The Microstructure, Mechanical Properties, and Precipitation Behavior of 1000 MPa Grade GEN3 Steel after Various Quenching Processes
Abstract
1. Introduction
2. Experimental
3. Results
3.1. Microstructure
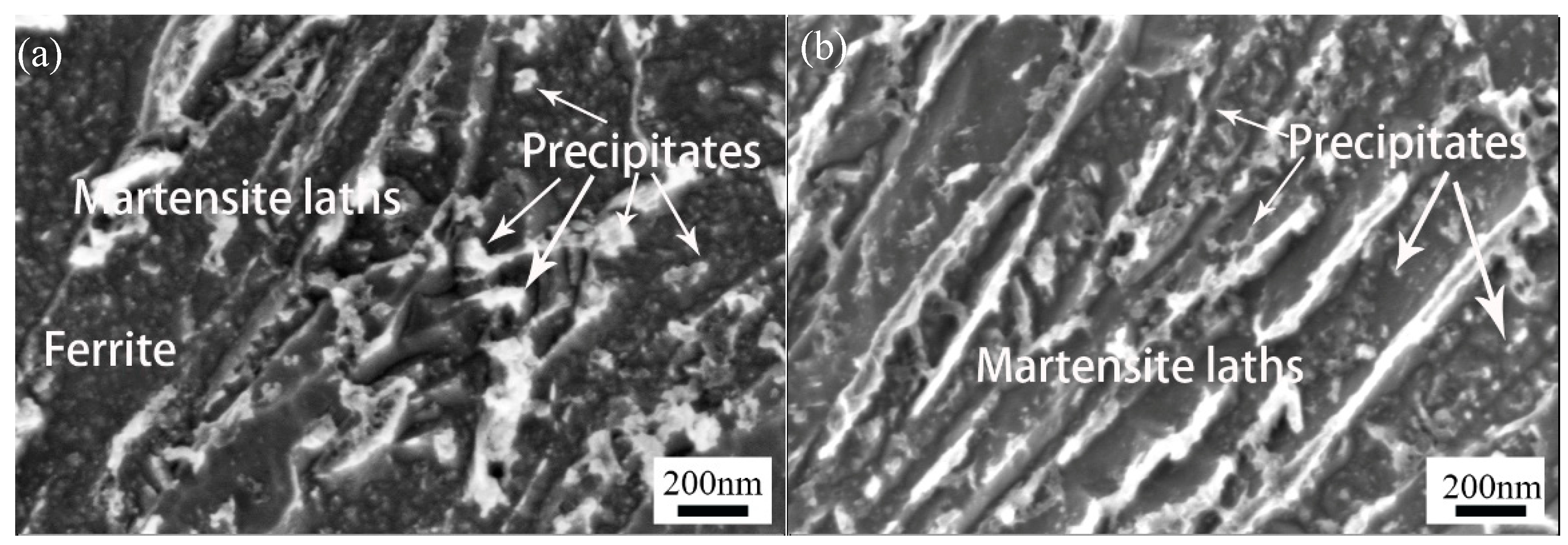
3.1.1. SEM Results
3.1.2. EBSD and XRD Results
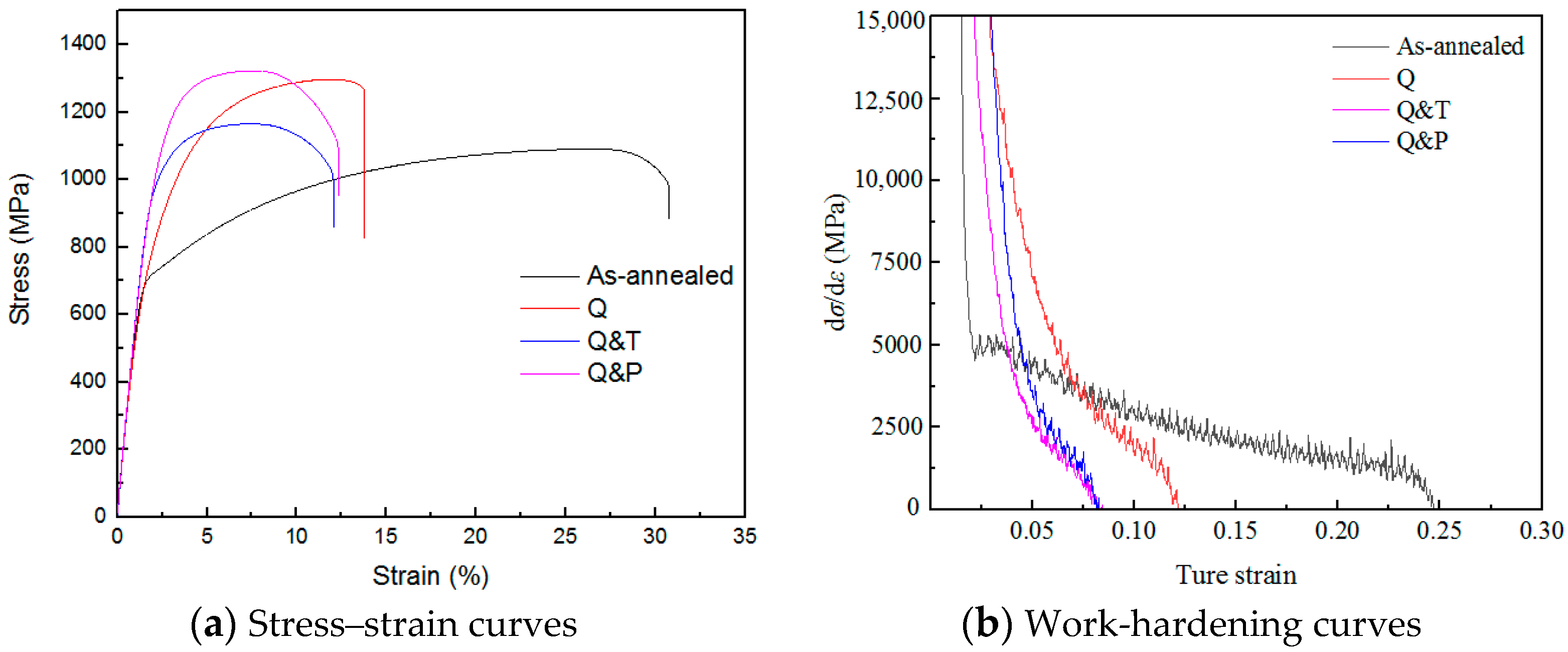
3.2. Mechanical Properties
3.3. Dislocation Density Measurement
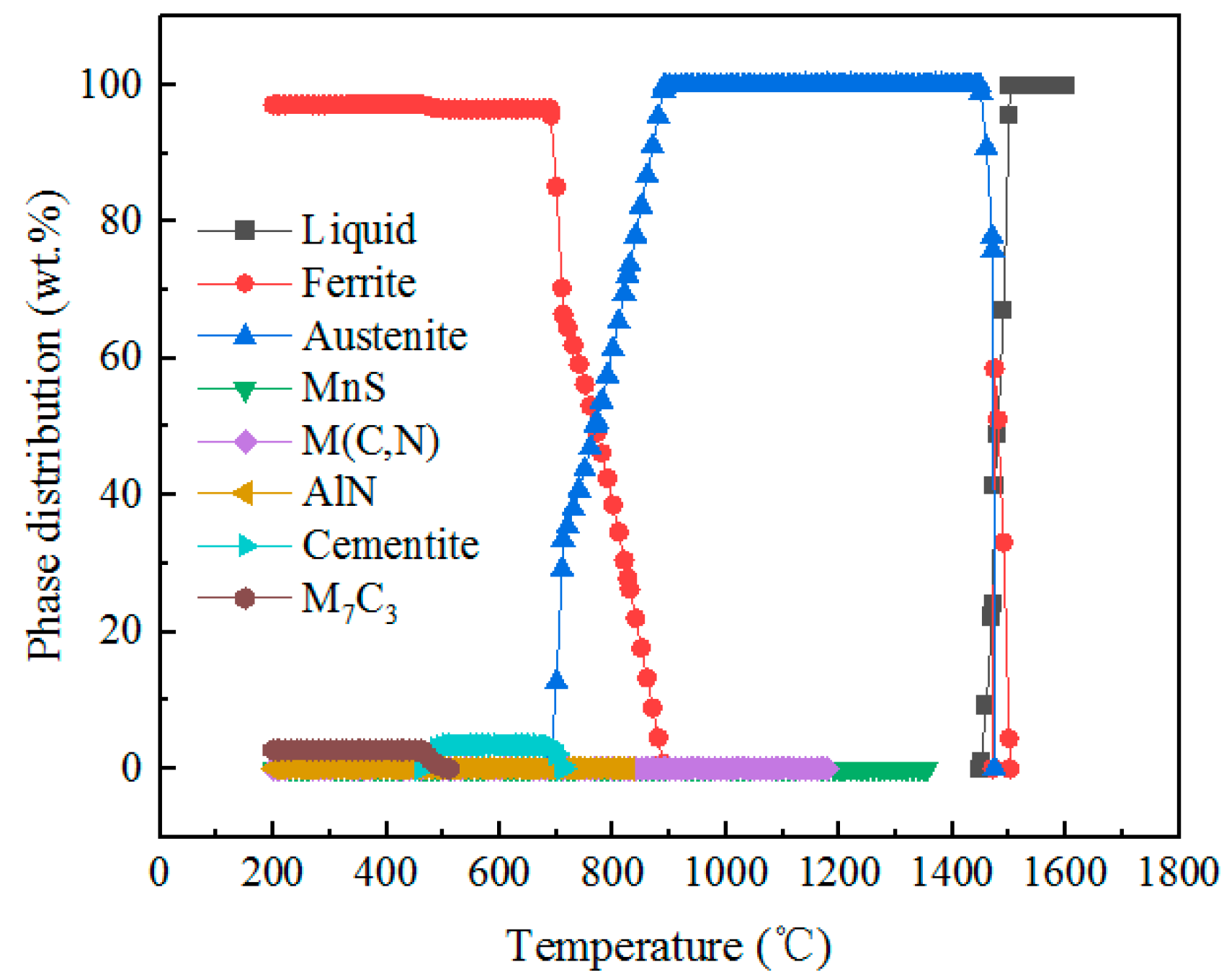
3.4. Precipitates
3.4.1. Calculation of Precipitations
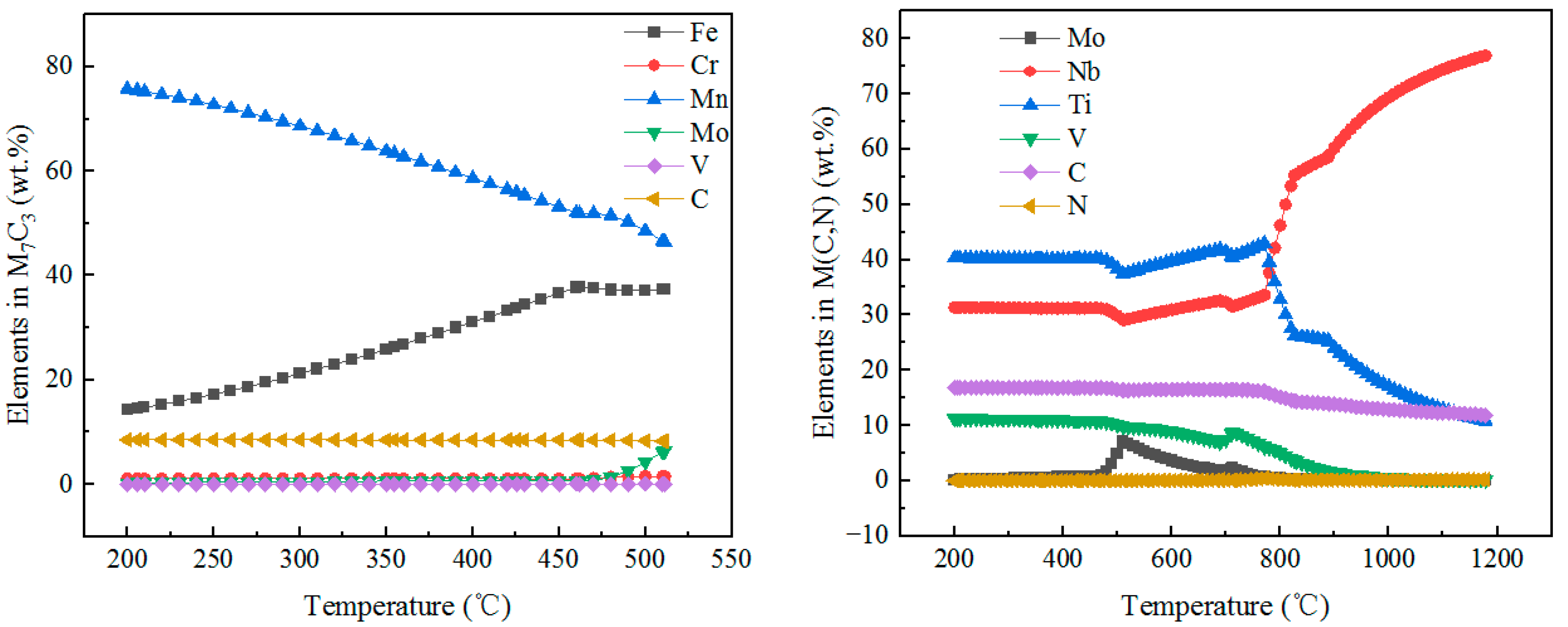
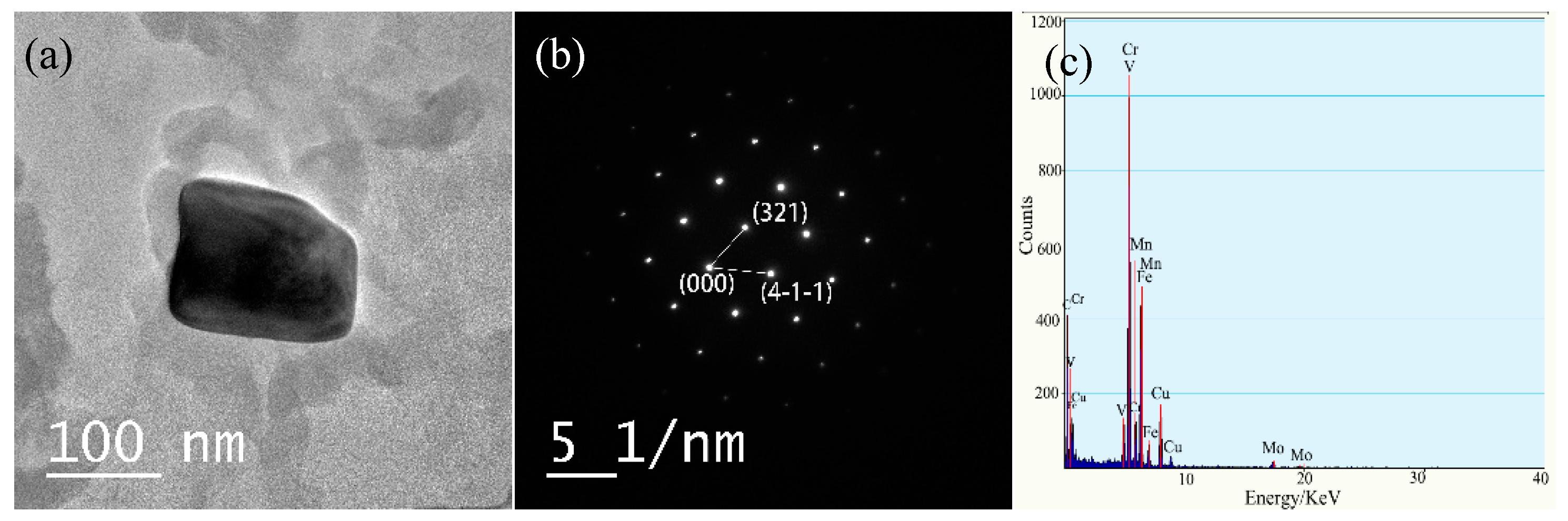
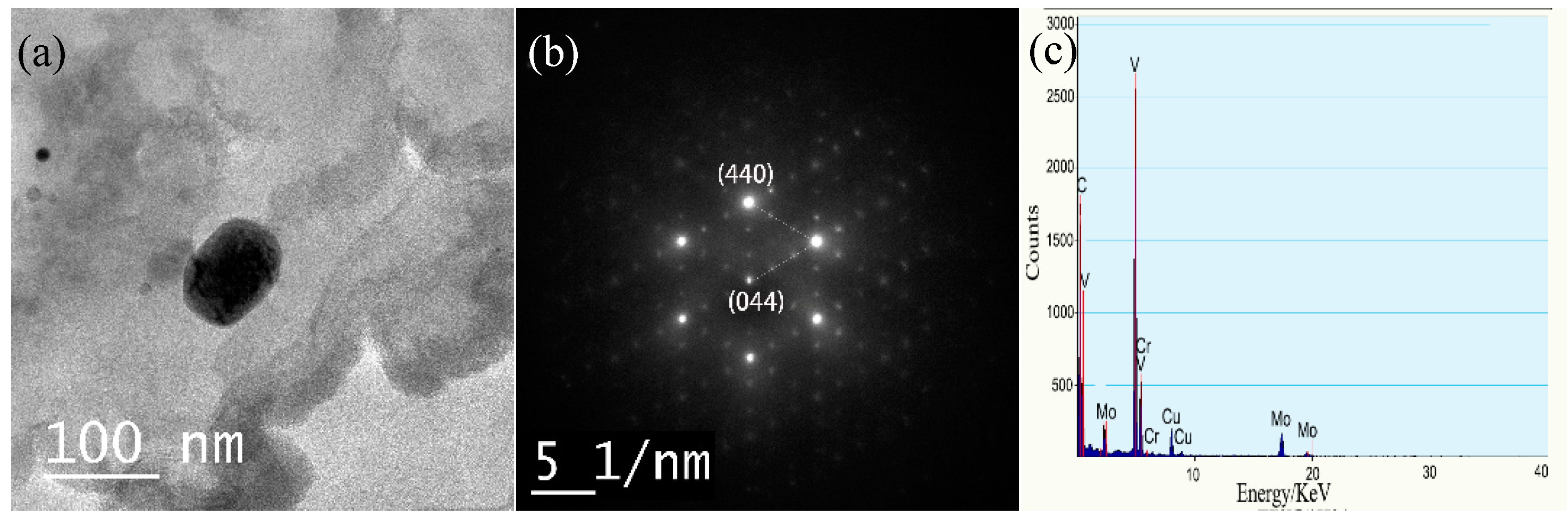
3.4.2. Types of Precipitates
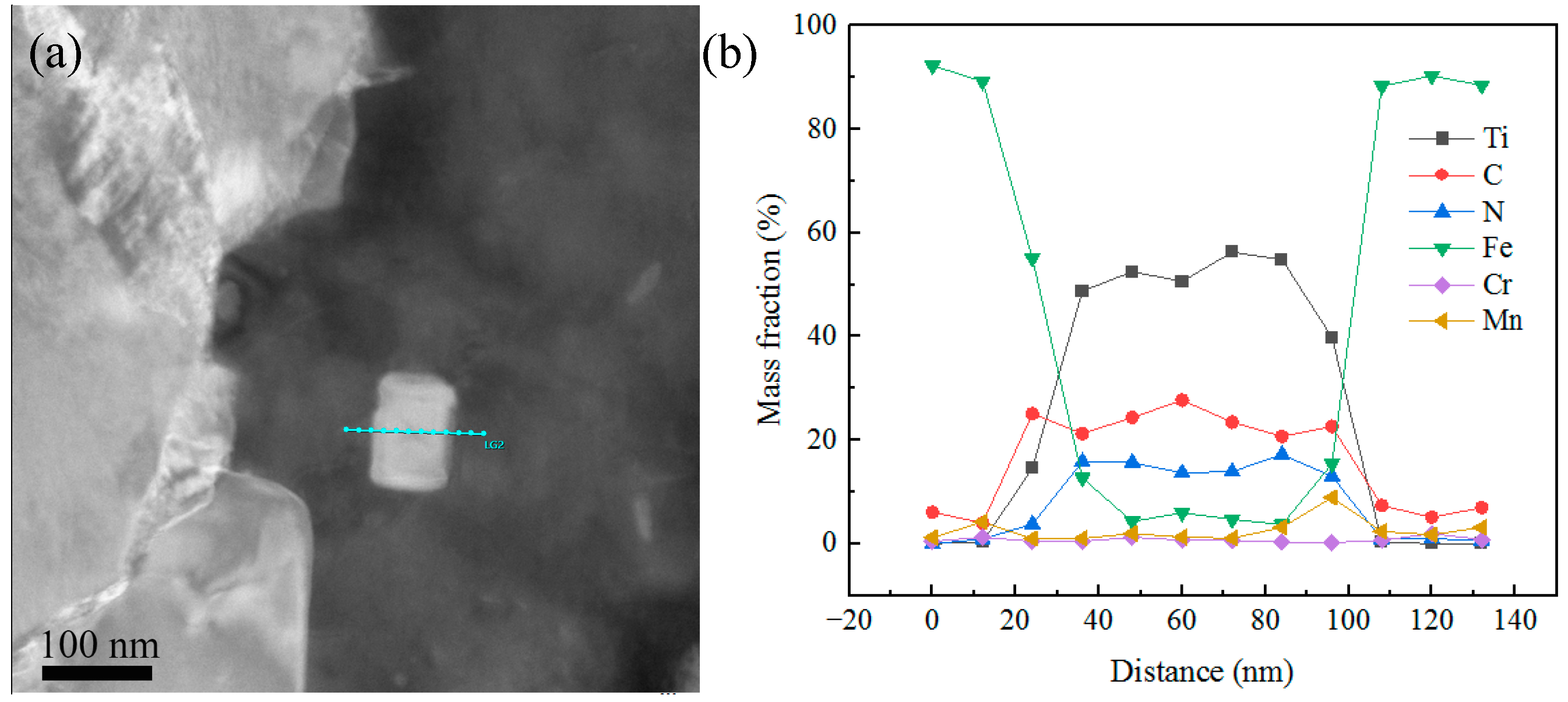
3.4.3. Distribution of Precipitates
4. Discussion
4.1. Microstructure Comparisons of Q&T and Q&P Specimens
4.2. Discussion on Strengthening Mechanism
4.2.1. Grain Boundary Strengthening
4.2.2. Dislocation Strengthening
