental Process | Ohmic Heating Treatment | Highlight | References |
|---|---|---|---|---|
| Soy protein isolate | They assessed the effects of ohmic and conventional heating on structural, thermal, and physical changes in soy protein isolate edible films. | The electrical frequency for treatment was 50 Hz, and the electric field intensities used for ohmic treatment were 3, 6, 9, and 12 V·cm−1, compared to conventional heating. | Ohmic-heated films exhibited reduced α-helix content, increased random coil content, smaller average particle size, lower fluorescence intensity, and higher surface hydrophobicity. | [36] |
| Sunflower protein isolate | They analyzed the structural, thermal, and physical changes in sunflower protein isolate compared to sodium caseinate when subjected to the application of non-thermal moderate electric field (MEF). | Electric field application: sample had 10 V/cm for 1 h (V1) and 10 V/cm for 2 h (V2) applied; sample had 150 V/cm for 5 s (V5) and 150 V/cm for 20 s (V20) applied. | Greater homogeneity was achieved with V20. The secondary and tertiary structures of sunflower protein changed primarily with V20, gaining a bulkier and more flexible structure and increased surface hydrophobicity. V5 had the lowest denaturation enthalpy. V1 also produced sodium caseinate solution with the lowest denaturation enthalpy. | [10] |
| Soy protein isolate | They studied the effect of ohmic heating on the structure and immunoreactive properties of soy proteins. | Ohmic heating studies were conducted at various frequencies, including 50 Hz, 500 Hz, 2 kHz, and 20 kHz. The electrode distance was consistently maintained at 30 mm for all assessed conditions. When necessary, the electric field intensities during the procedure ranged from 2 V/cm to 20 V/cm. Soybean trypsin inhibitor allergenic protein (STI), also known as Gly m TI, was selected as the allergen of interest due to its remarkable resistance to thermal treatments | Ohmic heating applications at frequencies ranging from 50 to 500 Hz can lead to conformational changes in soy protein fractions, as evidenced by the reduced fluorescence of aromatic amino acids. This effect may be directly associated with the formation of complexes with metals present in the protein solution during ohmic heating. However, there is a lack of detailed information on how electrical variables, such as electric field and frequency, affect soy protein fractions in this specific context. | [31] |
| Lactoferrin | The purpose of study was to create gel-like gelatin emulsions using lactoferrin dispersions heated through ohmic treatment. The impact of both ohmic and conventional heating on the protein’s secondary structure and the subsequent thermal aggregation of lactoferrin was assessed. | Ohmic heating was applied using a 4 cm electrode gap, with voltages ranging from 36 to 86 V. This treatment was compared to conventional heating. A double-walled glass reactor vessel was used, and a 20 mL lactoferrin dispersion was heated to 90 °C for 30 min for both treatments. | Both ohmic and conventional heating influenced the thermal unfolding and aggregation of lactoferrin molecules. Ohmic heating likely affected the molecular flexibility or the stability of lactoferrin’s hydrophobic groups. Ohmic heating resulted in fewer aggregated protein molecules compared to conventional heating. The difference in aggregation patterns was confirmed by a smaller increase in particle size, turbidity, intrinsic and extrinsic fluorescence, and a distinct dichroic signal, impacting the structural and mechanical properties of the prepared emulsions. | [37] |
