Solid-State Fermentation-Assisted Extraction of Flavonoids from Grape Pomace Using Co-Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Raw Material
2.3. Characterization of the Raw Material
2.4. Solid State Fermentation
2.4.1. Microorganisms
2.4.2. Preparation of Inoculum
2.4.3. Condition for SSF
2.4.4. SSF in Monoculture with Fungus
2.4.5. SSF in Monoculture with Yeasts
2.5. Assessments
2.5.1. Hydrolysable Tannins
2.5.2. Condensed Tannins
2.5.3. Total Flavonoids
2.5.4. Total Sugars
2.5.5. DPPH Radical Inhibition Test
2.5.6. Iron Ion Reduction Test FRAP
2.6. High-Performance Liquid Chromatography and Mass Spectroscopy (HPLC-MS)
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analysis
3. Results
3.1. Characterization of Raw Material
Physicochemical Characterization
3.2. SSF
3.2.1. SSF with Fungus
3.2.2. HPLC-MS for SSF with Fungus
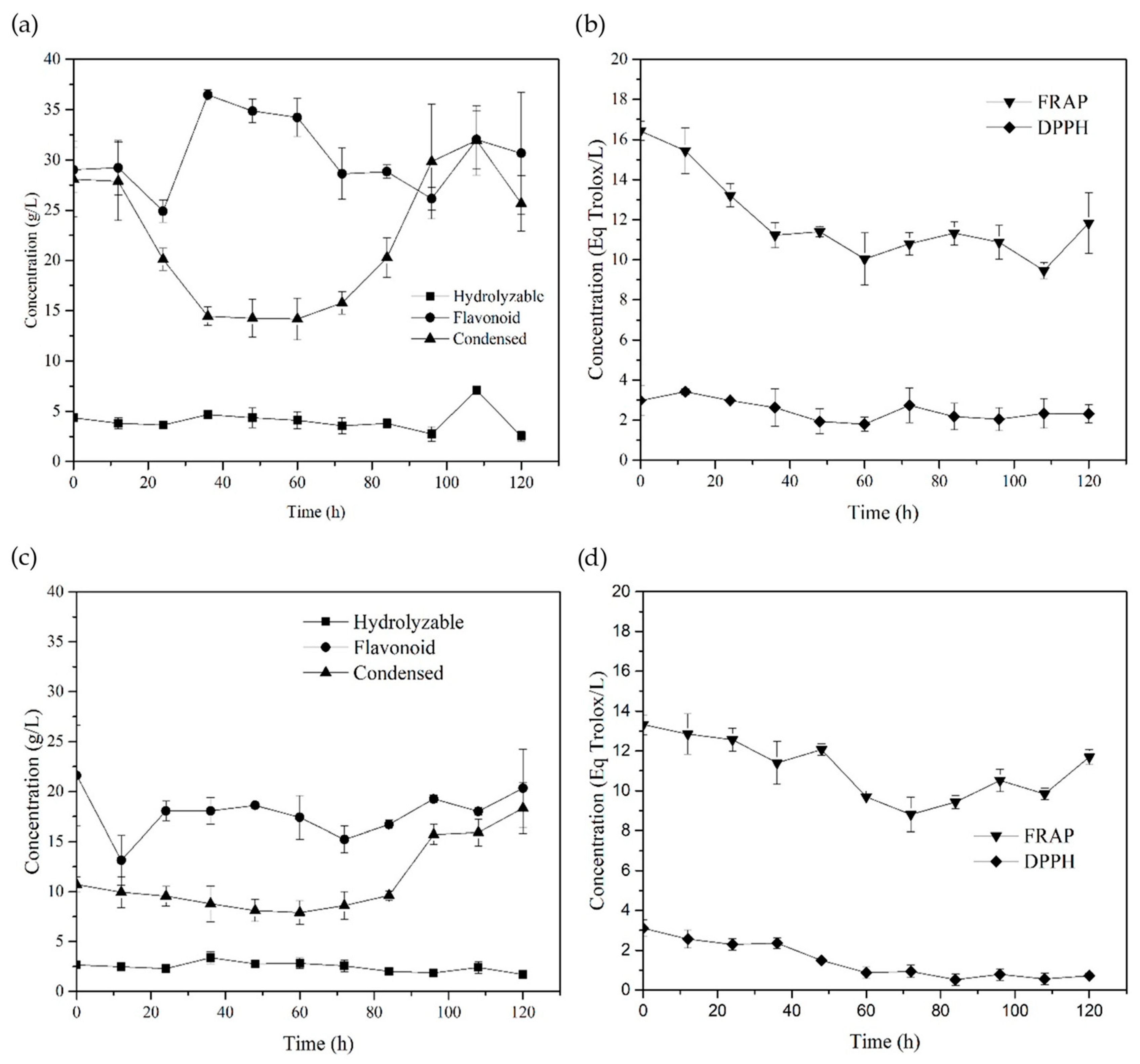
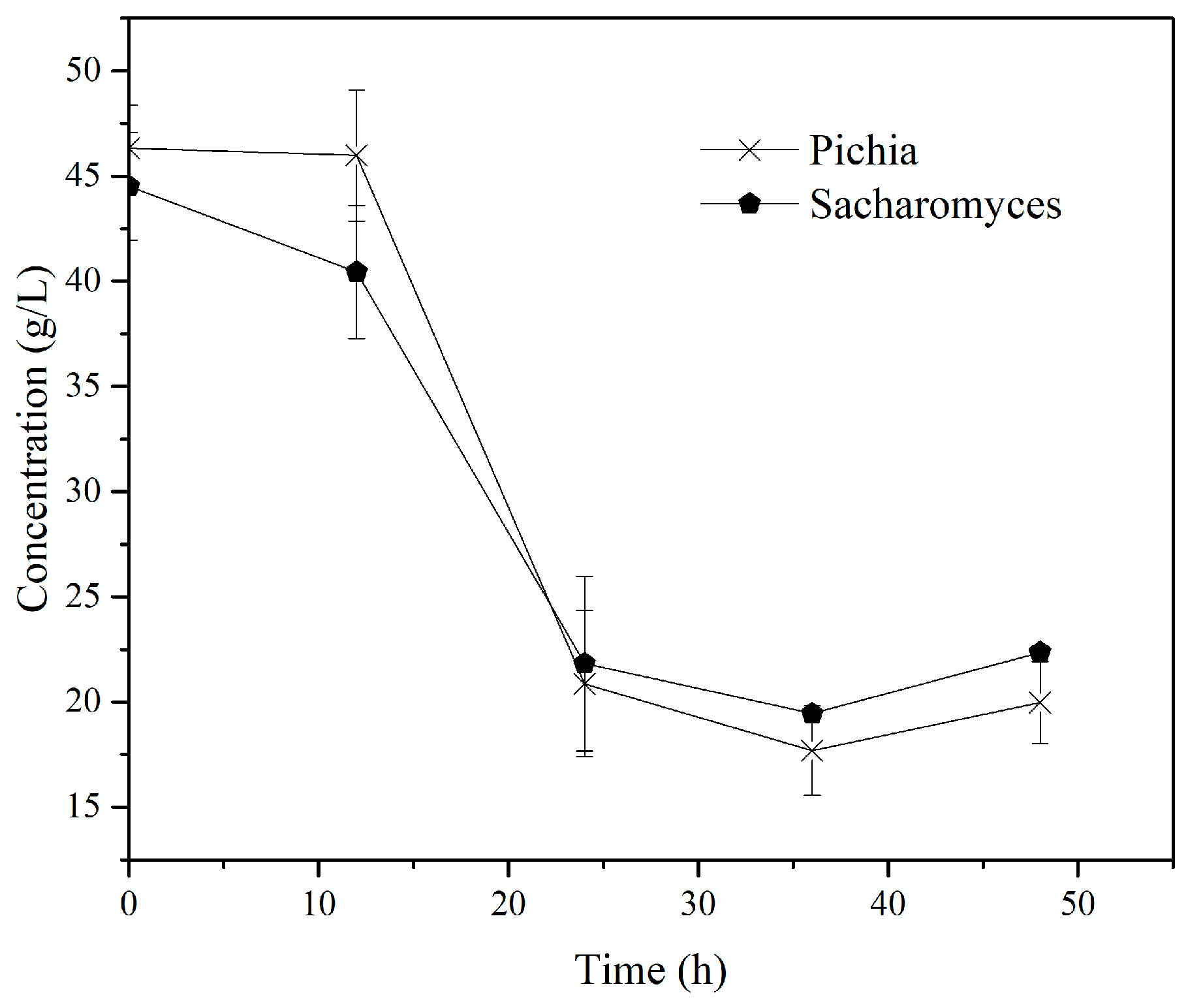
3.2.3. SSF with Yeast
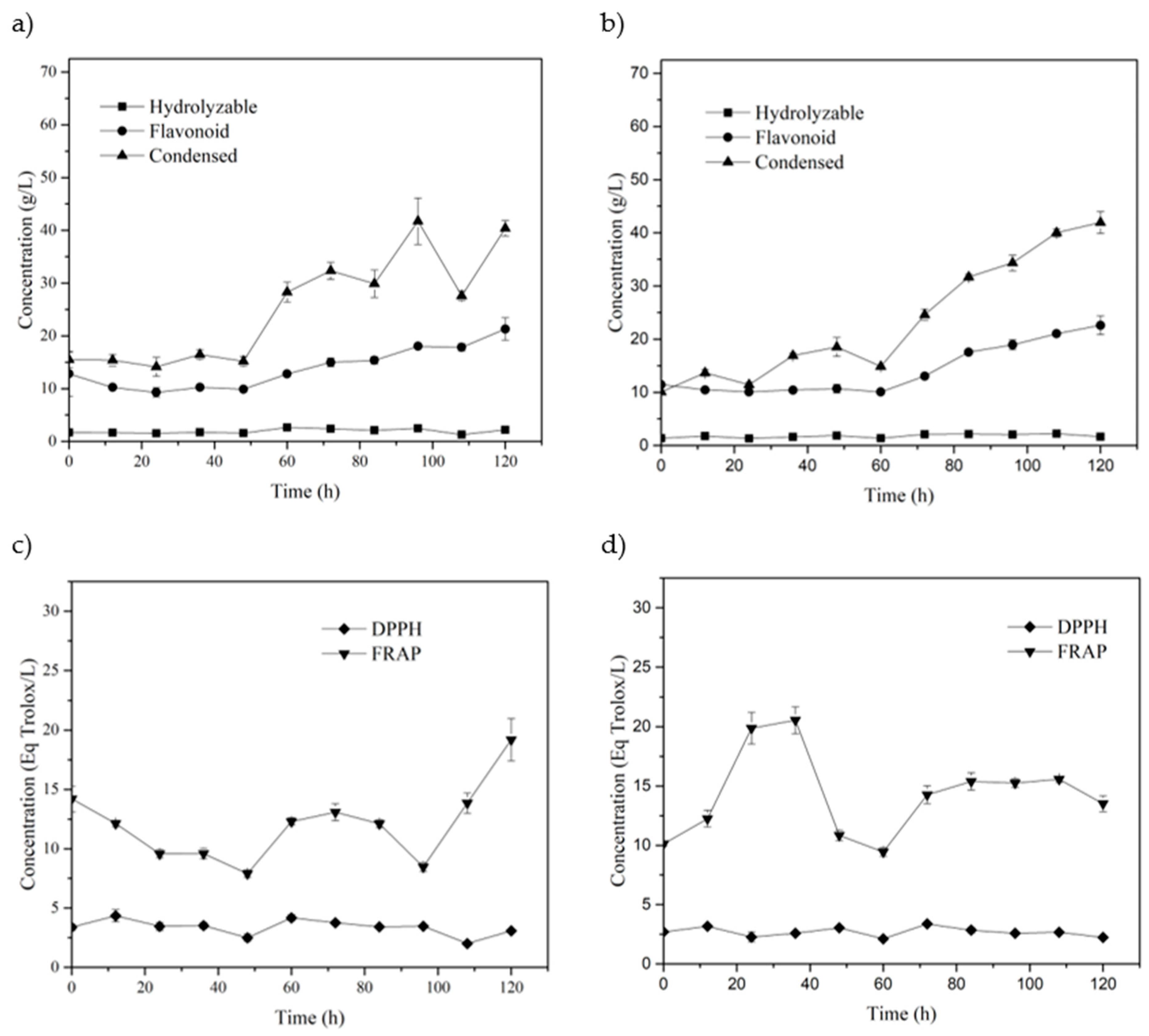
3.3. SSF with Co-Culture
HPLC-MS for Co-Culture
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Organization of Vine and Wine, OIV. Available online: https://www.oiv.int/what-we-do/global-report?oiv (accessed on 15 August 2024).
