The Physical Properties and Crystal Structure Changes of Stabilized Ice Cream Affected by Ultrasound-Assisted Freezing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ice Cream Mixes
2.3. Physicochemical Property Evaluation
2.3.1. Density
2.3.2. Overrun
2.3.3. pH
2.3.4. Freezing Point Analysis
2.4. Freezer Ice Cream Production
2.5. Ultrasound-Assisted Freezing
- Cryostat without the US for samples with (CS) and without stabilizers (CNS);
- Cryostat 21.5 kHz for samples with (CS21) and without stabilizers (CNS21), 10 min of US treatment, chopped operation;
- Cryostat 40 kHz for samples with (CS40) and without stabilizers (CNS40), 10 min of US treatment, chopped operation.
2.6. Microscopy Structure Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Ice Cream
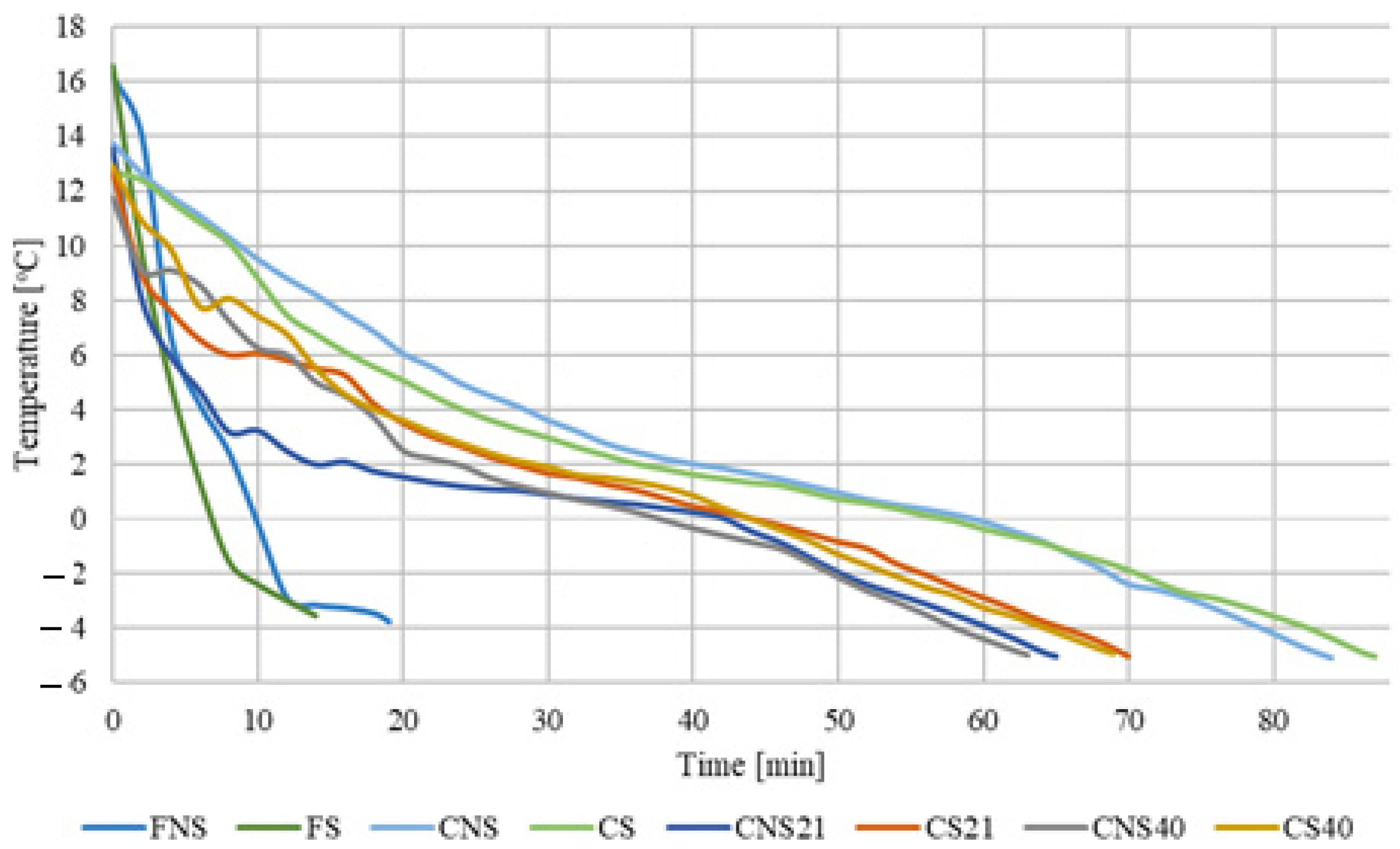
3.2. Freezing Course Analysis
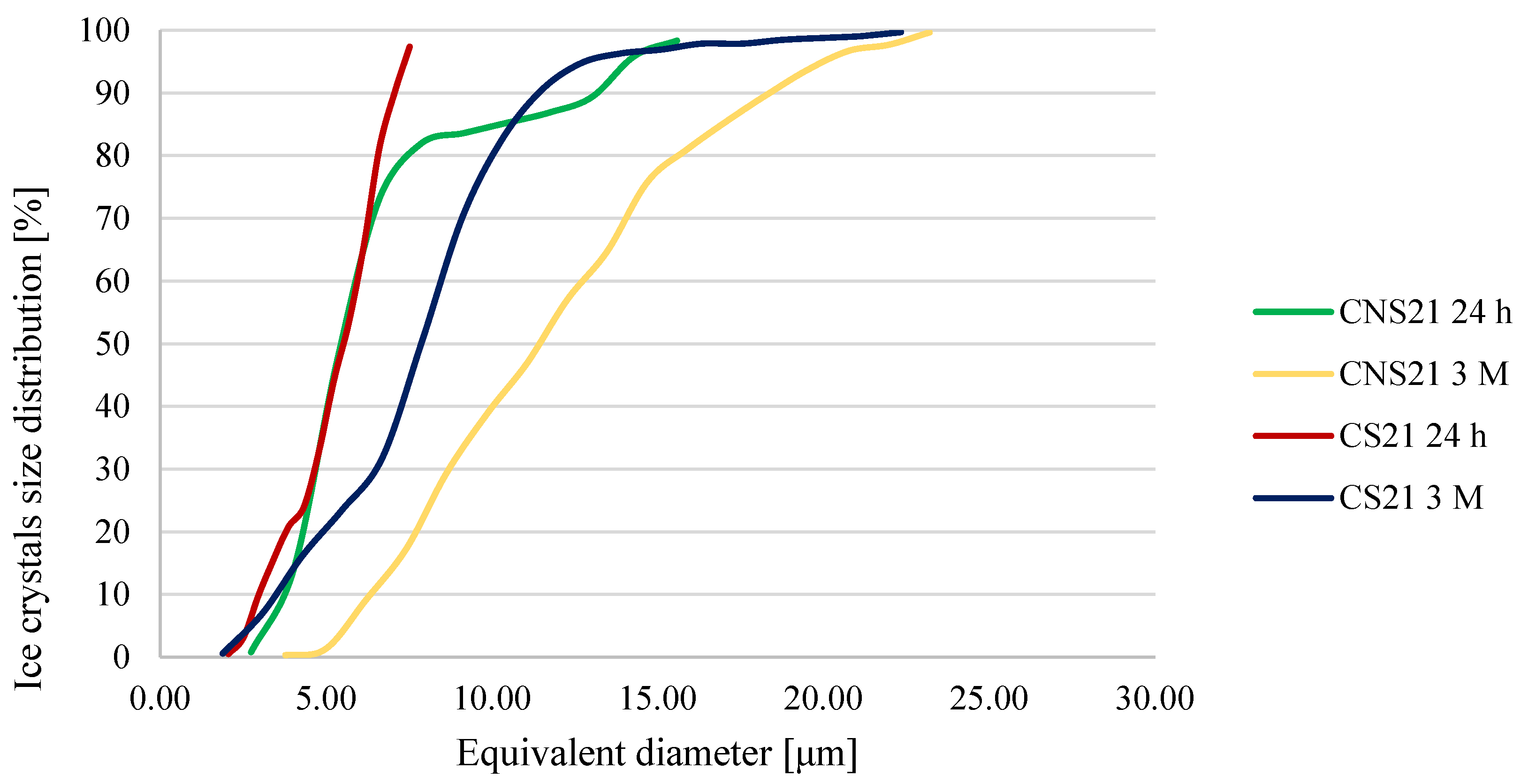
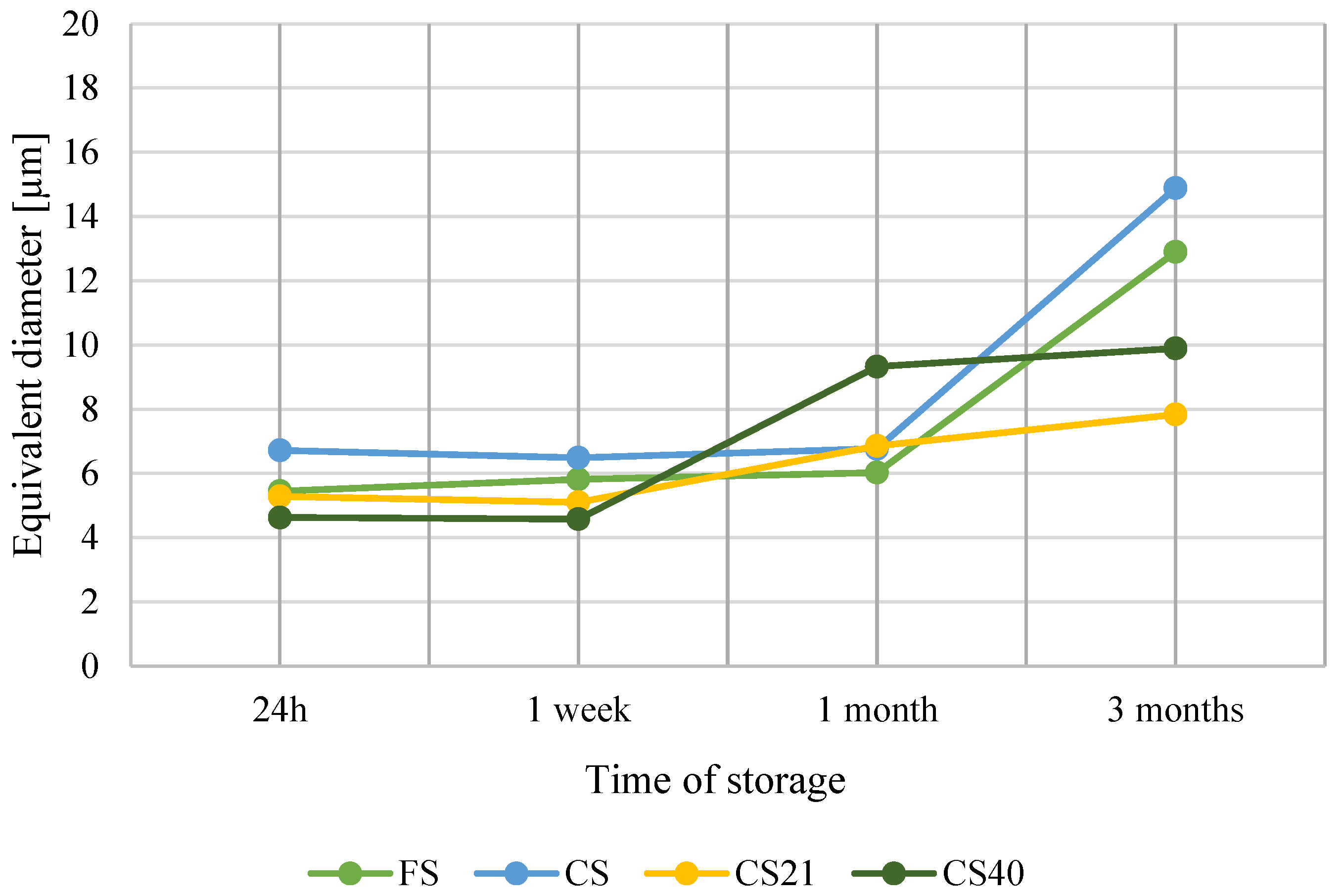
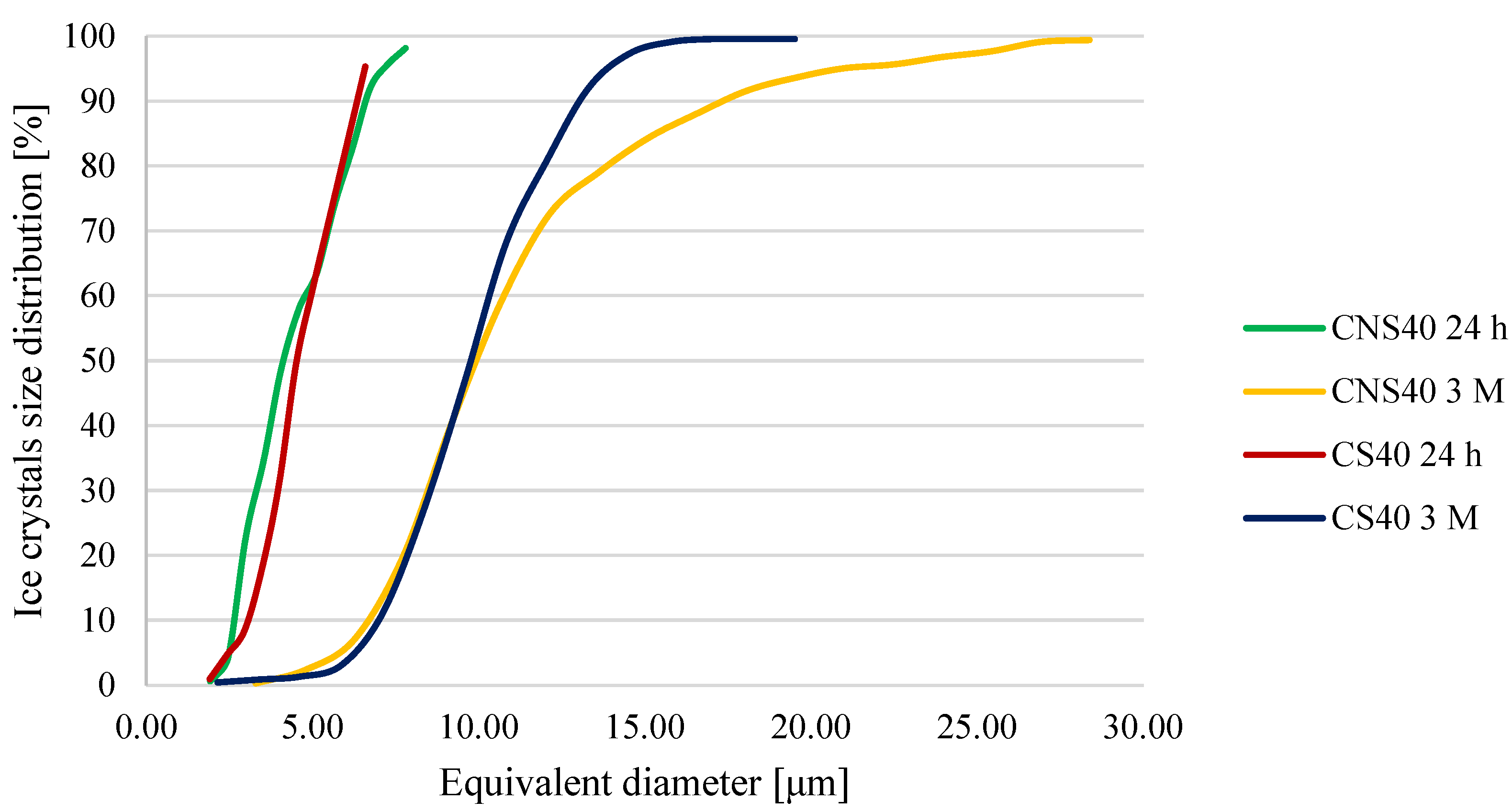
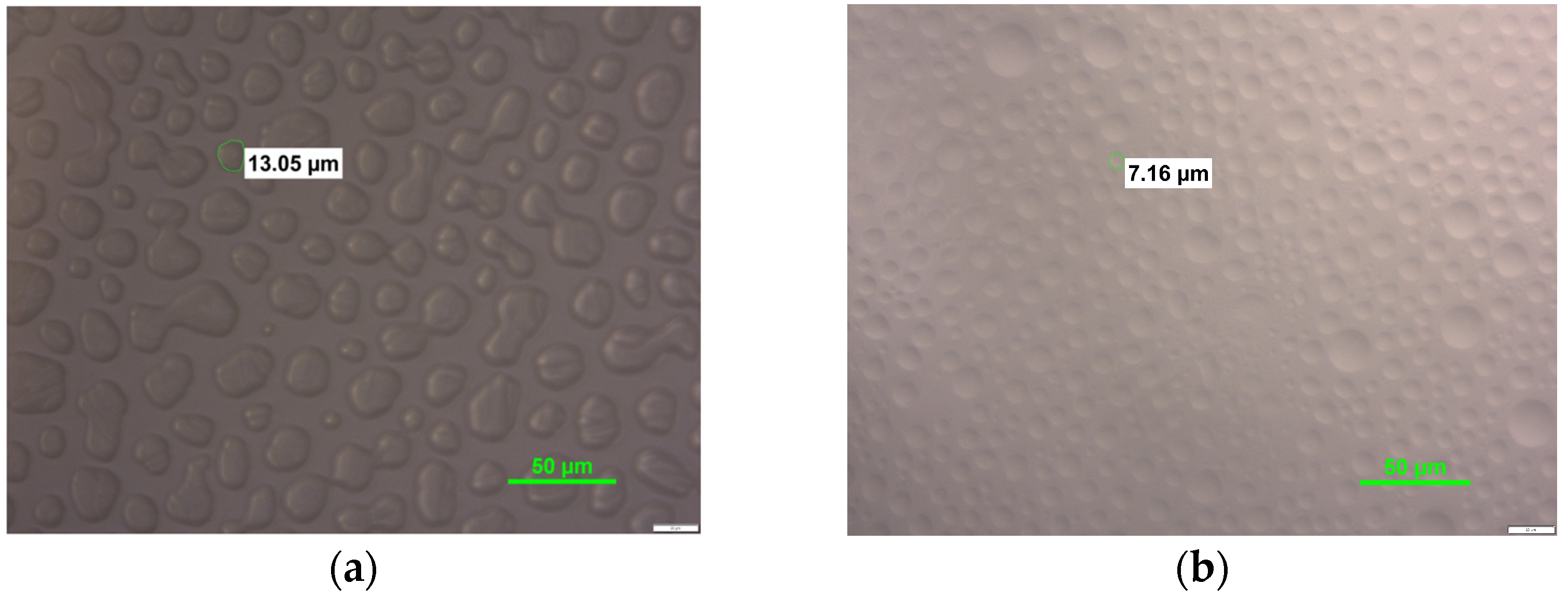
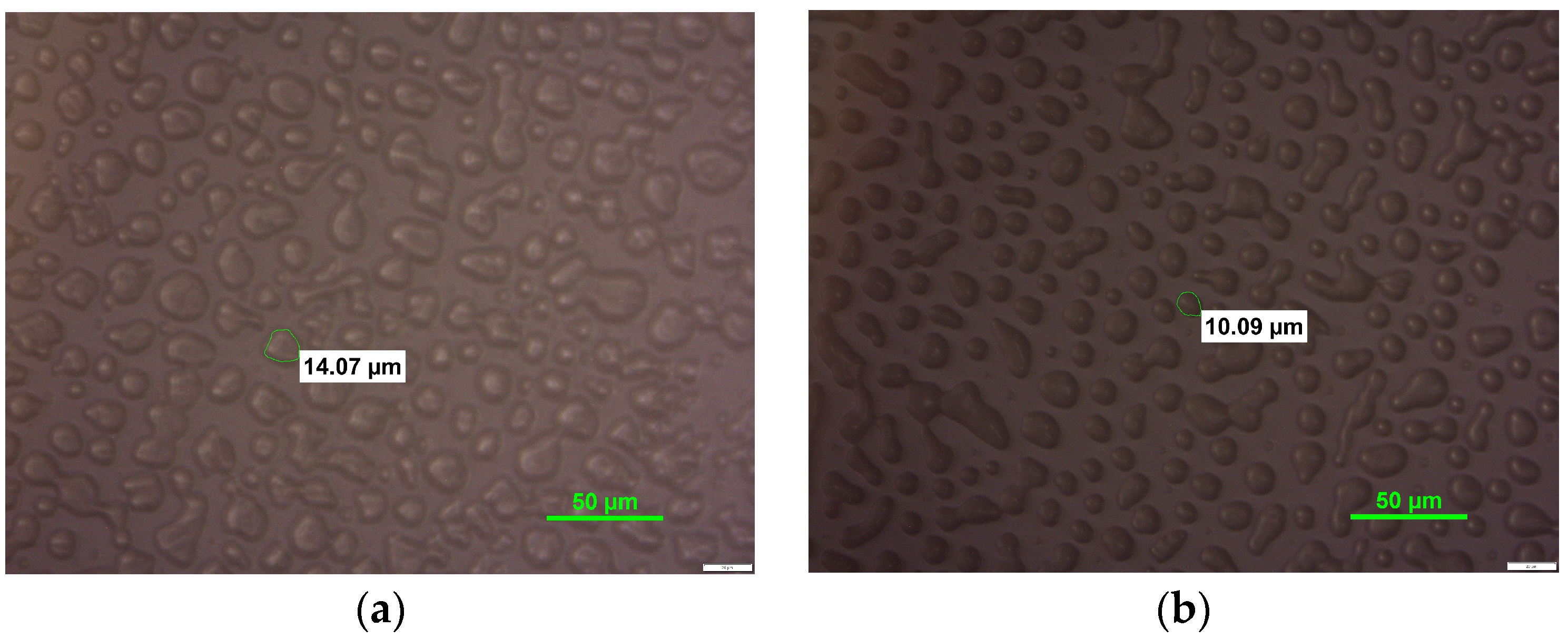
3.3. Ice Crystal Structure Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamińska-Dwórznicka, A.; Łaba, S.; Jakubczyk, E. The effects of selected stabilizers addition on physical properties and changes in crystal structure of whey ice cream. LWT 2022, 154, 112841. [Google Scholar] [CrossRef]