| β—Lactoglobulin | They investigated the effect of moderate electric fields (MEF) during the ohmic heating of purified β-lactoglobulin fractions under different physicochemical conditions of pH and temperature. | The treatments were conducted at temperatures of 50, 60, 70, 80, and 90 °C for 10 min. The applied electric field intensity varied from 80 V/cm during the heating phase to 20 V/cm during the holding step. The electric frequency remained constant at 20 kHz, as the choice of frequencies within the kHz range was adopted to prevent unwanted electrochemical effects, such as electrolysis and electrode oxidation. | The results indicated that the effects of moderate electric field (MEF) are limited to unfolded protein conformations. This suggests that under the specific conditions tested, including electric field intensity, frequency, and exposure period, the imposed disturbances are not significant enough to impede the protein folding process. Thus, the effects of MEF appear to act synergistically with thermal effects. Furthermore, their specific influence and scope varied according to the pH, which may be related to the flexibility of β-lactoglobulin in response to environmental conditions. These findings support theories presented in other studies, which had already demonstrated that moderate electric field (MEF) effects impact the aggregation and gelation of serum proteins. | [38] |
| Whey protein isolate | They evaluated the formation of soluble whey protein aggregates from whey protein isolated under conditions of near-neutral pH, both in the presence and absence of a moderate electric field (MEF). | The applied voltage and temperature were controlled using a function generator connected to an amplifier system. During the heating and holding phases, the applied voltage ranged from 15 to 22 V/cm and 4 to 8 V/cm, respectively. The electrical frequency was 25 kHz, chosen to reduce electrochemical reactions at the electrode interface, minimizing the potential for corrosion and metal leakage into the medium. The results were compared to conventional heating. | The results indicated that the application of moderate electric fields impacts the unfolding and aggregation processes of whey protein at relatively high temperatures. After heating at 85 °C for 30 s, treatments with moderate electric fields resulted in whey protein isolate solutions with a higher amount of β-Lactoglobulin (8%) and α-Lactalbumin (10%) in their native form, compared to a traditional heating method. The protein aggregates in solutions treated with a moderate electric field increased by up to 78 nm, while conventional heating led to an 86 nm increase in the size of these aggregates. The aggregation of whey proteins under the influence of the moderate electric field was not intense enough to create an elastic gel network. This is due to reductions in the value of storage and loss moduli after treatment with the moderate electric field. | [39] |
| Lentil protein isolate | They assessed the impact of ohmic heating (temperature, electric field intensity, and frequency) on the structure of lentil protein obtained through alkaline extraction and protein–pectin interactions | The heating was conducted at temperatures of 40, 50, 60, 70, and 80 °C. After reaching the desired temperature, a waiting period of 30 s was observed. The voltages used for heating and maintaining a constant temperature were 5 and 75 V/cm, respectively. In the ohmic heating treatments, the initial phase involved the application of an electric field of 75 V/cm until the solution reached 80 °C. In the first ohmic treatment, the voltage was reduced to 5 V/cm, and the temperature was kept constant at 80 °C. In the second treatment (OH-75 V/cm), electric fields ranging from 10 to 20 V/cm were used while maintaining the temperature at 80 °C. | The results indicated that the characteristics of lentil protein were modified by the thermal processes, with the extent of these modifications being influenced by the treatment temperature, electric field intensity, and pH during protein fraction extraction. Lentil protein extracted at pH 9 showed greater stability to temperature variations during ohmic heating. Under low electric field conditions (5 V/cm), there was an increase in protein surface hydrophobicity and accessibility to sulfhydryl groups. In contrast, lentil protein extracted at pH 7 was significantly affected by all thermal treatments. While some structural changes were observed in specific protein characteristics, no influences on protein–pectin interactions in lentil were identified. | [40] |
3.1. Ohmic Heating in Coagulated Products
