Advancing Sustainability: Utilizing Bacterial Polyhydroxyalkanoate for Food Packaging
Abstract
:1. Introduction
2. Important Properties of Food Packaging Films
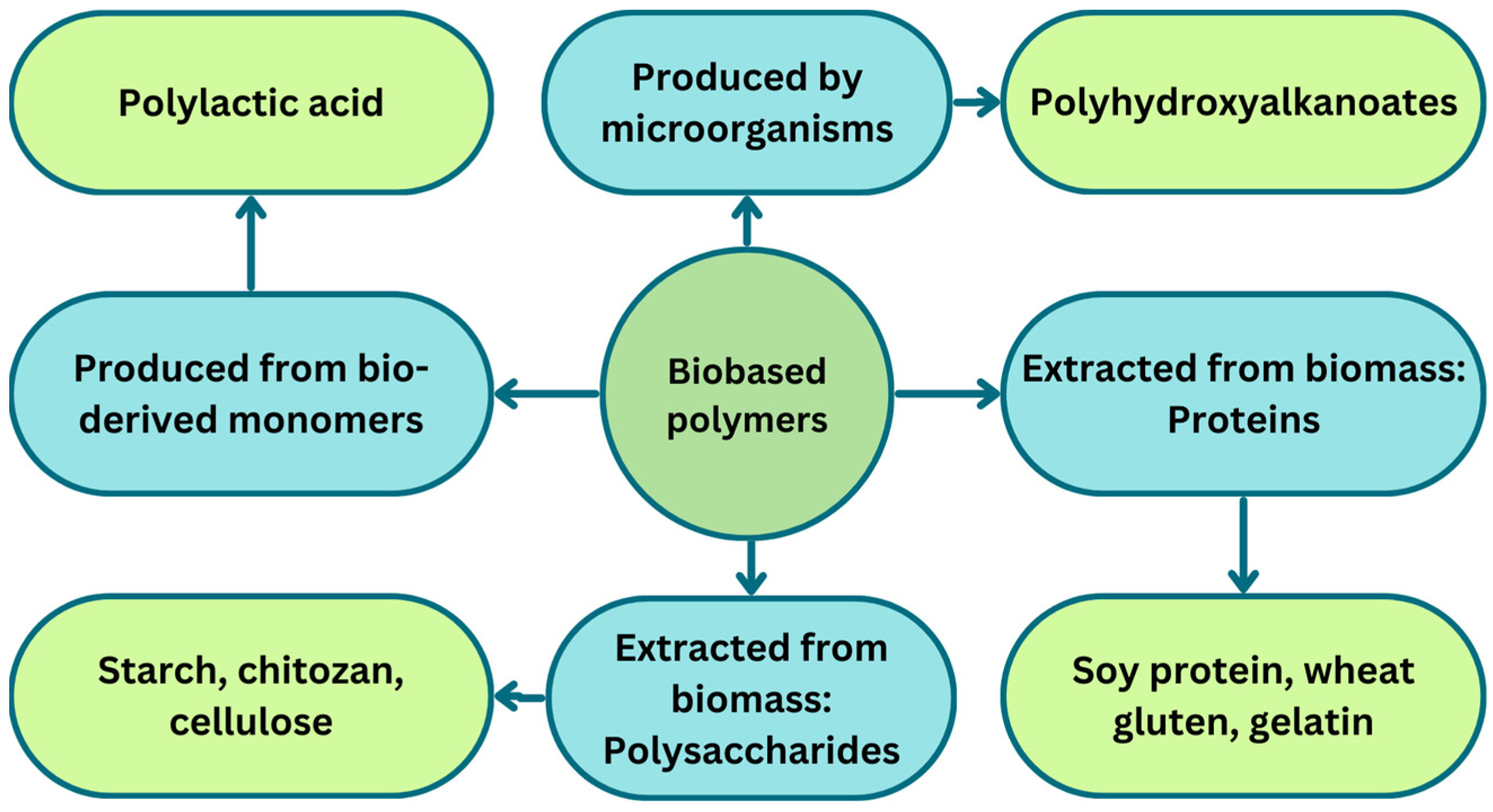
3. Biobased and Biodegradable Polymers
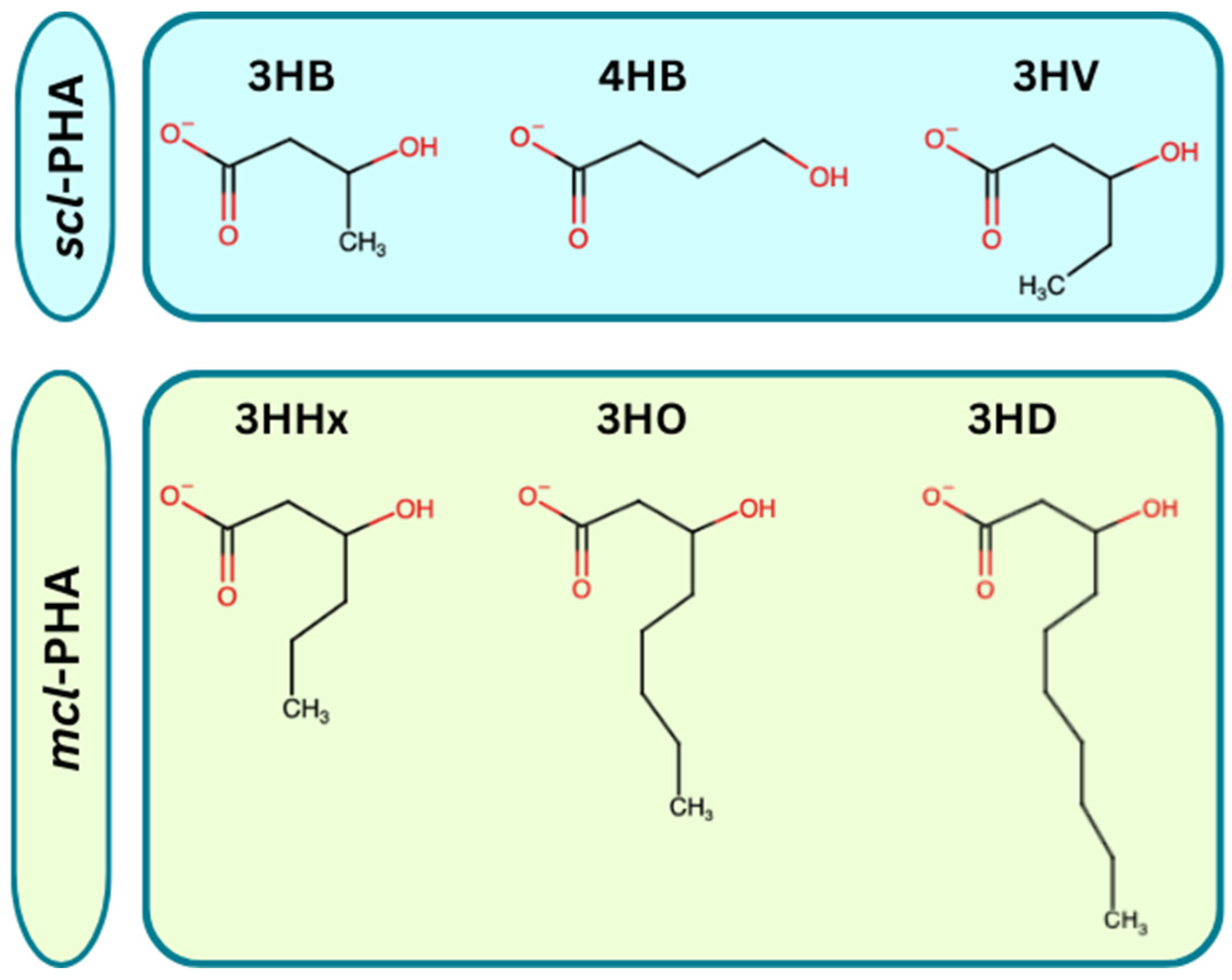
3.1. Polyhydroxyalkanoates
3.2. Polylactic Acid
3.3. Starch
3.4. Chitosan
3.5. Cellulose
3.6. Protein
3.7. Polycaprolactone
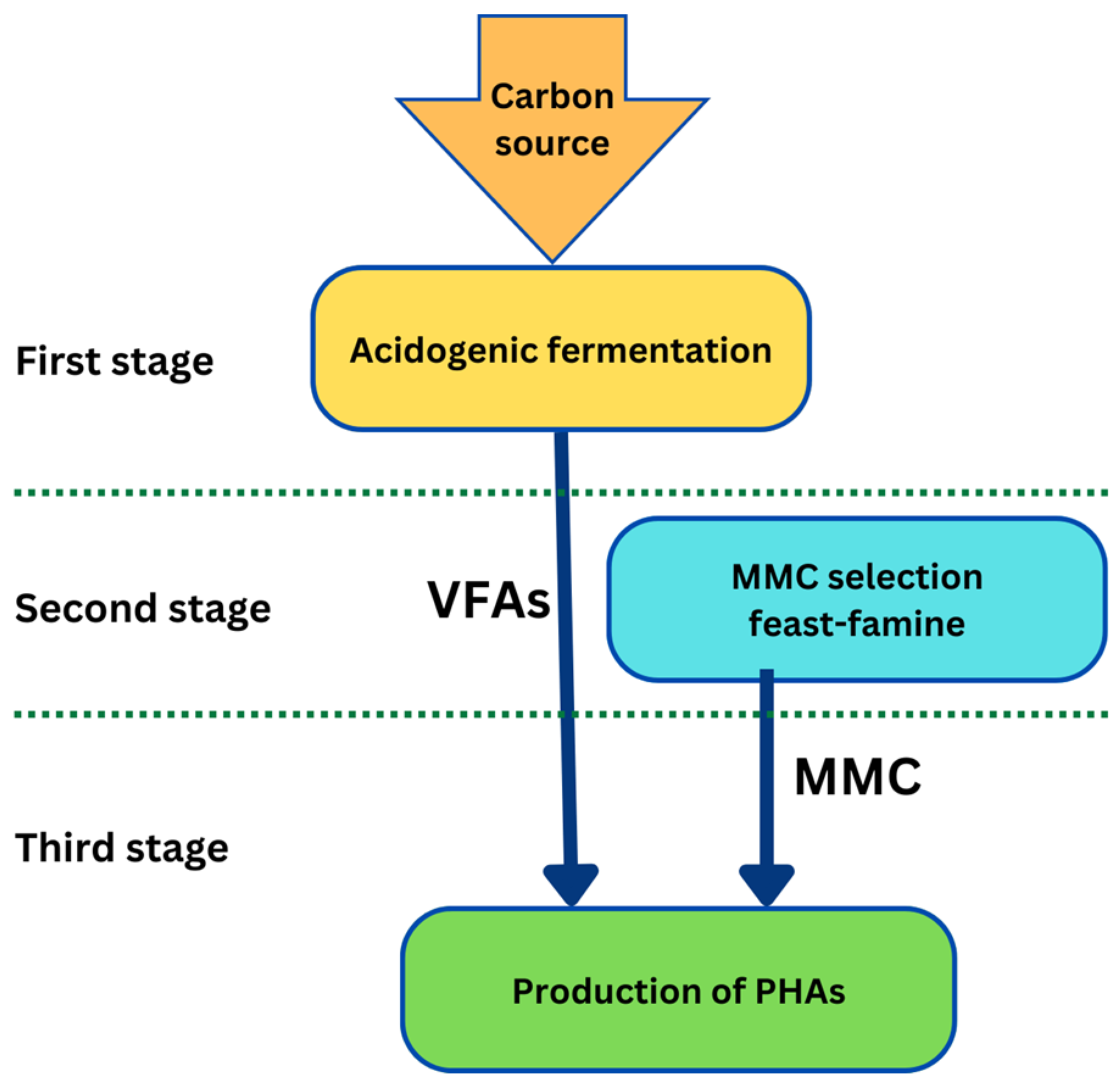
4. Production of PHAs from Biomass
4.1. Pure Cultures vs. Mixed Microbial Cultures
4.2. Submerged Fermentation vs. Solid-State Fermentation
4.3. Carbon Sources for PHA Production
4.4. Extraction and Purification Methods
4.5. Identification and Analysis of PHAs
5. Active Packaging Based on PHAs
5.1. Biodegradable PHA-Based Blends
5.2. Antimicrobial Active Packaging PHA-Based Materials
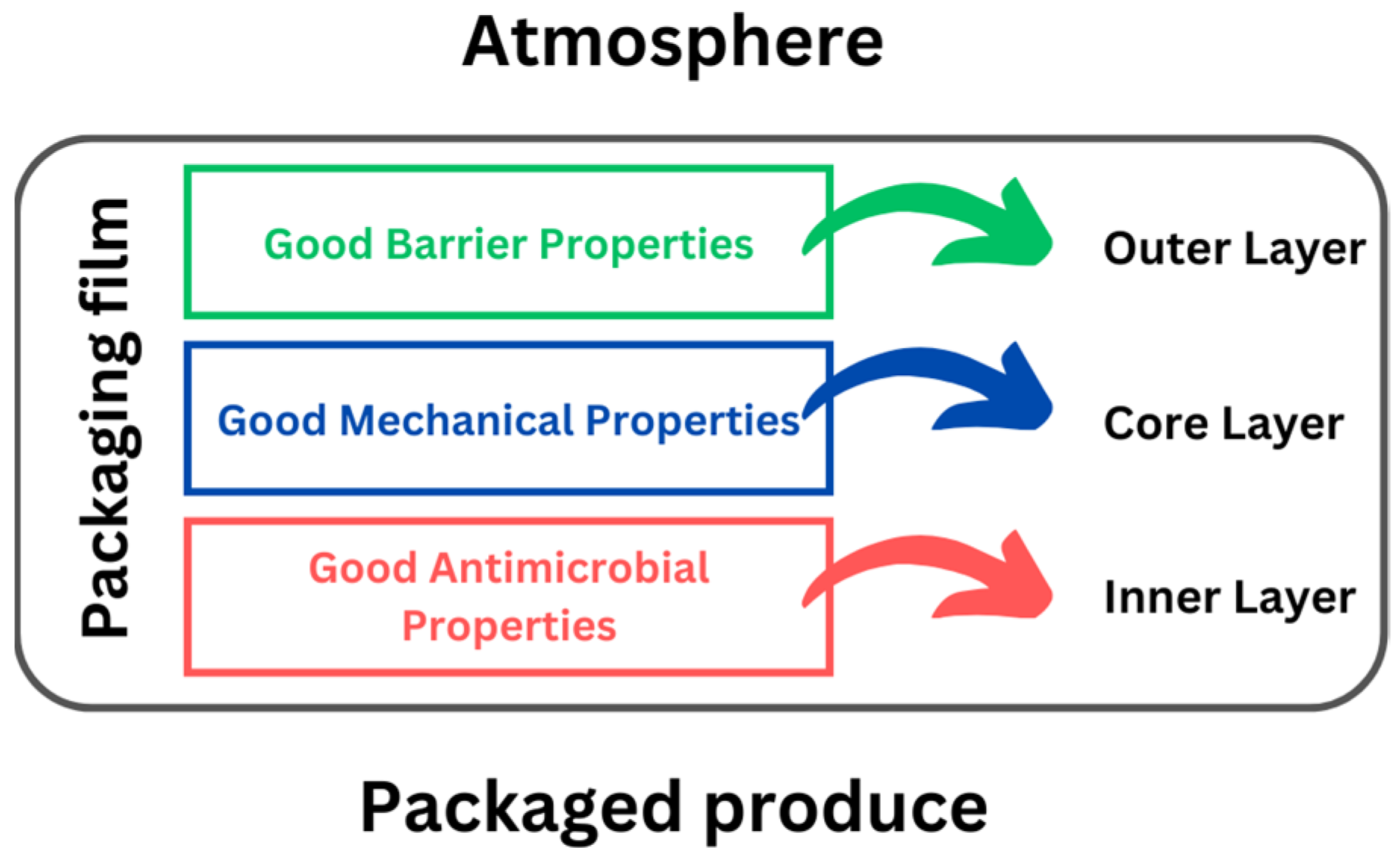
5.3. Multilayer PHA-Based Packaging
6. Life Cycle Assessment of the Biobased Polymers in Food Packaging
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Varghese, S.A.; Pulikkalparambil, H.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Novel biodegradable polymer films based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Ceiba pentandra natural fibers for packaging applications. Food Packag. Shelf Life 2020, 25, 100538. [Google Scholar] [CrossRef]
- Valdés García, A.; Juárez Serrano, N.; Beltrán Sanahuja, A.; Garrigós, M.C. Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants 2020, 9, 629. [Google Scholar] [CrossRef]
- Elfaleh, I.; Abbassi, F.; Habibi, M.; Ahmad, F.; Guedri, M.; Nasri, M.; Garnier, C. A comprehensive review of natural fibers and their composites: An eco-friendly alternative to conventional materials. Results Eng. 2023, 19, 101271. [Google Scholar] [CrossRef]
- Park, J.-Y.; Jung, S.; Na, Y.-G.; Jeon, C.-H.; Cheon, H.-Y.; Yun, E.-Y.; Lee, S.; Kwon, E.E.; Kim, J.-K. Biodiesel production from the black soldier fly larvae grown on food waste and its fuel property characterization as a potential transportation fuel. Environ. Eng. Res. 2021, 27, 200704. [Google Scholar] [CrossRef]
- Rana, P.; Inbaraj, B.S.; Gurumayum, S.; Sridhar, K. Sustainable Production of Lignocellulolytic Enzymes in Solid-State Fermentation of Agro-Industrial Waste: Application in Pumpkin (Cucurbita maxima) Juice Clarification. Agronomy 2021, 11, 2379. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Visco, A.; Scolaro, C.; Facchin, M.; Brahimi, S.; Belhamdi, H.; Gatto, V.; Beghetto, V. Agri-Food Wastes for Bioplastics: European Prospective on Possible Applications in Their Second Life for a Circular Economy. Polymers 2022, 14, 2752. [Google Scholar] [CrossRef]