- Innocente, N.; Comparin, D.; Corradini, C. Proteose-peptone whey fraction as emulsifier in ice-cream preparation. Int. Dairy J. 2002, 12, 69–74. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Gondek, E.; Laba, S.; Jakubczyk, E.; Samborska, K. Characteristics of Instrumental Methods to Describe and Assess the Recrystallization Process in Ice Cream Systems. Foods 2019, 8, 117. [Google Scholar] [CrossRef]
- Akdeniz, V.; Akalın, A.S. New approach for yoghurt and ice cream production: High-intensity ultrasound. Trends Food Sci. Technol. 2019, 86, 392–398. [Google Scholar] [CrossRef]
- Dadan, M.; Matys, A.; Kaminska-Dworznicka, A.; Lammerskitten, A.; Toepfl, S.; Parniakov, O. Improvement of freezing processes assisted by ultrasound. In Design and Optimization of Innovative Food Processing Techniques Assisted by Ultrasound; Elsevier: Amsterdam, The Netherlands, 2021; pp. 217–273. [Google Scholar]
- Zhu, Z.; Zhang, P.; Sun, D.-W. Effects of multi-frequency ultrasound on freezing rates and quality attributes of potatoes. Ultrason. Sonochem. 2020, 60, 104733. [Google Scholar] [CrossRef]
- Kot, A.; Kamińska-Dwórznicka, A.; Jakubczyk, E. Study on the Influence of Ultrasound Homogenisation on the Physical Properties of Vegan Ice Cream Mixes. Appl. Sci. 2022, 12, 8492. [Google Scholar] [CrossRef]
- Kot, A.; Jakubczyk, E.; Kamińska-Dwórznicka, A. The Effectiveness of Combination Stabilizers and Ultrasound Homogenization in Milk Ice Cream Production. Appl. Sci. 2023, 13, 7561. [Google Scholar] [CrossRef]