3.2. Thermal Treatment Using Ohmic Heating to Improve Protein Stability and Protein–Molecule Interactions
3.3. Effect on Chemical Bonds
3.4. Techno-Functional Properties
3.4.1. Water-Holding Capacity and Solubility
3.4.2. Oil-Holding Capacity and Emulsification
3.4.3. Gelling Properties
4. Final Considerations
Author Contributions
Funding
Conflicts of Interest
References
- Liu, F.; Li, M.; Wang, Q.; Yan, J.; Han, S.; Ma, C.; Ma, P.; Liu, X.; McClements, D.J. Future Foods: Alternative Proteins, Food Architecture, Sustainable Packaging, and Precision Nutrition. Crit. Rev. Food Sci. Nutr. 2023, 63, 6423–6444. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M. Plant Protein Ingredients with Food Functionality Potential. Nutr. Bull. 2020, 45, 321–327. [Google Scholar] [CrossRef]
- Qin, P.; Wang, T.; Luo, Y. A Review on Plant-Based Proteins from Soybean: Health Benefits and Soy Product Development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification Approaches of Plant-Based Proteins to Improve Their Techno-Functionality and Use in Food Products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhao, M.; Waterhouse, G.I.N. Protein Modification During Ingredient Preparation and Food Processing: Approaches to Improve Food Processability and Nutrition. Food Bioprocess. Technol. 2014, 7, 1853–1893. [Google Scholar] [CrossRef]
- Venkateswara Rao, M.; Sunil, C.K.; Rawson, A.; Chidanand, D.V.; Venkatachlapathy, N. Modifying the Plant Proteins Techno-Functionalities by Novel Physical Processing Technologies: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 4070–4091. [Google Scholar] [CrossRef]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond Oil: Press Cakes and Meals Supplying Global Protein Requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Ravindran, N.; Kumar Singh, S.; Singha, P. A Comprehensive Review on the Recent Trends in Extractions, Pretreatments and Modifications of Plant-Based Proteins. Food Res. Int. 2024, 190, 114575. [Google Scholar] [CrossRef]
- Albe Slabi, S.; Mathe, C.; Basselin, M.; Framboisier, X.; Ndiaye, M.; Galet, O.; Kapel, R. Multi-Objective Optimization of Solid/Liquid Extraction of Total Sunflower Proteins from Cold Press Meal. Food Chem. 2020, 317, 126423. [Google Scholar] [CrossRef]
- Subaşı, B.G.; Casanova, F.; Capanoglu, E.; Ajalloueian, F.; Sloth, J.J.; Mohammadifar, M.A. Protein Extracts from De-Oiled Sunflower Cake: Structural, Physico-Chemical and Functional Properties after Removal of Phenolics. Food Biosci. 2020, 38, 100749. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Effect of pH and Holding Time on the Characteristics of Protein Isolates from Chenopodium Seeds and Study of Their Amino Acid Profile and Scoring. Food Chem. 2019, 272, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Van De Vondel, J.; Lambrecht, M.A.; Delcour, J.A. Osborne Extractability and Chromatographic Separation of Protein from Quinoa (Chenopodium Quinoa Willd.) Wholemeal. LWT 2020, 126, 109321. [Google Scholar] [CrossRef]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition Through Plant-Based Foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef] [PubMed]
- Indiarto, R.; Rezaharsamto, B. A Review on Ohmic Heating and Its Use in Food. Int. J. Sci. Technol. Res. 2020, 9, 485–490. [Google Scholar]
- Ramaswamy, H.S.; Marcotte, M.; Sastry, S.; Abdelrahim, K. (Eds.) Ohmic Heating in Food Processing; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-0-429-14996-2. [Google Scholar]
- Knirsch, M.C.; Alves Dos Santos, C.; Martins De Oliveira Soares Vicente, A.A.; Vessoni Penna, T.C. Ohmic Heating—A Review. Trends Food Sci. Technol. 2010, 21, 436–441. [Google Scholar] [CrossRef]
- Assiry, A.; Sastry, S.K.; Samaranayake, C. Degradation Kinetics of Ascorbic Acid during Ohmic Heating with Stainless Steel Electrodes. J. Appl. Electrochem. 2003, 33, 187–196. [Google Scholar] [CrossRef]
- Jaeger, H.; Roth, A.; Toepfl, S.; Holzhauser, T.; Engel, K.-H.; Knorr, D.; Vogel, R.F.; Bandick, N.; Kulling, S.; Heinz, V.; et al. Opinion on the Use of Ohmic Heating for the Treatment of Foods. Trends Food Sci. Technol. 2016, 55, 84–97. [Google Scholar] [CrossRef]