- Gulsunoglu-Konuskan, Z.; Kilic-Akyilmaz, M. Microbial Bioconversion of Phenolic Compounds in Agro-industrial Wastes: A Review of Mechanisms and Effective Factors. J. Agric. Food Chem. 2022, 70, 6901–6910. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Singh, A.; Ahmed, M.; Iqbal, U.; Srivastava, U. Sustainability of Biodegradable Polymers for the Environment: An Alternative Approach for the Future. In Advances in Environmental Engineering and Green Technologies; Danish, M.S.S., Senjyu, T.S., Eds.; IGI Global: Hershey, PA, USA, 2021; pp. 65–87. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Qu, Z. A review on polyhydroxyalkanoate production from agricultural waste Biomass: Development, Advances, circular Approach, and challenges. Bioresour. Technol. 2021, 342, 126008. [Google Scholar] [CrossRef]
- Zytner, P.; Kumar, D.; Elsayed, A.; Mohanty, A.; Ramarao, B.V.; Misra, M. A review on polyhydroxyalkanoate (PHA) production through the use of lignocellulosic biomass. RSC Sustain. 2023, 1, 2120–2134. [Google Scholar] [CrossRef]
- Scarfato, P.; Di Maio, L.; Incarnato, L. Recent advances and migration issues in biodegradable polymers from renewable sources for food packaging. J. Appl. Polym. Sci. 2015, 132, 42597. [Google Scholar] [CrossRef]
- Lopez-Gil, A.; Rodriguez-Perez, M.A.; De Saja, J.A.; Bellucci, F.S.; Ardanuy, M. Strategies to Improve the Mechanical Properties of Starch-Based Materials: Plasticization and Natural Fibers Reinforcement. Polímeros Ciênc. Tecnol. 2014, 24, 36–42. [Google Scholar] [CrossRef]
- Mayuri, T.; Shukla, R.N.; Balaji, J. Biobased Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical Packaging Materials: A Review. Asian J. Dairy Food Res. 2022, 42, 137–143. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Cruz-Galvez, A.M.; Gomez-Aldapa, C.A.; Falfan-Cortes, R.N.; Guzman-Ortiz, F.A.; Rodríguez-Marín, M.L. Biopolymer films and the effects of added lipids, nanoparticles and antimicrobials on their mechanical and barrier properties: A review. Int. J. Food Sci. Technol. 2016, 51, 1967–1978. [Google Scholar] [CrossRef]
- Mushi, N.E.; Utsel, S.; Berglund, L.A. Nanostructured biocomposite films of high toughness based on native chitin nanofibers and chitosan. Front. Chem. 2014, 2, 99. [Google Scholar] [CrossRef]
- Wójtowicz, A. Selected Properties of Multilayer Films Applied for Vacuum and Modified Atmosphere Packaging Systems. Agric. Eng. 2018, 22, 89–98. [Google Scholar] [CrossRef]
- López-Martínez, E.D.; Martínez-Colunga, J.G.; Ramírez-Vargas, E.; Sanchez-Valdes, S.; Ramos De Valle, L.F.; Benavides-Cantu, R.; Rodríguez-Gonzalez, J.A.; Mata-Padilla, J.M.; Cruz-Delgado, V.J.; Borjas-Ramos, J.J.; et al. Influence of carbon structures on the properties and photodegradation of LDPE/LLDPE films. Polym. Adv. Technol. 2022, 33, 1727–1741. [Google Scholar] [CrossRef]
- Carosio, F.; Colonna, S.; Fina, A.; Rydzek, G.; Hemmerlé, J.; Jierry, L.; Schaaf, P.; Boulmedais, F. Efficient Gas and Water Vapor Barrier Properties of Thin Poly(lactic acid) Packaging Films: Functionalization with Moisture Resistant Nafion and Clay Multilayers. Chem. Mater. 2014, 26, 5459–5466. [Google Scholar] [CrossRef]