- Nazarewicz, S.; Kozłowicz, K.; Kobus, Z.; Gładyszewska, B.; Matwijczuk, A.; Ślusarczyk, L.; Skrzypek, T.; Sujka, M.; Kozłowicz, N. The Use of Ultrasound in Shaping the Properties of Ice Cream with Oleogel Based on Oil Extracted from Tomato Seeds. Appl. Sci. 2022, 12, 9165. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Kot, A.; Jakubczyk, E.; Buniowska-Olejnik, M.; Nowacka, M. Effect of ultrasound-assisted freezing on the crystal structure of mango sorbet. Crystals 2023, 13, 396. [Google Scholar] [CrossRef]
- Dłużewska, E.; Gazda, B.; Leszczyński, K. Wpływ wybranych hydrokoloidów polisacharydowych na jakość koncentratów lodów owocowych. Acta Sci. Pol. Technol. Aliment. 2003, 2, 97–107. [Google Scholar]
- Góral, M.; Kozłowicz, K.; Pankiewicz, U.; Góral, D.; Kluza, F.; Wójtowicz, A. Impact of stabilizers on the freezing process, and physicochemical and organoleptic properties of coconut milk-based ice cream. LWT 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Janczewska-Dupczyk, A.; Kot, A.; Laba, S.; Samborska, K. The impact of iota- and kappa-carrageenan addition on freezing process and ice crystals structure of strawberry sorbet frozen by various methods. J. Food Sci. 2020, 85, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Goff, H.D.; Hartel, R.W. Ice cream structure. In Ice Cream; Springer: Boston, MA, USA, 2013; pp. 313–352. [Google Scholar]