- Guo, W.; Llave, Y.; Jin, Y.; Fukuoka, M.; Sakai, N. Mathematical Modeling of Ohmic Heating of Two-Component Foods with Non-Uniform Electric Properties at High Frequencies. Innov. Food Sci. Emerg. Technol. 2017, 39, 63–78. [Google Scholar] [CrossRef]
- Alkanan, Z.T.; Altemimi, A.B.; Al-Hilphy, A.R.S.; Watson, D.G.; Pratap-Singh, A. Ohmic Heating in the Food Industry: Developments in Concepts and Applications during 2013–2020. Appl. Sci. 2021, 11, 2507. [Google Scholar] [CrossRef]
- Fryer, P.J.; De Alwis, A.A.P.; Koury, E.; Stapley, A.G.F.; Zhang, L. Ohmic Processing of Solid-Liquid Mixtures: Heat Generation and Convection Effects. J. Food Eng. 1993, 18, 101–125. [Google Scholar] [CrossRef]
- Halden, K. Changes in the Electrical Conductivity of Foods during Ohmic Heating. Int. J. Food Sci. Technol. 1990, 25, 9–25. [Google Scholar] [CrossRef]
- Varghese, K.S.; Pandey, M.C.; Radhakrishna, K.; Bawa, A.S. Technology, Applications and Modelling of Ohmic Heating: A Review. J. Food Sci. Technol. 2014, 51, 2304–2317. [Google Scholar] [CrossRef]
- Stancl, J.; Zitny, R. Milk Fouling at Direct Ohmic Heating. J. Food Eng. 2010, 99, 437–444. [Google Scholar] [CrossRef]
- Kamonpatana, P.; Gavahian, M.; Sastry, S.K. Ohmic Heating for Food Processing: Methods and Procedures Related to Process Parameters. In Emerging Food Processing Technologies; Gavahian, M., Ed.; Methods and Protocols in Food Science; Springer: New York, NY, USA, 2022; pp. 181–193. ISBN 978-1-07-162135-6. [Google Scholar]
- Rocha, R.S.; Silva, R.; Ramos, G.L.P.; Cabral, L.A.; Pimentel, T.C.; Campelo, P.H.; Blumer Zacarchenco, P.; Freitas, M.Q.; Esmerino, E.A.; Silva, M.C.; et al. Ohmic Heating Treatment in High-Protein Vanilla Flavored Milk: Quality, Processing Factors, and Biological Activity. Food Res. Int. 2022, 161, 111827. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.A.; Guimarães, J.D.T.; Cabral, L.A.; Silva, M.C.; Raices, R.S.L.; Ramos, G.L.P.A.; Pimentel, T.C.; Esmerino, E.A.; Cruz, A.G.D.; Freitas, M.Q.D. Effect of Ohmic Heating Temperature and Voltage on Liquid Whole Egg Processing. Innov. Food Sci. Emerg. Technol. 2023, 89, 103490. [Google Scholar] [CrossRef]
- Cappato, L.P.; Ferreira, M.V.S.; Guimaraes, J.T.; Portela, J.B.; Costa, A.L.R.; Freitas, M.Q.; Cunha, R.L.; Oliveira, C.A.F.; Mercali, G.D.; Marzack, L.D.F.; et al. Ohmic Heating in Dairy Processing: Relevant Aspects for Safety and Quality. Trends Food Sci. Technol. 2017, 62, 104–112. [Google Scholar] [CrossRef]
- Avelar, Z.; Vicente, A.A.; Saraiva, J.A.; Rodrigues, R.M. The Role of Emergent Processing Technologies in Tailoring Plant Protein Functionality: New Insights. Trends Food Sci. Technol. 2021, 113, 219–231. [Google Scholar] [CrossRef]
- Avelar, Z.; Monge-Morera, M.; Delcour, J.A.; Saraiva, J.A.; Vicente, A.A.; Rodrigues, R.M. Ohmic Heating as an Innovative Strategy to Modulate Protein Fibrillation. Innov. Food Sci. Emerg. Technol. 2024, 92, 103587. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Machado, L.; Ferreira, S.; Costa, J.; Villa, C.; Barreiros, M.P.; Mafra, I.; Teixeira, J.A.; Vicente, A.A. Influence of Ohmic Heating on the Structural and Immunoreactive Properties of Soybean Proteins. LWT 2021, 148, 111710. [Google Scholar] [CrossRef]
- Moreira, T.C.P.; Pereira, R.N.; Vicente, A.A.; Da Cunha, R.L. Effect of Ohmic Heating on Functionality of Sodium Caseinate—A Relationship with Protein Gelation. Food Res. Int. 2019, 116, 628–636. [Google Scholar] [CrossRef]
- Shimoyamada, M.; Itabashi, Y.; Sugimoto, I.; Kanauchi, M.; Ishida, M.; Tsuzuki, K.; Egusa, S.; Honda, Y. Characterization of Soymilk Prepared by Ohmic Heating and the Effects of Voltage Applied. Food Sci. Technol. Res. 2015, 21, 439–444. [Google Scholar] [CrossRef]
- Wang, L.-J.; Li, D.; Tatsumi, E.; Liu, Z.-S.; Chen, X.D.; Li, L.-T. Application of Two-Stage Ohmic Heating to Tofu Processing. Chem. Eng. Process. Process Intensif. 2007, 46, 486–490. [Google Scholar] [CrossRef]