- Abreu, A.S.; Oliveira, M.; De Sá, A.; Rodrigues, R.M.; Cerqueira, M.A.; Vicente, A.A.; Machado, A.V. Antimicrobial nanostructured starch based films for packaging. Carbohydr. Polym. 2015, 129, 127–134. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- De Beukelaer, H.; Hilhorst, M.; Workala, Y.; Maaskant, E.; Post, W. Overview of the mechanical, thermal and barrier properties of biobased and/or biodegradable thermoplastic materials. Polym. Test. 2022, 116, 107803. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Ali, S.S.; Abdelkarim, E.A.; Elsamahy, T.; Al-Tohamy, R.; Li, F.; Kornaros, M.; Zuorro, A.; Zhu, D.; Sun, J. Bioplastic production in terms of life cycle assessment: A state-of-the-art review. Environ. Sci. Ecotechnol. 2023, 15, 100254. [Google Scholar] [CrossRef] [PubMed]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging: A Review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Gumienna, M.; Górna, B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial food packaging: Potential and pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and intelligent packaging systems for a modern society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed; Oves, M.M.; Khan; Habib, S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated Charge Carriers and Reactive Oxygen Species in ZnO/Au Hybrid Nanostructures with Enhanced Photocatalytic and Antibacterial Activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Escuin, J.; Bourbon, A.; Cabedo, L.; Nevo, Y.; Cerqueira, M.; Lagaron, J. Development of Active Barrier Multilayer Films Based on Electrospun Antimicrobial Hot-Tack Food Waste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Cellulose Nanocrystal Interlayers. Nanomaterials 2020, 10, 2356. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Reis, M.A.M.; Carvalheira, M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Development and Characterization of Electrospun Biopapers of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Derived from Cheese Whey with Varying 3-Hydroxyvalerate Contents. Biomacromolecules 2021, 22, 2935–2953. [Google Scholar] [CrossRef]
- HRN EN 13432:2003; Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. 2003. Available online: https://repozitorij.hzn.hr/norm/HRN+EN+13432%3A2003 (accessed on 29 August 2024).
- Aliotta, L.; Gigante, V.; Acucella, O.; Signori, F.; Lazzeri, A. Thermal, Mechanical and Micromechanical Analysis of PLA/PBAT/POE-g-GMA Extruded Ternary Blends. Front. Mater. 2020, 7, 130. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Wemyss, A.M.; Iacovidou, E.; Wan, C. Design and Control of Compostability in Synthetic Biopolyesters. ACS Sustain. Chem. Eng. 2021, 9, 9151–9164. [Google Scholar] [CrossRef]
- Directive (EU) 2018/852. Available online: https://eur-lex.europa.eu/eli/dir/2018/852/oj (accessed on 10 July 2024).
- Directive 94/62/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31994L0062 (accessed on 10 July 2024).
- Directive (EU) 2019/904. Available online: https://eur-lex.europa.eu/eli/dir/2019/904/oj (accessed on 10 July 2024).
- REACH Regulation (EC) No 1907/2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:136:0003:0280:en:PDF (accessed on 10 July 2024).
- (EC) No 66/2010. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32010R0066 (accessed on 10 July 2024).