- Servicio de Información Agroalimentaria y Pesquera, SIAP Cierre Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 15 August 2024).
- Perra, M.; Cuena-Lombraña, A.; Bacchetta, G.; Manca, M.L.; Manconi, M.; Maroun, R.G.; Muntoni, A.; Tuberoso, C.I.G.; Gil, K.A.; De Gioannis, G. Combining Different Approaches for Grape Pomace Valorization: Polyphenols Extraction and Composting of the Exhausted Biomass. Sustainability 2022, 14, 10690. [Google Scholar] [CrossRef]
- Ianni, A.; Martino, G. Dietary grape pomace supplementation in dairy cows: Effect on nutritional quality of milk and its derived dairy products. Foods 2020, 9, 168. [Google Scholar] [CrossRef]
- Fontana, M.; Murowaniecki Otero, D.; Pereira, A.M.; Santos, R.B.; Gularte, M.A. Grape pomace flour for incorporation into cookies: Evaluation of nutritional, sensory and technological characteristics. J. Culin. Sci. Technol. 2022, 22, 850–869. [Google Scholar] [CrossRef]
- Amaya-Chantaca, D.; Flores-Gallegos, A.C.; Iliná, A.; Aguilar, C.N.; Sepúlveda-Torre, L.; Ascacio-Vadlés, J.A.; Chávez-González, M.L. Comparative extraction study of grape pomace bioactive compounds by submerged and solid-state fermentation. J. Chem. Technol. Biotechnol. 2022, 97, 1494–1505. [Google Scholar] [CrossRef]
- González, M.; Barrios, S.; Budelli, E.; Pérez, N.; Lema, P.; Heinzen, H. Ultrasound assisted extraction of bioactive compounds in fresh and freeze-dried Vitis vinifera cv Tannat grape pomace. Food Bioprod. Process. 2020, 124, 378–386. [Google Scholar] [CrossRef]
- Vakilian, K.; Nateghi, L.; Javadi, A.; Anarjan, N. Optimization of conventional and ultrasound-assisted extraction of pectin from unripe grape pomace: Extraction yield, degree of esterification, and galacturonic acid content. J. Food Meas. Charact. 2023, 17, 5777–5793. [Google Scholar] [CrossRef]
- Neto, R.T.; Santos, S.A.; Oliveira, J.; Silvestre, A.J. Impact of eutectic solvents utilization in the microwave assisted extraction of proanthocyanidins from grape pomace. Molecules 2021, 27, 246. [Google Scholar] [CrossRef]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Carpentieri, S.; Ferrari, G.; Pataro, G. Pulsed electric fields-assisted extraction of valuable compounds from red grape pomace: Process optimization using response surface methodology. Front. Nutr. 2023, 10, 1158019. [Google Scholar] [CrossRef]
- Silva, J.T.D.P.; Borges, M.H.; de Souza, C.A.C.; Fávaro-Trindade, C.S.; Sobral, P.J.D.A.; de Oliveira, A.L.; Martelli-Tosi, M. Grape Pomace Rich-Phenolics and Anthocyanins Extract: Production by Pressurized Liquid Extraction in Intermittent Process and Encapsulation by Spray-Drying. Foods 2024, 13, 279. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; De Vietro, N.; Massari, F.; Zambonin, C. Determination of polyphenols and vitamins in wine-making by-products by supercritical fluid extraction (SFE). Anal. Lett. 2020, 53, 2585–2595. [Google Scholar] [CrossRef]
- Xavier Machado, T.D.O.; Portugal, I.B.M.; Padilha, C.V.D.S.; Ferreira Padilha, F.; dos Santos Lima, M. New trends in the use of enzymes for the recovery of polyphenols in grape byproducts. J. Food Biochem. 2021, 45, e13712. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Carmona, J.Y.; Ramírez-Guzman, K.N.; Ascacio-Valdes, J.A.; Sepulveda, L.; Aguilar, C.N. Solid-state fermentation for recovery of carotenoids from tomato waste. Innov. Food Sci. Emerg. Technol. 2022, 80, 103108. [Google Scholar] [CrossRef]