4.2.3. Precipitation Strengthening
4.2.4. Solid Solution Strengthening
4.2.5. Comprehensive Strengthening
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varshney, A.; Sangal, S.; Kundu, S.; Mondal, K. Super strong and highly ductile low alloy multiphase steels consisting of bainite, ferrite, and retained austenite. Mater. Des. 2016, 95, 75–88. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zhao, X.M.; Zhao, X.Y.; Dong, C.Y.; Kang, S.X. Effects of nucleation site and morphology of carbide-free bainite on microstructures and properties of bainite/martensite multi-phase steels. Mater. Sci. Eng. A 2019, 744, 86–93. [Google Scholar] [CrossRef]
- Caballero, F.; Bhadeshia, H. Very strong bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251–257. [Google Scholar] [CrossRef]
- Rementeria, R.; Morales-Rivas, L.; Kuntz, M.; Garcia-Mateo, C.; Kerscher, E.; Sourmail, T.; Caballero, F. On the role of microstructure in governing the fatigue behaviour of nanostructured bainitic steels. Mater. Sci. Eng. A 2015, 630, 71–77. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F. Ultra-high-strength bainitic steels. ISIJ Int. 2005, 45, 1736–1740. [Google Scholar] [CrossRef]
- Speer, J.; Edmonds, D.; Rizzo, F.; Matlock, D. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Curr. Opin. Solid State Mater. Sci. 2004, 8, 219–237. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, H.; Gui, X.; Tan, Z.; Bai, B.; Weng, Y. Enhanced strain hardening capacity in a lean alloy steel treated by a “disturbed” bainitic austempering process. Acta Mater. 2015, 101, 31–39. [Google Scholar] [CrossRef]
- Pierman, A.P.; Bouaziz, O.; Pardoen, T.; Jacques, P.J.; Brassart, L. The influence of microstructure and composition on the plastic behaviour of dual-phase steels. Acta Mater. 2014, 73, 298–311. [Google Scholar] [CrossRef]