- Barron, A.; Sparks, T.D. Commercial Marine-Degradable Polymers for Flexible Packaging. iScience 2020, 23, 101353. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, Y.; Hu, X.; Bao, Q.; Zhao, Q.; Zhang, F.; Guo, K.; Li, S.; Li, T. Effect of Starch Nanoparticles and Tea Polyphenol Inclusion on Physicochemical and Mechanical Properties of Starch-Based Films. Starch-Stärke 2024, 76, 2300140. [Google Scholar] [CrossRef]
- Gregory, D.A.; Taylor, C.S.; Fricker, A.T.R.; Asare, E.; Tetali, S.S.V.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates and their advances for biomedical applications. Trends Mol. Med. 2022, 28, 331–342. [Google Scholar] [CrossRef]
- Zhila, N.; Shishatskaya, E. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int. J. Biol. Macromol. 2018, 111, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Miu, D.-M.; Vladu, M.-G.; Jinga, S.-I. Studies on mcl-Polyhydroxyalkanoates Using Different Carbon Sources for New Biomedical Materials. Chem. Proc. 2020, 3, 143. [Google Scholar] [CrossRef]
- Koller, M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef]
- Cai, F.; Lin, M.; Jin, W.; Chen, C.; Liu, G. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxvalerate) from volatile fatty acids by Cupriavidus necator. J. Basic Microbiol. 2023, 63, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Cho, I.J.; Lee, Y.; Kim, Y.; Kim, K.; Lee, S.Y. Microbial Polyhydroxyalkanoates and Nonnatural Polyesters. Adv. Mater. 2020, 32, 1907138. [Google Scholar] [CrossRef]
- Prakash, P.; Lee, W.-H.; Loo, C.-Y.; Wong, H.S.J.; Parumasivam, T. Advances in Polyhydroxyalkanoate Nanocarriers for Effective Drug Delivery: An Overview and Challenges. Nanomaterials 2022, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Alwuhaib, A.S.; Zinkevich, V.; Kartvelishvili, T.; Asatiani, N.; Sapojnikova, N. Bioplastics against Microplastics: Screening of Environmental Bacteria for Bioplastics Production. In Environmental Sciences; Salama, E.-S., Ed.; IntechOpen: Rijeka, Croatia, 2023; Volume 8. [Google Scholar] [CrossRef]
- Khamkong, T.; Penkhrue, W.; Lumyong, S. Optimization of Production of Polyhydroxyalkanoates (PHAs) from Newly Isolated Ensifer sp. Strain HD34 by Response Surface Methodology. Processes 2022, 10, 1632. [Google Scholar] [CrossRef]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Shishatskaya, E.I.; Volova, T.G. Biosynthesis of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from Different 4-Hydroxybutyrate Precursors by New Wild-Type Strain Cupriavidus necator IBP/SFU-1. Processes 2023, 11, 1423. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef]
- Riaz, S.; Rhee, K.Y.; Park, S.J. Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries. Polymers 2021, 13, 253. [Google Scholar] [CrossRef]
- Pryadko, A.; Surmeneva, M.A.; Surmenev, R.A. Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications. Polymers 2021, 13, 1738. [Google Scholar] [CrossRef]
- Emaimo, A.J.; Olkhov, A.A.; Iordanskii, A.L.; Vetcher, A.A. Polyhydroxyalkanoates Composites and Blends: Improved Properties and New Applications. J. Compos. Sci. 2022, 6, 206. [Google Scholar] [CrossRef]
- Vahabi, H.; Rohani Rad, E.; Parpaite, T.; Langlois, V.; Saeb, M.R. Biodegradable polyester thin films and coatings in the line of fire: The time of polyhydroxyalkanoate (PHA)? Prog. Org. Coat. 2019, 133, 85–89. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.-Y.; Yang, H.; Chen, J.-N.; Lin, Y.; Han, S.-Y.; Cao, Q.; Zeng, H.-S.; Ye, J.-W. A Polyhydroxyalkanoates-Based Carrier Platform of Bioactive Substances for Therapeutic Applications. Front. Bioeng. Biotechnol. 2022, 9, 798724. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Díez García, A.; López, D.; Fiori, S.; Peponi, L. Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials 2019, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Kaniuk, Ł.; Stachewicz, U. Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications. ACS Biomater. Sci. Eng. 2021, 7, 5339–5362. [Google Scholar] [CrossRef]
- Ferri, M.; Papchenko, K.; Degli Esposti, M.; Tondi, G.; De Angelis, M.G.; Morselli, D.; Fabbri, P. Fully Biobased Polyhydroxyalkanoate/Tannin Films as Multifunctional Materials for Smart Food Packaging Applications. ACS Appl. Mater. Interfaces 2023, 15, 28594–28605. [Google Scholar] [CrossRef] [PubMed]
- Carpine, R.; Olivieri, G.; Hellingwerf, K.J.; Pollio, A.; Marzocchella, A. Industrial Production of Poly-β-hydroxybutyrate from CO2: Can Cyanobacteria Meet this Challenge? Processes 2020, 8, 323. [Google Scholar] [CrossRef]
- Brdlík, P.; Borůvka, M.; Běhálek, L.; Lenfeld, P. The Influence of Additives and Environment on Biodegradation of PHBV Biocomposites. Polymers 2022, 14, 838. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, B.; Zhu, J.; Gao, Y.; Deng, W.; Chen, R.; Wang, H.-L. Electrospinning of Biodegradable, Monolithic Membrane with Distinct Bimodal Micron-Sized Fibers and Nanofibers for High Efficiency PMs Removal. ACS Appl. Mater. Interfaces 2023, 15, 35507–35515. [Google Scholar] [CrossRef]
- Deroiné, M.; César, G.; Le Duigou, A.; Davies, P.; Bruzaud, S. Natural Degradation and Biodegradation of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) in Liquid and Solid Marine Environments. J. Polym. Environ. 2015, 23, 493–505. [Google Scholar] [CrossRef]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R.; Altstädt, V. The impact of morphology on thermal properties and aerobic biodegradation of physically compatibilized poly (lactic acid)/co-plasticized thermoplastic starch blends. Polym. Adv. Technol. 2018, 29, 2880–2889. [Google Scholar] [CrossRef]
- Nevoralová, M.; Koutný, M.; Ujčić, A.; Starý, Z.; Šerá, J.; Vlková, H.; Šlouf, M.; Fortelný, I.; Kruliš, Z. Structure Characterization and Biodegradation Rate of Poly(ε-caprolactone)/Starch Blends. Front. Mater. 2020, 7, 141. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Castro-Aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Impact of Nanoclays on the Biodegradation of Poly(Lactic Acid) Nanocomposites. Polymers 2018, 10, 202. [Google Scholar] [CrossRef]
- Meng, M.; Wang, S.; Xiao, M.; Meng, Y. Recent Progress in Modification and Preparations of the Promising Biodegradable Plastics: Polylactide and Poly(butylene adipate-co-terephthalate). Sustain. Polym. Energy 2023, 1, 10006. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Xu, C.; Huang, H.-D.; Lin, H.; Zhong, G.-J.; Li, Z.-M. Polylactide Films with Superhydrophobicity and Superior Mechanical Properties Constructed by Combining Annealing Stretching and Nonsolvent-Induced Phase Separation. ACS Sustain. Chem. Eng. 2023, 11, 9498–9508. [Google Scholar] [CrossRef]
- Arias-Nava, E.H.; Valles-Rosales, D.J.; Sullivan, B.P. Biopolymer Non-Parametric Analysis: A Degradation Study under Accelerated Destructive Tests. Polymers 2023, 15, 620. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, R.; Biswas, S.; Rashid, T.U.; Afrin, S.; Jahan, R.A.; Haque, P.; Rahman, M.M. Preparation of Chitin-PLA laminated composite for implantable application. Bioact. Mater. 2017, 2, 199–207. [Google Scholar] [CrossRef]
- Gois, G.D.S.; Andrade, M.F.D.; Garcia, S.M.S.; Vinhas, G.M.; Santos, A.S.F.; Medeiros, E.S.; Oliveira, J.E.; Almeida, Y.M.B.D. Soil Biodegradation of PLA/CNW Nanocomposites Modified with Ethylene Oxide Derivatives. Mater. Res. 2018, 20, 899–904. [Google Scholar] [CrossRef]
- Fazita, M.R.; Jayaraman, K.; Bhattacharyya, D.; Hossain, M.; Haafiz, M.K.; H.P.S., A.K. Disposal Options of Bamboo Fabric-Reinforced Poly(Lactic) Acid Composites for Sustainable Packaging: Biodegradability and Recyclability. Polymers 2015, 7, 1476–1496. [Google Scholar] [CrossRef]
- Amor, A.; Okhay, N.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Gervais, M. Combined compatibilization and plasticization effect of low molecular weight poly(lactic acid) in poly(lactic acid)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) blends. Express Polym. Lett. 2018, 12, 114–125. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Udduttula, A.; Fan, Z.S.; Chen, J.H.; Sun, A.R.; Zhang, P. Microbial-Derived Polyhydroxyalkanoate-Based Scaffolds for Bone Tissue Engineering: Biosynthesis, Properties, and Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 763031. [Google Scholar] [CrossRef] [PubMed]