- Li, X.; Ye, C.; Tian, Y.; Pan, S.; Wang, L. Effect of Ohmic Heating on Fundamental Properties of Protein in Soybean Milk. J. Food Process Eng. 2018, 41, e12660. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Hu, X.; Zhu, X.; Wang, L.; Zhang, N.; Yu, D. Structural and Physical Properties of Soybean Protein Isolate Films with Ohmic Heating Treatment: Impacts of Electric Field. Innov. Food Sci. Emerg. Technol. 2022, 82, 103213. [Google Scholar] [CrossRef]
- De Figueiredo Furtado, G.; Pereira, R.N.C.; Vicente, A.A.; Cunha, R.L. Cold Gel-like Emulsions of Lactoferrin Subjected to Ohmic Heating. Food Res. Int. 2018, 103, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Avelar, Z.; Vicente, A.A.; Petersen, S.B.; Pereira, R.N. Influence of Moderate Electric Fields in β-Lactoglobulin Thermal Unfolding and Interactions. Food Chem. 2020, 304, 125442. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Martins, A.J.; Ramos, O.L.; Malcata, F.X.; Teixeira, J.A.; Vicente, A.A.; Pereira, R.N. Influence of Moderate Electric Fields on Gelation of Whey Protein Isolate. Food Hydrocoll. 2015, 43, 329–339. [Google Scholar] [CrossRef]
- Miranda, C.G.; Rodrigues, R.M.; Pereira, R.N.; Speranza, P.; Kurozawa, L.E.; Vicente, A.A.; Sato, A.C.K. Influence of Ohmic Heating on Lentil Protein Structure and Protein-Pectin Interactions. Innov. Food Sci. Emerg. Technol. 2023, 87, 103413. [Google Scholar] [CrossRef]
- Mefleh, M.; Pasqualone, A.; Caponio, F.; Faccia, M. Legumes as Basic Ingredients in the Production of Dairy-free Cheese Alternatives: A Review. J. Sci. Food Agric. 2022, 102, 8–18. [Google Scholar] [CrossRef]
- Pare, A.; Sivashankari, M.; Madan, A. Effect of Different Heating Methods on the Yield and Quality of Tofu. Soybean Res. 2014, 12, 99–109. [Google Scholar]
- Joeres, E.; Drusch, S.; Töpfl, S.; Juadjur, A.; Bindrich, U.; Völker, T.; Heinz, V.; Terjung, N. Ohmic vs. Conventional Heating: Influence of Moderate Electric Fields on Properties of Potato Protein Isolate Gels. Innov. Food Sci. Emerg. Technol. 2023, 85, 103333. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, R.; Hu, J.; Luan, Y.; Liu, R.; Ge, Q.; Yu, H.; Wu, M. Moderate Pulsed Electric Field-Induced Structural Unfolding Ameliorated the Gelling Properties of Porcine Muscle Myofibrillar Protein. Innov. Food Sci. Emerg. Technol. 2022, 81, 103145. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Luo, S.; Wang, N.; Wang, L.; Zhang, N.; Yu, D. Evaluation of Ohmic Heating Modified Soybean Protein Isolate Structure and Antioxidant Film under Different Catechin Concentrations. LWT 2023, 186, 115224. [Google Scholar] [CrossRef]
- De Vargas, V.H.; Marczak, L.D.F.; Flôres, S.H.; Mercali, G.D. Advanced Technologies Applied to Enhance Properties and Structure of Films and Coatings: A Review. Food Bioprocess. Technol. 2022, 15, 1224–1247. [Google Scholar] [CrossRef]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive Peptides Derived from Plant Origin By-Products: Biological Activities and Techno-Functional Utilizations in Food Developments—A Review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Çelik, M.; Güzel, M.; Yildirim, M. Effect of pH on Protein Extraction from Sour Cherry Kernels and Functional Properties of Resulting Protein Concentrate. J. Food Sci. Technol. 2019, 56, 3023–3032. [Google Scholar] [CrossRef]
- Kasapis, S.; Norton, I.T.; Ubbink, J.B. Modern Biopolymer Science; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 978-0-12-374195-0. [Google Scholar]
- Lima-Becerra, I.; María, B.-A.; Dorantes-Campuzano, F.; Mojica, L.; Loarca-Piña, G.; Morales-Sánchez, E.; Ramírez-Jiménez, A.K.; Gaytán-Martínez, M. Ohmic Heating as an Emerging Technology for the Improvement of the Techno-Functional Properties of Common Bean Flour. In Proceedings of the 2nd International Electronic Conference on Foods—“Future Foods and Food Technologies for a Sustainable World”, Online, 14 October 2021; p. 95. [Google Scholar]
- Joshi, M.; Timilsena, Y.; Adhikari, B. Global Production, Processing and Utilization of Lentil: A Review. J. Integr. Agric. 2017, 16, 2898–2913. [Google Scholar] [CrossRef]