- Romulo, A.; Meindrawan, B. Marpietylie Effect of Dairy and Non-Dairy Ingredients on the Physical Characteristic of Ice Cream: Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 794, 012145. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A. Wpływ stabilizatorów na ograniczenie rekrystalizacji w lodach typu sorbet. PrzemysŁ SpoŻywczy 2016, 1, 34–37. [Google Scholar] [CrossRef]
- Ndoye, F.T.; Alvarez, G. Characterization of ice recrystallization in ice cream during storage using the focused beam reflectance measurement. J. Food Eng. 2015, 148, 24–34. [Google Scholar] [CrossRef]
- Syed, Q.A.; Anwar, S.; Shukat, R.; Zahoor, T. Effects of different ingredients on texture of ice cream. J. Nutr. Health Food Eng. 2018, 8, 422–435. [Google Scholar] [CrossRef]
- Brown, J.R.; Seymour, J.D.; Brox, T.I.; Skidmore, M.L.; Wang, C.; Christner, B.C.; Luo, B.H.; Codd, S.L. Recrystallization inhibition in ice due to ice binding protein activity detected by nuclear magnetic resonance. Biotechnol. Rep. 2014, 3, 60–64. [Google Scholar] [CrossRef]
- Raymond, J.A.; Knight, C.A. Ice binding, recrystallization inhibition, and cryoprotective properties of ice-active substances associated with Antarctic sea ice diatoms. Cryobiology 2003, 46, 174–181. [Google Scholar] [CrossRef]
- Mortazavi, A.; Tabatabaie, F. Study of ice cream freezing process after treatment with ultrasound. World Appl. Sci. J. 2008, 4, 188–190. [Google Scholar]