- Caminero, M.; Chacón, J.; García-Plaza, E.; Núñez, P.; Reverte, J.; Becar, J. Additive Manufacturing of PLA-Based Composites Using Fused Filament Fabrication: Effect of Graphene Nanoplatelet Reinforcement on Mechanical Properties, Dimensional Accuracy and Texture. Polymers 2019, 11, 799. [Google Scholar] [CrossRef]
- Le Gars, M.; Dhuiège, B.; Delvart, A.; Belgacem, M.N.; Missoum, K.; Bras, J. High-Barrier and Antioxidant Poly(lactic acid)/Nanocellulose Multilayered Materials for Packaging. ACS Omega 2020, 5, 22816–22826. [Google Scholar] [CrossRef]
- Luo, F.; Fortenberry, A.; Ren, J.; Qiang, Z. Recent Progress in Enhancing Poly(Lactic Acid) Stereocomplex Formation for Material Property Improvement. Front. Chem. 2020, 8, 688. [Google Scholar] [CrossRef]
- Shafqat, A.; Tahir, A.; Mahmood, A.; Tabinda, A.B.; Yasar, A.; Pugazhendhi, A. A review on environmental significance carbon foot prints of starch based bio-plastic: A substitute of conventional plastics. Biocatal. Agric. Biotechnol. 2020, 27, 101540. [Google Scholar] [CrossRef]
- Luchese, C.L.; Pavoni, J.M.F.; Dos Santos, N.Z.; Quines, L.K.; Pollo, L.D.; Spada, J.C.; Tessaro, I.C. Effect of chitosan addition on the properties of films prepared with corn and cassava starches. J. Food Sci. Technol. 2018, 55, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Othman, S.H.; Majid, N.A.; Tawakkal, I.S.M.A.; Basha, R.K.; Nordin, N.; Shapi’I, R.A. Tapioca starch films reinforced with microcrystalline cellulose for potential food packaging application. Food Sci. Technol. 2019, 39, 605–612. [Google Scholar] [CrossRef]
- Chi, K.; Wang, H.; Catchmark, J.M. Sustainable starch-based barrier coatings for packaging applications. Food Hydrocoll. 2020, 103, 105696. [Google Scholar] [CrossRef]
- Surendren, A.; Mohanty, A.K.; Liu, Q.; Misra, M. A review of biodegradable thermoplastic starches, their blends and composites: Recent developments and opportunities for single-use plastic packaging alternatives. Green Chem. 2022, 24, 8606–8636. [Google Scholar] [CrossRef]
- Tedeschi, G.; Guzman-Puyol, S.; Ceseracciu, L.; Benitez, J.J.; Cataldi, P.; Bissett, M.; Heredia, A.; Athanassiou, A.; Heredia-Guerrero, J.A. Sustainable, High-Barrier Polyaleuritate/Nanocellulose Biocomposites. ACS Sustain. Chem. Eng. 2020, 8, 10682–10690. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Martins, H.H.; Manrich, A.; Otoni, C.G.; Carvalho, E.E.N.; Piccolli, R.H.; Oliveira, J.E.; Pinheiro, A.C.M.; Mattoso, L.H.C. Development of quaternary nanocomposites made up of cassava starch, cocoa butter, lemongrass essential oil nanoemulsion, and brewery spent grain fibers. J. Food Sci. 2021, 86, 1979–1996. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.C.D.S.; Silva, R.B.S.; Carvalho, C.W.P.; Rossi, A.L.; Teixeira, J.A.; Freitas-Silva, O.; Cabral, L.M.C. Cellulose nanocrystals from grape pomace and their use for the development of starch-based nanocomposite films. Int. J. Biol. Macromol. 2020, 159, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tebar, N.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A Review. Polymers 2023, 15, 396. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-Based Agronanochemicals as a Sustainable Alternative in Crop Protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef]
- Yan, M.R.; Hsieh, S.; Ricacho, N. Innovative Food Packaging, Food Quality and Safety, and Consumer Perspectives. Processes 2022, 10, 747. [Google Scholar] [CrossRef]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Fourie, J.; Taute, F.; Du Preez, L.; De Beer, D. Novel chitosan-poly(vinyl acetate) biomaterial suitable for additive manufacturing and bone tissue engineering applications. J. Bioact. Compat. Polym. 2021, 36, 394–413. [Google Scholar] [CrossRef]
- Gallyamov, M.O.; Chaschin, I.S.; Bulat, M.V.; Bakuleva, N.P.; Badun, G.A.; Chernysheva, M.G.; Kiselyova, O.I.; Khokhlov, A.R. Chitosan coatings with enhanced biostability in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 270–277. [Google Scholar] [CrossRef]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef]
- De Moraes Crizel, T.; De Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Hickmann Flôres, S. Active food packaging prepared with chitosan and olive pomace. Food Hydrocoll. 2018, 74, 139–150. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production From Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Xia, G.; Zhou, Q.; Xu, Z.; Zhang, J.; Ji, X.; Zhang, J.; Nawaz, H.; Wang, J.; Peng, J. Cellulose-Based Films with Ultraviolet Shielding Performance Prepared Directly from Waste Corrugated Pulp. Polymers 2021, 13, 3359. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Wang, H.; Liu, C.; Pan, X. Preparation and Characterization of Regenerated Cellulose Film from a Solution in Lithium Bromide Molten Salt Hydrate. Polymers 2018, 10, 614. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, C.; Zhu, X.; Li, B.; Huang, C. Lignin-enhanced wet strength of cellulose-based materials: A sustainable approach. Green Chem. 2023, 25, 4995–5009. [Google Scholar] [CrossRef]
- Ye, G.; Gu, T.; Chen, B.; Bi, H.; Hu, Y. Mechanical, thermal properties and shape memory behaviors of PLA/PCL/PLA-g-GMA blends. Polym. Eng. Sci. 2023, 63, 2084–2092. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Wu, S.; Liu, T.; Lin, G.; Shang, B.; Ma, J.; Lu, W.; Zhang, F.; Li, J.; et al. Biological Application of Novel Biodegradable Cellulose Composite as a Hemostatic Material. Mediat. Inflamm. 2022, 2022, 4083477. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 2015, 45–54. [Google Scholar] [CrossRef]
- Mohan, D.; Teong, Z.K.; Bakir, A.N.; Sajab, M.S.; Kaco, H. Extending Cellulose-Based Polymers Application in Additive Manufacturing Technology: A Review of Recent Approaches. Polymers 2020, 12, 1876. [Google Scholar] [CrossRef] [PubMed]
- Ruiken, C.J.; Breuer, G.; Klaversma, E.; Santiago, T.; Van Loosdrecht, M.C.M. Sieving wastewater—Cellulose recovery, economic and energy evaluation. Water Res. 2013, 47, 43–48. [Google Scholar] [CrossRef]
- Behera, C.R.; Santoro, D.; Gernaey, K.V.; Sin, G. Organic carbon recovery modeling for a rotating belt filter and its impact assessment on a plant-wide scale. Chem. Eng. J. 2018, 334, 1965–1976. [Google Scholar] [CrossRef]
- Ansari, M.M.; Heo, Y.; Do, K.; Ghosh, M.; Son, Y.-O. Nanocellulose derived from agricultural biowaste by-products–Sustainable synthesis, biocompatibility, biomedical applications, and future perspectives: A review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100529. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, B.; Sarkar, K. Nanocellulose as sustainable biomaterials for drug delivery. Sens. Int. 2022, 3, 100135. [Google Scholar] [CrossRef]
- Said, N.S.; Mhd Sarbon, N. A comparative study: Development and characterization of active biodegradable chicken skin and mammalian gelatin composite films incorporated with curcumin extracts. J. Food Process. Preserv. 2021, 45, e15771. [Google Scholar] [CrossRef]
- Insaward, A.; Duangmal, K.; Mahawanich, T. Mechanical, Optical, and Barrier Properties of Soy Protein Film As Affected by Phenolic Acid Addition. J. Agric. Food Chem. 2015, 63, 9421–9426. [Google Scholar] [CrossRef] [PubMed]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef]
- Patil, R.D.; Dalev, P.G.; Mark, J.E.; Vassileva, E.; Fakirov, S. Biodegradation of chemically modified gelatin films in lake and river waters. J. Appl. Polym. Sci. 2000, 76, 29–37. [Google Scholar] [CrossRef]
- Zhang, W.; Hedayati, S.; Tarahi, M.; Can Karaca, A.; Hadidi, M.; Assadpour, E.; Jafari, S.M. Advances in transglutaminase cross-linked protein-based food packaging films; a review. Int. J. Biol. Macromol. 2023, 253, 127399. [Google Scholar] [CrossRef]
- Bhaskar, R.; Zo, S.M.; Narayanan, K.B.; Purohit, S.D.; Gupta, M.K.; Han, S.S. Recent development of protein-based biopolymers in food packaging applications: A review. Polym. Test. 2023, 124, 108097. [Google Scholar] [CrossRef]
- Rashid, A.B.; Haque, M.; Islam, S.M.M.; Uddin Labib, K.M.R. Nanotechnology-enhanced fiber-reinforced polymer composites: Recent advancements on processing techniques and applications. Heliyon 2024, 10, e24692. [Google Scholar] [CrossRef]