- Mark, A.F.; Wang, X.; Essadiqi, E.; Embury, J.D.; Boyd, J.D. Development and characterisation of model TRIP steel microstructures. Mater. Sci. Eng. A 2013, 576, 108–117. [Google Scholar] [CrossRef]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Edmonds, D.V.; He, K.; Rizzo, F.C.; De Cooman, B.C.; Matlock, D.K.; Speer, J. Quenching and partitioning martensite—A novel steel heat treatment. Mater. Sci. Eng. A 2006, 438–440, 25–34. [Google Scholar] [CrossRef]
- Tan, X.D.; Xu, Y.; Yang, X.; Wu, D. Microstructure–properties relationship in a one-step quenched and partitioned steel. Mater. Sci. Eng. A 2014, 589, 101–111. [Google Scholar] [CrossRef]
- Mori, K.; Bariani, P.F.; Behren, B.A.; Brosius, A.; Bruschi, S.; Maeno, T.; Merklein, M.; Yanagimoto, J. Hot stamping of ultra-high strength steel parts. CIRP Ann. 2017, 66, 755–777. [Google Scholar] [CrossRef]
- Leslie, W.C. The Physical Metallurgy of Steels; Hemisphere Publishing Corporation: Washington, DC, USA, 1981. [Google Scholar]
- Zhou, S.; Zhang, K.; Wang, Y.; Gu, J.F.; Rong, Y.H. High strength-elongation product of Nb-microalloyed low-carbon steel by a novel quenching-partitioning-tempering process. Mater. Sci. Eng. A 2011, 528, 8006–8012. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, K.; Chen, N.; Gu, J.; Rong, Y. Investigation on high strength hot-rolled plates by quenching-partitioning-tempering process suitable for engineering. ISIJ Int. 2022, 51, 1688–1695. [Google Scholar] [CrossRef]
- Alexander, L.; Klug, H.P. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials; John Wiley & Sons: New York, NY, USA, 1974; pp. 150–170. [Google Scholar]
- Zhao, Y.H.; Liao, X.Z.; Jin, Z.; Valiev, R.Z.; Zhu, Y.T. Microstructures and mechanical properties of ultrafine grained 7075 Al alloy processed by ECAP and their evolutions during annealing. Acta Mater. 2004, 52, 4589–4599. [Google Scholar] [CrossRef]