- Jambrak, A.; Lerda, D.; Mirčeta, R.; Šimunek, M.; Lelas, V.; Chemat, F.; Herceg, Z.; Batur, V. Experimental design and optimization of ultrasound treatment: Functional and physical properties of sonicated ice cream model mixtures. J. Food Process. Technol. 2012, 3, 145. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Impact of incorporation of CO2 on the melting, texture and sensory attributes of soft-serve ice cream. Int. Dairy J. 2020, 109, 104789. [Google Scholar] [CrossRef]
- Astráin-Redín, L.; Abad, J.; Rieder, A.; Kirkhus, B.; Raso, J.; Cebrián, G.; Álvarez, I. Direct contact ultrasound assisted freezing of chicken breast samples. Ultrason. Sonochem. 2021, 70, 105319. [Google Scholar] [CrossRef] [PubMed]
- Lomolino, G.; Zannoni, S.; Zabara, A.; Da Lio, M.; De Iseppi, A. Ice recrystallisation and melting in ice cream with different proteins levels and subjected to thermal fluctuation. Int. Dairy J. 2020, 100, 104557. [Google Scholar] [CrossRef]
- Xu, B.-g.; Zhang, M.; Bhandari, B.; Sun, J.; Gao, Z. Infusion of CO2 in a solid food: A novel method to enhance the low-frequency ultrasound effect on immersion freezing process. Innov. Food Sci. Emerg. Technol. 2016, 35, 194–203. [Google Scholar] [CrossRef]
- Ohsaka, K.; Trinh, E. Apparatus for measuring the growth velocity of dendritic ice in undercooled water. J. Cryst. Growth 1998, 194, 138–142. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, Y.; Li, X.; Yan, T.; Cui, J. Effects of milk protein-polysaccharide interactions on the stability of ice cream mix model systems. Food Hydrocoll. 2015, 45, 327–336. [Google Scholar] [CrossRef]