- Buenrostro-Figueroa, J.J.; Velázquez, M.; Flores-Ortega, O.; Ascacio-Valdés, J.A.; Huerta-Ochoa, S.; Aguilar, C.N.; Prado-Barragán, L.A. Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem. 2017, 62, 16–23. [Google Scholar] [CrossRef]
- Yepes-Betancur, D.P.; Márquez-Cardozo, C.J.; Cadena-Chamorro, E.M.; Martinez-Saldarriaga, J.; Torres-León, C.; Ascacio-Valdes, A.; Aguilar, C.N. Solid-state fermentation–assisted extraction of bioactive compounds from hass avocado seeds. Food Bioprod. Process. 2021, 126, 155–163. [Google Scholar] [CrossRef]
- Rodríguez, A.; Gea, T.; Sánchez, A.; Font, X. Agro-wastes and inert materials as supports for the production of biosurfactants by solid-state fermentation. Waste Biomass Valorization 2021, 12, 1963–1976. [Google Scholar] [CrossRef]
- Arora, D.; Gupta, P.; Jaglan, S.; Roullier, C.; Grovel, O.; Bertrand, S. Expanding the chemical diversity through microorganisms co-culture: Current status and outlook. Biotechnol. Adv. 2020, 40, 107521. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1. [Google Scholar] [CrossRef]
- Phaiboonsilpa, N.; Chysirichote, T.; Champreda, V.; Laosiripojana, N. Fermentation of xylose, arabinose, glucose, their mixtures and sugarcane bagasse hydrolyzate by yeast Pichia stipitis for ethanol production. Energy Rep. 2020, 6, 710–713. [Google Scholar] [CrossRef]
- Yu, R.; Liu, J.; Wang, Y.; Wang, H.; Zhang, H. Aspergillus niger as a secondary metabolite factory. Front. Chem. 2021, 9, 701022. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdes, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Muñiz-Márquez, D.B.; Aguilar-Zárate, P.; Rodríguez-Herrera, R.; Aguilar, C.N. Microplate quantification of total phenolic content from plant extracts obtained by conventional and ultrasound methods. Phytochem Anal. 2014, 5, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Perumal, R.; Bean, S.R.; Wilson, J.D. High-throughput micro-plate HCl–vanillin assay for screening tannin content in sorghum grain. J. Sci. Food Agric. 2014, 94, 2133–2136. [Google Scholar] [CrossRef]
- de la Rosa, L.A.; Vazquez-Flores, A.A.; Alvarez-Parrilla, E.; Rodrigo-García, J.; Medina-Campos, O.N.; Ávila-Nava, A.; Pedraza-Chaverri, J. Content of major classes of polyphenolic compounds, antioxidant, antiproliferative, and cell protective activity of pecan crude extracts and their fractions. J. Funct. Foods 2014, 7, 219–228. [Google Scholar] [CrossRef]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and sensitive anthrone–sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Buenrostro, J.J.; Prado-Barragán, A.; Rodríguez-Herrera, R.; Aguilar, C.N. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J. Basic Microbiol. 2016, 56, 329–336. [Google Scholar] [CrossRef]
- Tavares, P.; Nascimento, R.; Andrade, R.; Biasoto, A.; Druzian, J.; Souza, C.D. Physicochemical Characterization of Agro-Industrial Waste from BRS Magna Grape Cultivar. 2020. Available online: https://www.alice.cnptia.embrapa.br/alice/handle/doc/1127018 (accessed on 15 August 2024).