- Gomes, M.C.; Mano, J.F. Chemical modification strategies to prepare advanced protein-based biomaterials. Biomater. Biosyst. 2021, 1, 100010. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Marco, I.D.; Perego, P. Optimization of PCL Polymeric Films as Potential Matrices for the Loading of Alpha-Tocopherol by a Combination of Innovative Green Processes. Processes 2021, 9, 2244. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Hermanová, S.; Bálková, R.; Voběrková, S.; Chamradová, I.; Omelková, J.; Richtera, L.; Mravcová, L.; Jančář, J. Biodegradation study on poly(ε-caprolactone) with bimodal molecular weight distribution. J. Appl. Polym. Sci. 2013, 127, 4726–4735. [Google Scholar] [CrossRef]
- Cesur, S.; Köroğlu, C.; Yalçın, H.T. Antimicrobial and biodegradable food packaging applications of polycaprolactone/organo nanoclay/chitosan polymeric composite films. J. Vinyl Addit. Technol. 2018, 24, 376–387. [Google Scholar] [CrossRef]
- Kumar, V.; Sehgal, R.; Gupta, R. Blends and composites of polyhydroxyalkanoates (PHAs) and their applications. Eur. Polym. J. 2021, 161, 110824. [Google Scholar] [CrossRef]
- Carvalheira, M.; Amorim, C.L.; Oliveira, A.C.; Guarda, E.C.; Costa, E.; Ribau Teixeira, M.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. Valorization of Brewery Waste through Polyhydroxyalkanoates Production Supported by a Metabolic Specialized Microbiome. Life 2022, 12, 1347. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R. Solid-state Fermentation for the Production of Poly(hydroxyalkanoates). Chem. Biochem. Eng. Q. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Khor, J.W.; Yoon, L.W.; Nguyen-Huynh, T.-T.; Ng, W.L.; Low, J.H. Effect of pH control and uncoupled carbon and nitrogen feeding strategy on enrichment of mixed microbial culture for polyhydroxyalkanoates production. J. Phys. Conf. Ser. 2023, 2523, 012001. [Google Scholar] [CrossRef]
- Valentino, F.; Karabegovic, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Polyhydroxyalkanoate (PHA) storage within a mixed-culture biomass with simultaneous growth as a function of accumulation substrate nitrogen and phosphorus levels. Water Res. 2015, 77, 49–63. [Google Scholar] [CrossRef]
- Szacherska, K.; Oleskowicz-Popiel, P.; Ciesielski, S.; Mozejko-Ciesielska, J. Volatile Fatty Acids as Carbon Sources for Polyhydroxyalkanoates Production. Polymers 2021, 13, 321. [Google Scholar] [CrossRef]
- Titz, M.; Kettl, K.-H.; Shahzad, K.; Koller, M.; Schnitzer, H.; Narodoslawsky, M. Process optimization for efficient biomediated PHA production from animal-based waste streams. Clean Technol. Environ. Policy 2012, 14, 495–503. [Google Scholar] [CrossRef]
- Bosco, F.; Cirrincione, S.; Carletto, R.; Marmo, L.; Chiesa, F.; Mazzoli, R.; Pessione, E. PHA Production from Cheese Whey and “Scotta”: Comparison between a Consortium and a Pure Culture of Leuconostoc mesenteroides. Microorganisms 2021, 9, 2426. [Google Scholar] [CrossRef]
- Amer, A.; Kim, Y. Modeling the growth of diverse microorganisms during feast-famine enrichment. Water Environ. Res. 2022, 94, e10803. [Google Scholar] [CrossRef]
- Carvalho, J.M.; Marreiros, B.C.; Reis, M.A.M. Polyhydroxyalkanoates Production by Mixed Microbial Culture under High Salinity. Sustainability 2022, 14, 1346. [Google Scholar] [CrossRef]
- Valentino, F.; Morgan-Sagastume, F.; Campanari, S.; Villano, M.; Werker, A.; Majone, M. Carbon recovery from wastewater through bioconversion into biodegradable polymers. New Biotechnol. 2017, 37, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Cruz, R.A.P.; Cardoso, P.; Silva, F.; Freitas, E.B.; Carvalho, G.; Reis, M.A.M. Combined Strategies to Boost Polyhydroxyalkanoate Production from Fruit Waste in a Three-Stage Pilot Plant. ACS Sustain. Chem. Eng. 2021, 9, 8270–8279. [Google Scholar] [CrossRef]
- Ganesh Saratale, R.; Cho, S.-K.; Dattatraya Saratale, G.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Naresh Bharagava, R.; Kumar, G.; Su Kim, D.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Freitas, F.; Paiva, A.; Mano, F.; Dionísio, M.; Ramos, A.M.; Reis, M.A.M. Valorization of fatty acids-containing wastes and byproducts into short- and medium-chain length polyhydroxyalkanoates. New Biotechnol. 2016, 33, 206–215. [Google Scholar] [CrossRef]
- Huzir, N.; Aslan, A.; Rosly, M.; Hussin, M.; Amin, A.; Tamunaidu, P. Production of polyhydroxyalkanoate from nipa sap using Cupriavidus necator DSM545. IOP Conf. Ser. Earth Environ. Sci. 2023, 1144, 012008. [Google Scholar] [CrossRef]
- Vigneswari, S.; Noor, M.S.M.; Amelia, T.S.M.; Balakrishnan, K.; Adnan, A.; Bhubalan, K.; Amirul, A.-A.A.; Ramakrishna, S. Recent Advances in the Biosynthesis of Polyhydroxyalkanoates from Lignocellulosic Feedstocks. Life 2021, 11, 807. [Google Scholar] [CrossRef]
- Raza, Z.A.; Tariq, M.R.; Majeed, M.I.; Banat, I.M. Recent developments in bioreactor scale production of bacterial polyhydroxyalkanoates. Bioprocess Biosyst. Eng. 2019, 42, 901–919. [Google Scholar] [CrossRef]
- Valentino, F.; Gottardo, M.; Micolucci, F.; Pavan, P.; Bolzonella, D.; Rossetti, S.; Majone, M. Organic Fraction of Municipal Solid Waste Recovery by Conversion into Added-Value Polyhydroxyalkanoates and Biogas. ACS Sustain. Chem. Eng. 2018, 6, 16375–16385. [Google Scholar] [CrossRef]
- Johnston, B.; Radecka, I.; Hill, D.; Chiellini, E.; Ilieva, V.; Sikorska, W.; Musioł, M.; Zięba, M.; Marek, A.; Keddie, D.; et al. The Microbial Production of Polyhydroxyalkanoates from Waste Polystyrene Fragments Attained Using Oxidative Degradation. Polymers 2018, 10, 957. [Google Scholar] [CrossRef]
- Zainab-L, I.; Uyama, H.; Li, C.; Shen, Y.; Sudesh, K. Production of Polyhydroxyalkanoates From Underutilized Plant Oils by Cupriavidus necator. CLEAN—Soil Air Water 2018, 46, 1700542. [Google Scholar] [CrossRef]
- Brojanigo, S.; Parro, E.; Cazzorla, T.; Favaro, L.; Basaglia, M.; Casella, S. Conversion of Starchy Waste Streams into Polyhydroxyalkanoates Using Cupriavidus necator DSM 545. Polymers 2020, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; De Carvalho, M.S.; Osajima, J.A.; Da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with essential oils as antimicrobial agents, packaging, repellents, and insecticides: An overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.; Freitas, F.; Cruz, M.V.; Paiva, A.; Dionísio, M.; Reis, M.A.M. Conversion of fat-containing waste from the margarine manufacturing process into bacterial polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 71, 68–73. [Google Scholar] [CrossRef]
- Liao, Q.; Guo, L.; Ran, Y.; Gao, M.; She, Z.; Zhao, Y.; Liu, Y. Optimization of polyhydroxyalkanoates (PHA) synthesis with heat pretreated waste sludge. Waste Manag. 2018, 82, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Lorini, L.; Martinelli, A.; Pavan, P.; Majone, M.; Valentino, F. Downstream processing and characterization of polyhydroxyalkanoates (PHAs) produced by mixed microbial culture (MMC) and organic urban waste as substrate. Biomass Convers. Biorefinery 2021, 11, 693–703. [Google Scholar] [CrossRef]
- Koller, M. Advances in Polyhydroxyalkanoate (PHA) Production, Volume 2. Bioengineering 2020, 7, 24. [Google Scholar] [CrossRef]
- Christensen, M.; Chiciudean, I.; Jablonski, P.; Tanase, A.-M.; Shapaval, V.; Hansen, H. Towards high-throughput screening (HTS) of polyhydroxyalkanoate (PHA) production via Fourier transform infrared (FTIR) spectroscopy of Halomonas sp. R5-57 and Pseudomonas sp. MR4-99. PLoS ONE 2023, 18, e0282623. [Google Scholar] [CrossRef]
- Afghan, I.G.; Hashmi, A.; Ali, A. General Overview of Previous Advances on Polyhydroxyalkanoates (PHA) Synthesis by Microorganism Utilizing Different Waste Carbon Sources. J. Res. Appl. Sci. Biotechnol. 2022, 1, 176–180. [Google Scholar] [CrossRef]
- Kucera, D.; Novackova, I.; Pernicova, I.; Sedlacek, P.; Obruca, S. Biotechnological Production of Poly(3-Hydroxybutyrate-co-4-Hydroxybutyrate-co-3-Hydroxyvalerate) Terpolymer by Cupriavidus sp. DSM 19379. Bioengineering 2019, 6, 74. [Google Scholar] [CrossRef]
- Tan, G.-Y.; Chen, C.-L.; Li, L.; Ge, L.; Wang, L.; Razaad, I.; Li, Y.; Zhao, L.; Mo, Y.; Wang, J.-Y. Start a Research on Biopolymer Polyhydroxyalkanoate (PHA): A Review. Polymers 2014, 6, 706–754. [Google Scholar] [CrossRef]