- Shi, Z.; Zhong, S.; Yan, W.; Liu, M.; Yang, Z.; Qiao, X. The effects of ultrasonic treatment on the freezing rate, physicochemical quality, and microstructure of the back muscle of grass carp (Ctenopharyngodon idella). LWT 2019, 111, 301–308. [Google Scholar] [CrossRef]
- Kiran-Yildirim, B.; Gaukel, V. Ice Crystal Growth in Sucrose Solutions Containing Kappa- and Iota-Carrageenans. Chem. Eng. Technol. 2020, 43, 1040–1047. [Google Scholar] [CrossRef]
- Aslan Türker, D.; Dogan, M. Effects of ultrasound homogenization on the structural and sensorial attributes of ice cream: Optimization with Taguchi and data envelopment analysis. J. Food Meas. Charact. 2021, 15, 4888–4898. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, X.; Liu, Q.; Chen, Q.; Kong, B. Changes in microstructure, quality and water distribution of porcine longissimus muscles subjected to ultrasound-assisted immersion freezing during frozen storage. Meat Sci. 2019, 151, 24–32. [Google Scholar] [CrossRef]
- Hu, S.-Q.; Liu, G.; Li, L.; Li, Z.-X.; Hou, Y. An improvement in the immersion freezing process for frozen dough via ultrasound irradiation. J. Food Eng. 2013, 114, 22–28. [Google Scholar] [CrossRef]


| Sample | Density | pH | Overrun | Freezing Point |
|---|---|---|---|---|
| NS | 1.161 ± 0.030 a | 6.35 ± 0.01 a | 16.4 ± 1.6 b | −1.8 ± 0.1 a |
| S | 1.157 ± 0.221 a | 6.31 ± 0.00 a | 25.2 ± 0.7 a | −2.4 ± 0.2 b |