- Granato, D.; de Magalhães Carrapeiro, M.; Fogliano, V.; van Ruth, S.M. Effects of geographical origin, varietal and farming system on the chemical composition and functional properties of purple grape juices: A review. Trends Food Sci. Technol. 2016, 52, 31–48. [Google Scholar] [CrossRef]
- Priyadarshini, A.; Sahoo, M.M.; Raut, P.R.; Mahanty, B.; Sahoo, N.K. Kinetic modelling and process engineering of phenolics microbial and enzymatic biodegradation: A current outlook and challenges. J. Water Process Eng. 2021, 44, 102421. [Google Scholar] [CrossRef]
- Fadel, M.; Hamed, A.A.; Abd-Elaziz, A.M.; Ghanem, M.M.; Roshdy, A.M. Cellulases and animal feed production by solid-state fermentation by Aspergillus fumigatus NRCF-122 mutant. Egypt J. Chem. 2021, 64, 3511–3520. [Google Scholar] [CrossRef]
- Manan, M.A.; Webb, C. Design aspects of solid-state fermentation as applied to microbial bioprocessing. J. Appl. Biotechnol. Bioeng. 2017, 4, 511–532. [Google Scholar]
- Chawla, P.; Kumar, V.; Bains, A.; Singh, R.; Sadh, P.K.; Kaushik, R.; Kumar, N. Improvement of mineral absorption and nutritional properties of Citrullus vulgaris seeds using solid-state fermentation. J. Am. Coll. Nutr. 2020, 39, 628–635. [Google Scholar] [CrossRef]
- Moran-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Production of cellulases and xylanases in solid-state fermentation by different strains of Aspergillus niger using sugarcane bagasse and brewery spent grain. Biochem. Eng. J. 2021, 172, 108060. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Özcan, M.M.; Al Juhaimi, F.; Babiker, E.F.E.; Ghafoor, K.; Banjanin, T.; Alqah, H.A. Chemical composition, bioactive compounds, mineral contents, and fatty acid composition of pomace powder of different grape varieties. J. Food Process. Preserv. 2020, 44, e14539. [Google Scholar] [CrossRef]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; Morais, S.M.D.; Lima, A.D.; Martins, C.G.; Rodrigues, L.L. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- Tanabe, C.K.; Nelson, J.; Boulton, R.B.; Ebeler, S.E.; Hopfer, H. The use of macro, micro, and trace elemental profiles to differentiate commercial single vineyard pinot noir wines at a sub-regional level. Molecules 2020, 25, 2552. [Google Scholar] [CrossRef]
- Chan, L.G.; Cohen, J.L.; de Moura Bell, J.M.L.N. Conversion of agricultural streams and food-processing by-products to value-added compounds using filamentous fungi. Annu. Rev. Food Sci. Technol. 2018, 9, 503–523. [Google Scholar] [CrossRef]
- Betts, R.E.; Walters, D.E.; Rosazza, J.P. Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J. Med. Chem. 1974, 17, 599–602. [Google Scholar] [CrossRef]
- Veiko, A.G.; Lapshina, E.A.; Zavodnik, I.B. Comparative analysis of molecular properties and reactions with oxidants for quercetin, catechin, and naringenin. Mol. Cell. Biochem. 2021, 476, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Machate, H.; Benali, T.; Sahib, N.; Jaouadi, I.; Omari, N.E.; Bouyahya, A. Natural sources and pharmacological properties of pinosylvin. Plants 2022, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]