- Mazaheri, Y.; Kermanpur, A.; Najafizadeh, A. Strengthening mechanisms of ultrafine grained dual phase steels developed by new thermomechanical processing. ISIJ Int. 2015, 55, 218–226. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Qin, Y.C. Metal Science and Heat Treatment; Machinery Industry Press: Beijing, China, 2007. [Google Scholar]
- Pellissier, G.E.; Purdy, S.M. Stereology and Quantitative Metallography; American Society for Testing & Materials: Philadelphia, PA, USA, 1972. [Google Scholar]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Cao, J.; Yong, Q.L.; Liu, Q.; Sun, X. Precipitation of MC phase and precipitation strengthening in hot rolled Nb–Mo and Nb–Ti steels. J. Mater. Sci. 2007, 42, 10080–10084. [Google Scholar] [CrossRef]
- Hasan, H.S.; Peet, M.J.; Avettand-Fenoel, M.N.; Bhadeshia, H.K.D.H. Effect of tempering upon the tensile properties of a nanostructured bainitic steel. Mater. Sci. Eng. A 2014, 615, 340–347. [Google Scholar] [CrossRef]
- Kneissl, A.C.; Garcia, C.I.; Deardo, A.J. HSLA Steels: Processing, Properties, and Applications; Geoffrey, T., Zhang, S., Eds.; The Minerals, Metals and Materials Society: San Diego, CA, USA, 1992; pp. 99–102. [Google Scholar]
- Fu, J.; Li, G.Q.; Mao, X.P.; Fang, K.M. Nanoscale cementite precipitates and comprehensive strengthening mechanism of steel. Metall. Mater. Trans. A 2011, 42, 3797–3812. [Google Scholar] [CrossRef]
- Li, Q. Modeling the microstructure–mechanical property relationship for a 12Cr-2W-V-Mo-Ni power plant steel. Mater. Sci. Eng. A 2003, 361, 385–391. [Google Scholar] [CrossRef]
- Hutchinson, B.; Hagstrom, J.; Karlsson, O.; Lindell, D.; Tornberg, M.; Lindberg, F.; Thuvander, M. Microstructures and hardness of as-quenched martensites (0.1–0.5%C). Acta Mater. 2011, 59, 5845–5858. [Google Scholar] [CrossRef]