| Sample | Time [min] | Sample | Time [min] |
|---|---|---|---|
| FNS | 19 ± 4 c | FS | 14 ± 0 c |
| CNS | 84 ± 0 a | CS | 87 ± 4 c |
| CNS21 | 65 ± 1 b | CS21 | 70 ± 3 b |
| CNS40 | 63 ± 4 b | CS40 | 69 ± 4 b |
| Variant | Minimal Diameter [µm] | Maximal Diameter [µm] | DA in Class with the Highest Frequency [µm] | |
|---|---|---|---|---|
| FNS | 24 h | 2.38 | 14.72 | 7.39 ± 2.23 d,e,f |
| 1 week | 3.03 | 16.10 | 7.53 ± 2.84 d | |
| 1 month | 4.57 | 24.49 | 7.45 ± 2.21 d,e | |
| 3 months | 8.02 | 29.75 | 17.99 ± 4.05 a | |
| CNS | 24 h | 1.98 | 18.38 | 6.60 ± 2.59 e,f |
| 1 week | 3.51 | 21.58 | 6.41 ± 2.20 f | |
| 1 month | 3.00 | 20.55 | 8.91 ± 3.39 c | |
| 3 months | 4.05 | 27.08 | 16.90 ± 4.27 a | |
| CNS21 | 24 h | 2.74 | 16.87 | 6.54 ± 3.28 e,f |
| 1 week | 2.03 | 15.25 | 6.78 ± 4.45 d,e,f | |
| 1 month | 4.12 | 22.32 | 6.86 ± 1.95 d,e,f | |
| 3 months | 3.77 | 24.42 | 11.84 ± 4.44 b | |
| CNS40 | 24 h | 1.93 | 8.34 | 4.44 ± 1.54 g |
| 1 week | 1.96 | 10.96 | 4.32 ± 1.68 g | |
| 1 month | 1.80 | 19.35 | 9.31 ± 4.40 b | |
| 3 months | 3.29 | 29.85 | 11.07 ± 4.64 b |
| Variant | Minimal Diameter [µm] | Maximal Diameter [µm] | DA in Class with the Highest Frequency [µm] | |
|---|---|---|---|---|
| FS | 24 h | 1.43 | 17.08 | 5.45 ± 3.08 h,i |
| 1 week | 2.21 | 17.27 | 5.82 ± 2.43 g,h | |
| 1 month | 3.17 | 12.65 | 6.03 ± 1.83 f,g,h | |
| 3 months | 2.70 | 28.58 | 12.90 ± 4.51 b | |
| CS | 24 h | 3.71 | 8.56 | 6.72 ± 1.07 e,f,g |