| Present Study (g/100 g) | Amaya-Chantaca [6] | Tavares [33] | |
|---|---|---|---|
| Ash | 3.26 ± 0.3 | 6.72 ± 0.023 | 3.27 ± 0.03 |
| Lipids | 7.56 ± 0.11 | 7.07 ± 0.38 | 4.54 ± 0.02 |
| Moisture | 6.05 ± 0.16 | 6.91 ± 0.27 | 10.28 ± 0.01 |
| Protein | 10.12 ± 0.38 | 2.12 ± 0.44 | 7.67 ± 0.04 |
| Fiber | 24.04 ± 0.77 | 27.75 ± 0.25 | 9.502 ± 0.005 |
| Carbohydrates | 48.97 ± 0.34 | 50.57 ± 0.27 | 74.24 ± 0.02 |
| IAA (g/g) | 3.45 ± 0.09 | 3.38 ± 0.1 | - |
| PCH (%) | 71.96 ± 1.38 | 72.24 ± 1.97 | - |
| aw | 0.472 ± 0.005 | - | - |
| Mineral | Present Study (mg/g ash) | Mohamed [40] | Sousa [41] |
|---|---|---|---|
| K | 81.08 ± 0.27 | 27.18 ± 2.47 | 1.40 ± 0.313 |
| Ca | 10.14 ± 0.14 | 6.87 ± 0.31 | 0.44 ± 0.715 |
| Mg | 5.02 ± 0.19 | 3.57 ± 0.76 | 0.13 ± 0.255 |
| P | 1.88 ± 0.004 | 31.57 ± 3.54 | 0.183 ± 0.255 |
| S | 0.97 ± 0.01 | 0.86 ± 0.09 | 0.089 ± 0.336 |
| Fe | 0.31 ± 0.006 | 21.54 ± 1.28 | 18.08 ± 0.03 |
| Family | Compound/Time (h) | 0 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 | 108 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydroxycoumarins | Esculin | * | * | * | * | * | |||||
| Alkylmethoxyphenols | 4-Vinylguaiacol | * | * | * | * | * | * | * | |||
| Hydroxycinnamic acids | Caffeoylaspartic acid | * | * | * | * | * | |||||
| Phenolic terpenes | Carnosol | * | * | ||||||||
| Lignans | Tigloylgomycin H | * | |||||||||
| Hydroxycinnamic acids | Caffeoyltartaric acid | * | |||||||||
| Metalloporphyrin | Phlorin | * | * | ||||||||
| Alkylphenols | 5-Heptadecylresorcinol | * | * | * | * | * | |||||
| Hydroxycinnamic acids | p-coumaroylmalic acid | * | * | * | * | * | |||||
| Hydroxybenzoic acids | Gallic acid 3-O-gallate | * | * | * | |||||||
| Methoxycinnamic acid | 5–5′-dehydrodiferulic acid | * | * | ||||||||
| Hydroxycinnamic acids | 3-p-coumaroylquinic acid | * | * | ||||||||
| Hydroxycoumarins | Scopoletin | * | |||||||||
| Hydroxybenzoic acids | Tetragalloyl glucose | * | |||||||||
| Methoxycinnamic acids | Feruloyl tartaric acid | * | |||||||||
| Hydroxycinnamic acids | 1-caffeoylquinic acid | * | |||||||||
| Flavonols | Quercetin | * | * | * | * | * | * | * | * | * | * |
| Time (h): 0 | 24 | 48 | 72 | 96 | 120 |
|---|---|---|---|---|---|
| Gallic acid 3-O-gallate | Rosmarinic acid | 5-Nonadecylresorcinol | p-Coumaric acid 4-O-glucoside | Gallic acid 3-O-gallate | Gallic acid 3-O-gallate |
| 4-Vinylguaiacol | 5-Nonadecylresorcinol | Gallic acid 3-O-gallate | Gallic acid 3-O-gallate | 5-Nonadecylresorcinol | 5-Nonadecylresorcinol |
| Rosmarinic acid | Dihydrocaffeic acid | Caffeic acid 4-O-glucoside | Caffeic acid 4-O-glucoside | Bisdemethoxycurcumin | Caffeic acid 4-O-glucoside |
| Gallic acid 4-O-glucoside | Gallic acid 4-O-glucoside | Medioresinol | Medioresinol | Caffeic acid 4-O-glucoside | Medioresinol |
| (+)-Catechin | 7,4′-Dihydroxyflavone | 3-O-Xylosyl-rutinoside of quercetin | 4-Vinylguaiacol | Medioresinol | 3-Feruloylquinic acid |