- Monilola, W.S.; Makinde, O.E. Production and Characterization of Polyhydroxyalkanoates from Lactic Acid Bacteria Isolated from Dairy Wastewater, Fermented Cow Milk and ‘Ogi’. J. Adv. Microbiol. 2020, 20, 31–46. [Google Scholar] [CrossRef]
- Omerović, N.; Djisalov, M.; Živojević, K.; Mladenović, M.; Vunduk, J.; Milenković, I.; Knežević, N.Ž.; Gadjanski, I.; Vidić, J. Antimicrobial nanoparticles and biodegradable polymer composites for active food packaging applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2428–2454. [Google Scholar] [CrossRef]
- Kanda, G.S.; Al-Qaradawi, I.; Luyt, A.S. Morphology and property changes in PLA/PHBV blends as function of blend composition. J. Polym. Res. 2018, 25, 196. [Google Scholar] [CrossRef]
- Arrieta, M.; Samper, M.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-based biodegradable active packaging and its application to salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Mangaraj, S.; Mohanty, S.; Swain, S.; Yadav, A. Development and Characterization of Commercial Biodegradable Film from PLA and Corn Starch for Fresh Produce Packaging. J. Packag. Technol. Res. 2019, 3, 127–140. [Google Scholar] [CrossRef]
- Magalhães, N.F.; Andrade, C.T.D. Properties of melt-processed poly(hydroxybutyrate-co-hydroxyvalerate)/starch 1:1 blend nanocomposites. Polímeros Ciênc. Tecnol. 2013, 23, 366–372. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez, M.A. Poly(3-hydroxybutyrate)–thermoplastic starch–organoclay bionanocomposites: Surface properties. J. Appl. Polym. Sci. 2017, 134, 45217. [Google Scholar] [CrossRef]
- Vernaez, O.; Neubert, K.J.; Kopitzky, R.; Kabasci, S. Compatibility of Chitosan in Polymer Blends by Chemical Modification of Bio-based Polyesters. Polymers 2019, 11, 1939. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Zhao, H.; Wang, J.; Yin, C.; Zhang, L.; Zhao, Y. Effects of Cellulose Nanocrystals and Cellulose Nanofibers on the Structure and Properties of Polyhydroxybutyrate Nanocomposites. Polymers 2019, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.S.; Frone, A.N.; Radu, I.C.; Stanescu, P.O.; Truşcă, R.; Rădiţoiu, V.; Nicolae, C.A.; Gabor, A.R.; Panaitescu, D.M. Microfibrillated Cellulose Grafted with Metacrylic Acid as a Modifier in Poly(3-hydroxybutyrate). Polymers 2021, 13, 3970. [Google Scholar] [CrossRef] [PubMed]
- Zytner, P.; Wu, F.; Misra, M.; Mohanty, A.K. Toughening of Biodegradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/Poly(ε-caprolactone) Blends by In Situ Reactive Compatibilization. ACS Omega 2020, 5, 14900–14910. [Google Scholar] [CrossRef] [PubMed]
- Morais, D.D.S.; Siqueira, D.D.; Luna, C.B.B.; Araújo, E.M.; Bezerra, E.B.; Wellen, R.M.R. Grafting maleic anhydride onto polycaprolactone: Influence of processing. Mater. Res. Express 2019, 6, 055315. [Google Scholar] [CrossRef]
- Zhao, X.; Ji, K.; Kurt, K.; Cornish, K.; Vodovotz, Y. Optimal mechanical properties of biodegradable natural rubber-toughened PHBV bioplastics intended for food packaging applications. Food Packag. Shelf Life 2019, 21, 100348. [Google Scholar] [CrossRef]
- Rodriguez-Uribe, A.; Wang, T.; Pal, A.K.; Wu, F.; Mohanty, A.K.; Misra, M. Injection moldable hybrid sustainable composites of BioPBS and PHBV reinforced with talc and starch as potential alternatives to single-use plastic packaging. Compos. Part C Open Access 2021, 6, 100201. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Antimicrobial Biodegradable Food Packaging Based on Chitosan and Metal/Metal-Oxide Bio-Nanocomposites: A Review. Polymers 2021, 13, 2790. [Google Scholar] [CrossRef]
- Cavalli, L.R.; Klein, J.M.; Sandri, I.G.; Brandalise, R. Este trabajo se centró en el desarrollo de envases activos biodegradables con mezclas de poli (ácido láctico) (PLA), poli (etileno-co-acetato de vinilo) (EVA), polietilenglicol (PEG) y quitosano (QUI). Res. Soc. Dev. 2021, 10, e50010916964. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and Antioxidant Performance of Various Essential Oils and Natural Extracts and Their Incorporation into Biowaste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Layers Made from Electrospun Ultrathin Fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun Antimicrobial Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Containing Eugenol Essential Oil Encapsulated in Mesoporous Silica Nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.C.; Daitx, T.S.; Mauler, R.S.; Da Silva, N.M.; Miotto, M.; Crespo, J.S.; Carli, L.N. Poly(hydroxybutyrate-co-hydroxyvalerate)-based nanocomposites for antimicrobial active food packaging containing oregano essential oil. Food Packag. Shelf Life 2020, 26, 100602. [Google Scholar] [CrossRef]
- Tamošaitis, A.; JaruševičienĖ, A.; StrykaitĖ, M.; Damašius, J. Analysis of antimicrobial whey protein-based biocomposites with lactic acid, tea tree (Melaleuca alternifolia) and garlic (Allium sativum) essential oils for Edam cheese coating. Int. J. Dairy Technol. 2022, 75, 611–618. [Google Scholar] [CrossRef]
- Abarca, R.L.; Medina, J.; Alvarado, N.; Ortiz, P.A.; Carrillo López, B. Biodegradable gelatin-based films with nisin and EDTA that inhibit Escherichia coli. PLoS ONE 2022, 17, e0264851. [Google Scholar] [CrossRef] [PubMed]
- Moshe Dvir, I.; Weizman, O.; Lewitus, D.; Weintraub, S.; Ophir, A.; Dotan, A. Antimicrobial active packaging combining essential oils mixture: Migration and odor control study. Polym. Adv. Technol. 2019, 30, 2558–2566. [Google Scholar] [CrossRef]
- Othman, S.H.; Wane, B.M.; Nordin, N.; Noor Hasnan, N.Z.; Talib, R.A.; Karyadi, J.N.W. Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers 2021, 13, 4060. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Fard, H.K. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Ilyas, S.; Hezam, A.; Mathur, S.; Byrappa, K. Smart Fortified PHBV-CS Biopolymer with ZnO–Ag Nanocomposites for Enhanced Shelf Life of Food Packaging. ACS Appl. Mater. Interfaces 2019, 11, 48309–48320. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Mufida, N.; Panatarani, C.; Joni, I.M. Enhanced multi functionality of semi-refined iota carrageenan as food packaging material by incorporating SiO2 and ZnO nanoparticles. Heliyon 2021, 7, e06963. [Google Scholar] [CrossRef]
- Ojha, N.; Das, N. Fabrication and characterization of biodegradable PHBV/SiO2 nanocomposite for thermo-mechanical and antibacterial applications in food packaging. IET Nanobiotechnol. 2020, 14, 785–795. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer packaging: Advances in preparation techniques and emerging food applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1156–1186. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Pratt, S.; Lant, P.A.; Levett, I.; Laycock, B. Polyhydroxyalkanoate coatings restrict moisture uptake and associated loss of barrier properties of thermoplastic starch films. J. Appl. Polym. Sci. 2018, 135, 46379. [Google Scholar] [CrossRef]
- Sanyang, M.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Morey, I.; Talens, P.; Chiralt, A. Active bilayer films of thermoplastic starch and polycaprolactone obtained by compression molding. Carbohydr. Polym. 2015, 127, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Cherpinski, A.; Torres-Giner, S.; Vartiainen, J.; Peresin, M.S.; Lahtinen, P.; Lagaron, J.M. Improving the water resistance of nanocellulose-based films with polyhydroxyalkanoates processed by the electrospinning coating technique. Cellulose 2018, 25, 1291–1307. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Hilliou, L.; Escuin, J.M.; Cabedo, L.; Nevo, Y.; Prieto, C.; Lagaron, J.M. High-Oxygen-Barrier Multilayer Films Based on Polyhydroxyalkanoates and Cellulose Nanocrystals. Nanomaterials 2021, 11, 1443. [Google Scholar] [CrossRef]
- Hernández-García, E.; Freitas, P.A.V.; Zomeño, P.; González-Martínez, C.; Torres-Giner, S. Multilayer Sheets Based on Double Coatings of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) on Paper Substrate for Sustainable Food Packaging Applications. Appl. Sci. 2022, 13, 179. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2020 An analysis of European Plastics Production, Demand and Waste Data. Available online: https://plasticseurope.org/wp-content/uploads/2021/09/Plastics_the_facts-WEB-2020_versionJun21_final.pdf (accessed on 10 July 2024).