- Miranda, C.G.; Speranza, P.; Kurozawa, L.E.; Kawazoe Sato, A.C. Lentil Protein: Impact of Different Extraction Methods on Structural and Functional Properties. Heliyon 2022, 8, e11775. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Avelar, Z.; Machado, L.; Pereira, R.N.; Vicente, A.A. Electric Field Effects on Proteins—Novel Perspectives on Food and Potential Health Implications. Food Res. Int. 2020, 137, 109709. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.J.; Du, H.; McManus, W.R.; Larsen, K.M. Microstructural Changes in Casein Supramolecules during Acidification of Skim Milk. J. Dairy Sci. 2009, 92, 5854–5867. [Google Scholar] [CrossRef] [PubMed]
- Joeres, E.; Schölzel, H.; Drusch, S.; Töpfl, S.; Heinz, V.; Terjung, N. Ohmic vs. Conventional Heating: Influence of Moderate Electric Fields on Properties of Egg White Protein Gels. Food Hydrocoll. 2022, 127, 107519. [Google Scholar] [CrossRef]
- Cassanelli, M.; Prosapio, V.; Norton, I.; Mills, T. Acidified/Basified Gellan Gum Gels: The Role of the Structure in Drying/Rehydration Mechanisms. Food Hydrocoll. 2018, 82, 346–354. [Google Scholar] [CrossRef]
| Advantages | Disadvantages |
|---|---|
| Fast and uniform heating: Ohmic heating can heat food quickly and evenly, preventing hot and cold spots. This helps maintain the quality of the final product. | High initial costs: The installation of ohmic heating equipment can be expensive, which can be a barrier for small food processing businesses. |
| Energy efficiency: Compared to some conventional heating methods, ohmic heating can be more energy-efficient as it directly converts electricity into heat in the food. | Potential quality issues: Depending on the food composition and processing conditions, ohmic heating can affect the texture, color, and taste of food undesirably. Another concern is the migration of metallic ions from the electrodes into the food. |
| Precise temperature control: Precise temperature control during ohmic heating is possible, which is important for ensuring food safety and the quality of the final product. | Requires conductive foods: Ohmic heating works best with foods that are good electrical conductors. Foods with low electrical conductivity (such as those high in fats) may be less effective in this process. |
| Reduced nutrient loss: In some cases, ohmic heating may result in less nutrient loss compared to more aggressive thermal processing methods, such as prolonged boiling. This can be achieved thanks to more uniform and rapid heating, which allows for reduced temperature and shorter exposure time of the food to heat. Additionally, the food is heated directly through the passage of electrical current, eliminating the need for heating mediums such as water, which can lead to nutrient loss through leaching. | Need for technical monitoring: Due to the precision required in temperature control, ohmic heating processes demand constant technical monitoring, which can increase operational costs. |
| Reduced need for additives: Since the process is quick and effective in reducing microorganisms, there may be a reduced need for chemical additives or preservatives. Additionally, the reduced exposure of the food to heat minimizes thermal damage to the food matrix, which decreases the need for added additives to compensate for sensory loss caused by heating. | Potential for electrical burns: There is a risk of electrical shock when using ohmic heating equipment, necessitating the implementation of appropriate safety measures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, I.F.; Pimentel, T.C.; da Cruz, A.G.; Stringheta, P.C.; Martins, E.; Campelo, P.H. Ohmic Heating in Food Processing: An Overview of Plant-Based Protein Modification. Processes 2024, 12, 1800. https://doi.org/10.3390/pr12091800
dos Santos IF, Pimentel TC, da Cruz AG, Stringheta PC, Martins E, Campelo PH. Ohmic Heating in Food Processing: An Overview of Plant-Based Protein Modification. Processes. 2024; 12(9):1800. https://doi.org/10.3390/pr12091800
Chicago/Turabian Styledos Santos, Israel Felipe, Tatiana Colombo Pimentel, Adriano Gomes da Cruz, Paulo César Stringheta, Evandro Martins, and Pedro Henrique Campelo. 2024. "Ohmic Heating in Food Processing: An Overview of Plant-Based Protein Modification" Processes 12, no. 9: 1800. https://doi.org/10.3390/pr12091800