| 1 week | 2.35 | 14.54 | 6.49 ± 2.85 e,f,g | |
| 1 month | 3.98 | 10.70 | 6.77 ± 1.38 e,f | |
| 3 months | 3.73 | 27.32 | 14.89 ± 4.55 a | |
| CS21 | 24 h | 2.06 | 7.98 | 5.29 ± 1.45 h,i,j |
| 1 week | 2.81 | 10.26 | 5.11 ± 1.96 h,i,j | |
| 1 month | 4.12 | 15.25 | 6.86 ± 3.08 e | |
| 3 months | 3.77 | 24.42 | 7.84 ± 3.35 d | |
| CS40 | 24 h | 1.91 | 7.12 | 4.36 ± 1.26 i,j |
| 1 week | 1.81 | 12.51 | 4.58 ± 1.80 j | |
| 1 month | 1.96 | 20.63 | 9.33 ± 2.92 c | |
| 3 months | 2.15 | 20.75 | 9.90 ± 2.47 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamińska-Dwórznicka, A.; Kot, A. The Physical Properties and Crystal Structure Changes of Stabilized Ice Cream Affected by Ultrasound-Assisted Freezing. Processes 2024, 12, 1957. https://doi.org/10.3390/pr12091957
Kamińska-Dwórznicka A, Kot A. The Physical Properties and Crystal Structure Changes of Stabilized Ice Cream Affected by Ultrasound-Assisted Freezing. Processes. 2024; 12(9):1957. https://doi.org/10.3390/pr12091957
Chicago/Turabian StyleKamińska-Dwórznicka, Anna, and Anna Kot. 2024. "The Physical Properties and Crystal Structure Changes of Stabilized Ice Cream Affected by Ultrasound-Assisted Freezing" Processes 12, no. 9: 1957. https://doi.org/10.3390/pr12091957
APA StyleKamińska-Dwórznicka, A., & Kot, A. (2024). The Physical Properties and Crystal Structure Changes of Stabilized Ice Cream Affected by Ultrasound-Assisted Freezing. Processes, 12(9), 1957. https://doi.org/10.3390/pr12091957