| (−)-Epicatechin | 4-Vinylguaiacol | Caffeoyl tartaric acid | Rosmarinic acid | Hydroxycaffeic acid | Cyanidin 3-O-(6″-dioxalyl-glucoside) |
| Feruloyl tartaric acid | 3,4-DHPEA-EDA | 24-Methylcholesteryl ferulate | Feruloyl tartaric acid | p-HPEA-EA | |
| 6,8-Dihydroxykaempferol | Apigenin 7-O-(6″-malonyl-apiosyl-glucoside) | p-HPEA-EA | Pinosylvin | p-Coumaroyl glycolic acid | |
| p-HPEA-EA | 1,2,2′-Triferuloylgentiobiose | Patulentin 3-O-glucoside | p-Coumaroyl glycolic acid | 1-Caffeoylquinic acid | |
| Delphinidin 3-O-(6″-acetyl-glucoside) | Cirsimaritin | 5-O-Galloylquinic acid | Patulentin 3-O-glucoside | (+)-Catechin | |
| Myricetin | Lariciresinol | p-Anisaldehyde | Quercetin |
| Time (h): 0 | 24 | 48 | 72 | 96 | 120 |
|---|---|---|---|---|---|
| Gallic acid 3-O-gallate | Pterostilbene | Gallic acid 3-O-gallate | Gallic acid 3-O-gallate | Rosmarinic acid | Caffeic acid 4-O-glucoside |
| 1-Sinapoyl-2-feruloylgentiobiose | Rosmarinic acid | 1-Caffeoylquinic acid | Quercetin 3-O-(6”-malonyl-glucoside) 7-O-glucoside | Caffeic acid 4-O-glucoside | Cinnamoylglucose |
| (+)-Catechin | 5-Nonadecylresorcinol | Caffeic acid 4-O-glucoside | Caffeic acid 4-O-glucoside | Medioresinol | 4-Vinylguaiacol |
| Secoisolariciresinol | 2,3-Dihydroxybenzoic acid | Cyanidin 3-O-(6″-malonyl-3″-glucosyl-glucoside) | Medioresinol | 4-Vinylguaiacol | Theaflavin 3′-O-gallate |
| 5-O-Galloylquinic acid | 3,7,4′-O-triglucósido de kaempferol Kaempferol 3,7,4′-O-triglucoside | Quercetin 3-O-(6”-malonyl-glucoside) 7-O-glucoside | Dihydrocaffeic acid | 6,8-Dihydroxykaempferol | Gallic acid 3-O-gallate |
| Procyanidin trimer C1 | Feruloyltartaric acid | Caffeoyltartaric acid | 4-Vinylguaiacol | Kaempferol 3-O-(2″-rhamnosyl-6″-acetyl-galactoside) 7-O-rhamnoside | 1-Caffeoylquinic acid |
| Feruloyltartaric acid | d-Viniferin | Gallic acid 3-O-galactoside | Feruloyltartaric acid | ||
| Kaempferol 3,7,4′-O-triglucoside | p-Coumaroylglycolic acid | (+)-Catechin | |||
| (+)-Catechin | Quercetin | Acetyl eugenol | |||
| Cyanidin 3-O-glucosyl-rutinoside | |||||
| (+)-Catechin | |||||
| Acetyl eugenol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siller-Sánchez, A.; Aguilar, C.N.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Kumar Verma, D.; Aguilar-González, M. Solid-State Fermentation-Assisted Extraction of Flavonoids from Grape Pomace Using Co-Cultures. Processes 2024, 12, 2027. https://doi.org/10.3390/pr12092027
Siller-Sánchez A, Aguilar CN, Chávez-González ML, Ascacio-Valdés JA, Kumar Verma D, Aguilar-González M. Solid-State Fermentation-Assisted Extraction of Flavonoids from Grape Pomace Using Co-Cultures. Processes. 2024; 12(9):2027. https://doi.org/10.3390/pr12092027
Chicago/Turabian StyleSiller-Sánchez, Arturo, Cristóbal N. Aguilar, Mónica L. Chávez-González, Juan A. Ascacio-Valdés, Deepak Kumar Verma, and Miguel Aguilar-González. 2024. "Solid-State Fermentation-Assisted Extraction of Flavonoids from Grape Pomace Using Co-Cultures" Processes 12, no. 9: 2027. https://doi.org/10.3390/pr12092027