- Zheng, J.; Suh, S. Strategies to reduce the global carbon footprint of plastics. Nat. Clim. Change 2019, 9, 374–378. [Google Scholar] [CrossRef]
| Property | Petroleum-Based Polymers | Biodegradable Polymers |
|---|---|---|
| Barrier Properties | High moisture, oxygen, and gas barriers (WVTR: 6.9 g/m2 day PET, 1.5 g/m2 day LDPE; OTR: 36 mLO2/m2 day bar PET, 1624 mLO2/m2 day bar LDPE) | Moderate to good, but generally lower than petroleum-based (e.g., PLA has moderate/low oxygen barrier properties (180 mLO2/m2 day bar), and low moisture barrier (35.5 g/m2 day); PHB has good oxygen barrier properties (22 mLO2/m2 day bar), but moderate to low moisture barrier (5.5 g/m2 day) |
| Mechanical Strength | High tensile strength (PET 59.4 MPa), good impact resistance | Varies; PHB has good strength (43.9 MPa), while PLA and starch blends are more brittle |
| Thermal Stability | High (PETs have high melting points, 245 °C) | Lower; PCL 70 °C, PLA and TPS melts around 140–160 °C, while PHB is more thermally stable (180 °C) but still lower than PET |
| Transparency | Excellent (especially PET (glass-like visibility)) | Good; PLA is highly transparent, PHA is less so, starch blends can be cloudy |
| Flexibility | High (especially LDPE) | Varies; PHA and some blends are flexible, but PLA is more rigid |
| Biodegradability | Non-biodegradable | Biodegradable under industrial composting conditions (PHA, PLA, starch blends) |
| Compostability | Not compostable | Compostable under specific conditions (e.g., industrial composting for PLA, PHA) |
| Recyclability | Recyclable (but limited recycling rates in practice) | Limited recyclability, typically more focused on composting or biodegradation |
| Cost | Relatively low and well-established (USD 800–USD 1600 per metric ton) Factors influencing cost: LDPE isLDPE is generally cost-effective, but prices can fluctuate based on crude oil prices, supply-demand dynamics, and regional production capabilities. | Higher than petroleum-based polymers, though costs are decreasing with scale (USD 2000–USD 6000 per metric ton) |
| Environmental Impact | High carbon footprint, non-renewable. | Lower carbon footprint, derived from renewable resources, fully biodegradable Due to natural conversion, PLA emits 2.8 kg CO2 kg−1 during its life cycle. PLA saves ~66% of the energy required to produce conventional plastics Starch utilization in bioplastics production causes a reduction in GHG emissions (>80%) and fossil fuel consumption (>60%). When compared to synthetic plastics, starch might cause an increase in eutrophication potential and land usage |
| Application Suitability | Widely suitable across various applications | Suitable for specific applications like food packaging where biodegradability is prioritized |
| Antimicrobial Agent | MIC | Microorganism Type | References |
|---|---|---|---|
| Chitosan | 0.1–1.0 mg/mL | Gram-positive bacteria (Staphylococcus aureus, lactic acid bacteria, Listeria innocua) | [27] |
| Chitosan | 0.5–2.0 mg/mL | Gram-negative bacteria (Escherichia coli, Pseudomonas spp., Salmonella spp.) | [27] |
| Silver nanoparticles | 0.001 and 0.1 μg/mL | Staphylococcus aureus, Escherichia coli | [28] |
| Essential oils | 0.25 to 2.0 μg/mL | Gram-positive (Listeria monocitogenes, Listeria innocua, Brochothrix thermosphacta) and Gram-negative bacteria (Escherichia coli, Salmonella spp.) | [29] |
| SiO2 nanoparticles | 0.156 mM | Streptococcus mutans | [30] |
| ZnO nanoparticles | 31.25 µg/mL | Escherichia coli | [31] |
| Polymer | Properties | Advantages | Disadvantages | Limitations | Real-Time Applications |
|---|---|---|---|---|---|
| PLA | Transparent, high strength, biodegradable, thermoplastic | Renewable source, compostable, good clarity, easy processing | Brittle, poor thermal stability, low impact resistance | Limited use in high-temperature applications | Food packaging, disposable cutlery, 3D printing, medical implants |
| PHA | Biodegradable, good barrier properties, thermoplastic | Biocompatible, high biodegradability, versatile mechanical properties | High production cost, brittle | High cost and limited commercial availability | Biodegradable packaging, medical sutures, agricultural films |
| TPS | Biodegradable, flexible, thermoplastic | Low cost, compostable, easily blended with other polymers | Poor moisture resistance, low mechanical strength | Limited durability, needs blending with other polymers for improved properties | Biodegradable bags, packaging films, disposable items |
| PBS | Biodegradable, good thermal stability, flexible | Good mechanical properties, heat resistant, compostable | Higher cost compared to other biobased polymers | Limited commercial availability | Packaging materials, agricultural films, biodegradable tableware |
| PBAT | Biodegradable, flexible, good impact resistance | Flexible, good mechanical properties, suitable for blending | Derived partially from fossil fuels | Still partly reliant on petrochemical sources | Biodegradable films, agricultural mulch, compostable bags |
| PCL | Biodegradable, low melting point, easy to process | Biocompatible, good flexibility, blends well with other polymers | Slow biodegradation rate, low melting point | Limited use in high-temperature applications | Medical devices, drug delivery systems, biodegradable packaging |
| Cellulose-based polymers | Biodegradable, good mechanical properties, moisture-sensitive | Abundant, renewable, good film-forming ability | Sensitive to moisture, difficult to process | Limited water resistance, requires additives for improved durability | Food packaging, films, textiles, coatings |
| Carbon Source | PHA Yield | Culture Type | Reference |
|---|---|---|---|
| Olive oil distillate | 0.9 g PHA/g | Cupriavidus necator | [142] |
| Starchy waste stream | 5.12 g PHA/L | Cupriavidus necator DSM 545 | [149] |
| Cheese whey waste stream | 0.12–0.20 g PHA/L | MMC | [150] |
| Cheese whey | 0.35–0.52 g PHA/L | Enterobacter cloacae, Raoultella ornithinolytica, Citrobacter freundii, Escherichia coli, Vibrio parahaemoliticus, Leuconostoc spp. | [136] |
| Waste frying oil | 0.19–0.34 g PHA/g | R. eutropha | [151] |
| Waste sludge and synthetic wastewater | 0.648 kg/m3 | MMC | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stublić, K.; Ranilović, J.; Ocelić Bulatović, V.; Kučić Grgić, D. Advancing Sustainability: Utilizing Bacterial Polyhydroxyalkanoate for Food Packaging. Processes 2024, 12, 1886. https://doi.org/10.3390/pr12091886
Stublić K, Ranilović J, Ocelić Bulatović V, Kučić Grgić D. Advancing Sustainability: Utilizing Bacterial Polyhydroxyalkanoate for Food Packaging. Processes. 2024; 12(9):1886. https://doi.org/10.3390/pr12091886
Chicago/Turabian StyleStublić, Krešimir, Jasmina Ranilović, Vesna Ocelić Bulatović, and Dajana Kučić Grgić. 2024. "Advancing Sustainability: Utilizing Bacterial Polyhydroxyalkanoate for Food Packaging" Processes 12, no. 9: 1886. https://doi.org/10.3390/pr12091886
APA StyleStublić, K., Ranilović, J., Ocelić Bulatović, V., & Kučić Grgić, D. (2024). Advancing Sustainability: Utilizing Bacterial Polyhydroxyalkanoate for Food Packaging. Processes, 12(9), 1886. https://doi.org/10.3390/pr12091886