- Islamgaliev, R.K.; Nikitina, M.A.; Ganeev, A.V.; Sitdikov, V.D. Strengthening mechanisms in ultrafine-grained ferritic/martensitic steel produced by equal channel angular pressing. Mater. Sci. Eng. A 2019, 744, 163–170. [Google Scholar] [CrossRef]
- Charleux, M.; Poole, W.J.; Militzer, M.; Deschamps, A. Precipitation behavior and its effect on strengthening of an HSLA-Nb/Ti steel. Metall. Mater. Trans. A 2001, 32, 1635–1647. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Bourne, G.R.; Kelly, M.S.; Matthews, T.E. Mechanical properties of nanocrystalline nickel produced by electrodeposition. Nanostruct. Mater. 1999, 11, 343–350. [Google Scholar] [CrossRef]
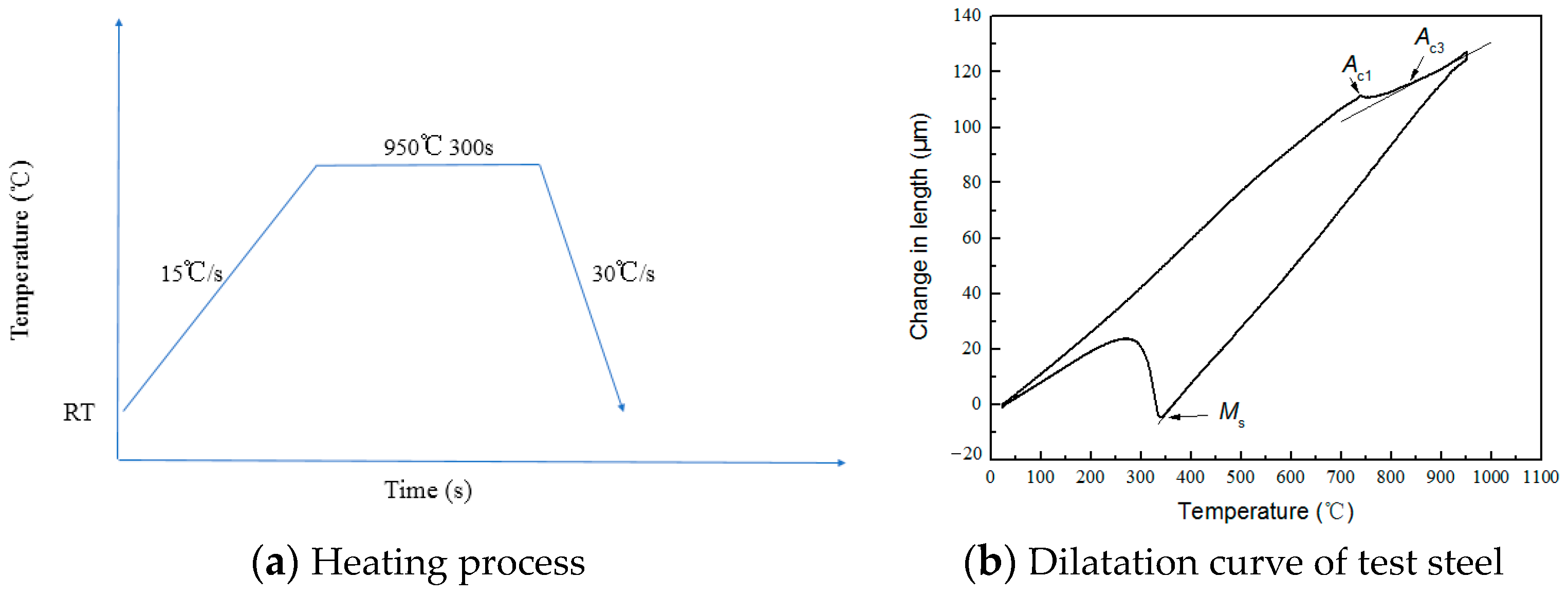
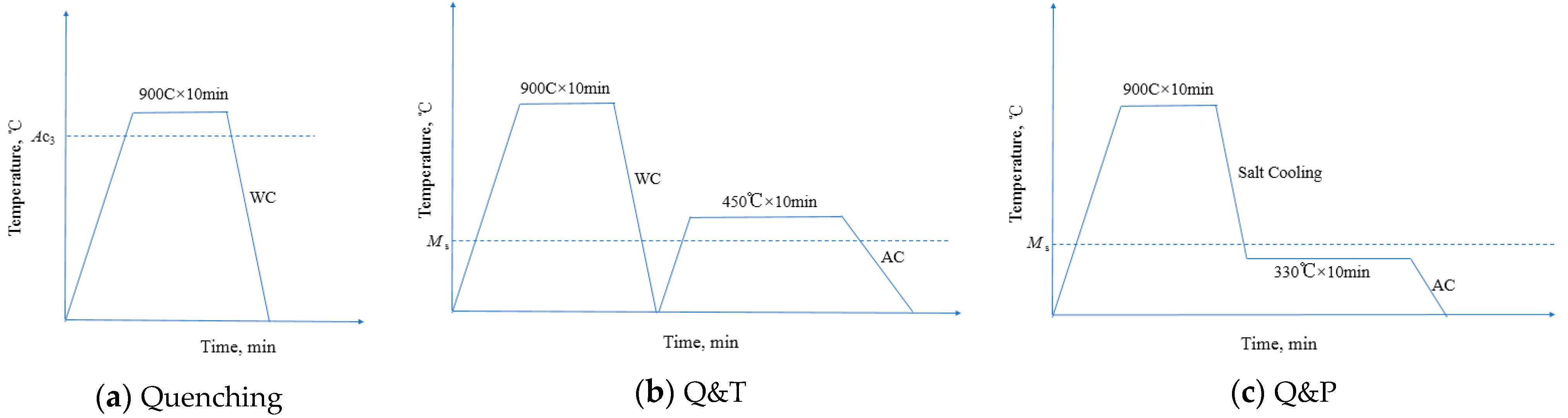
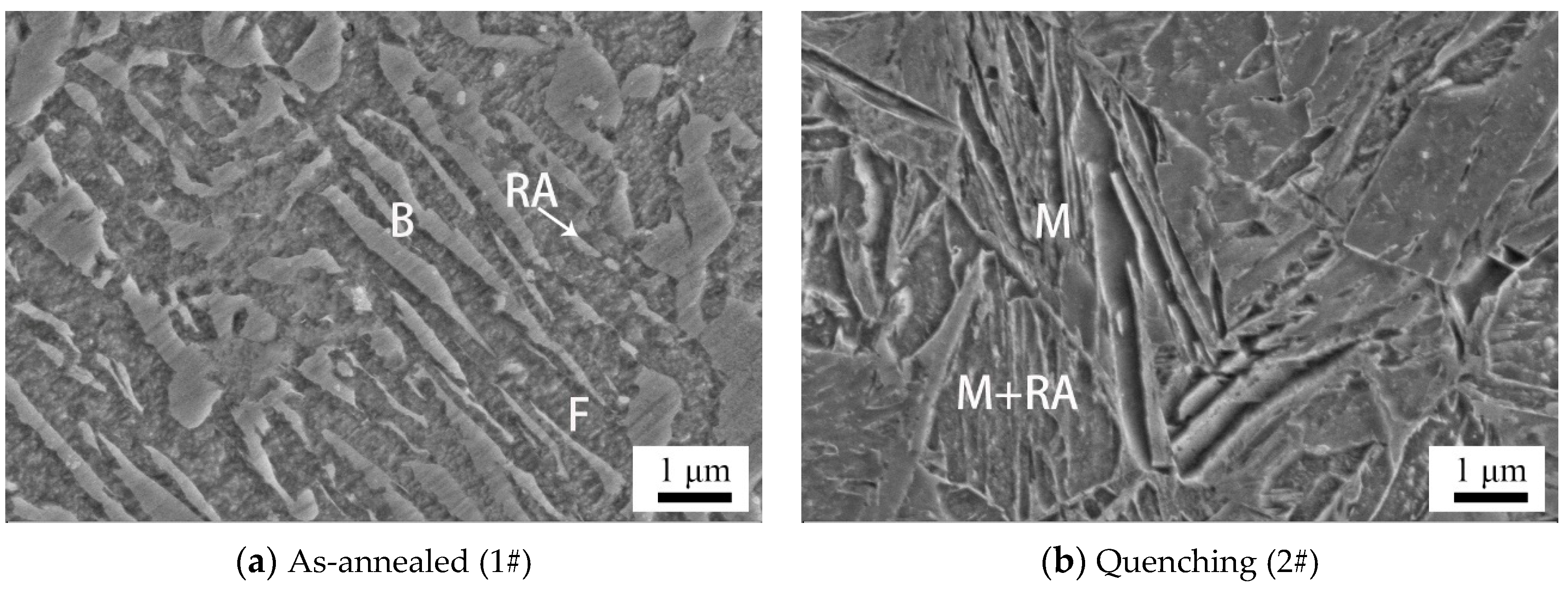

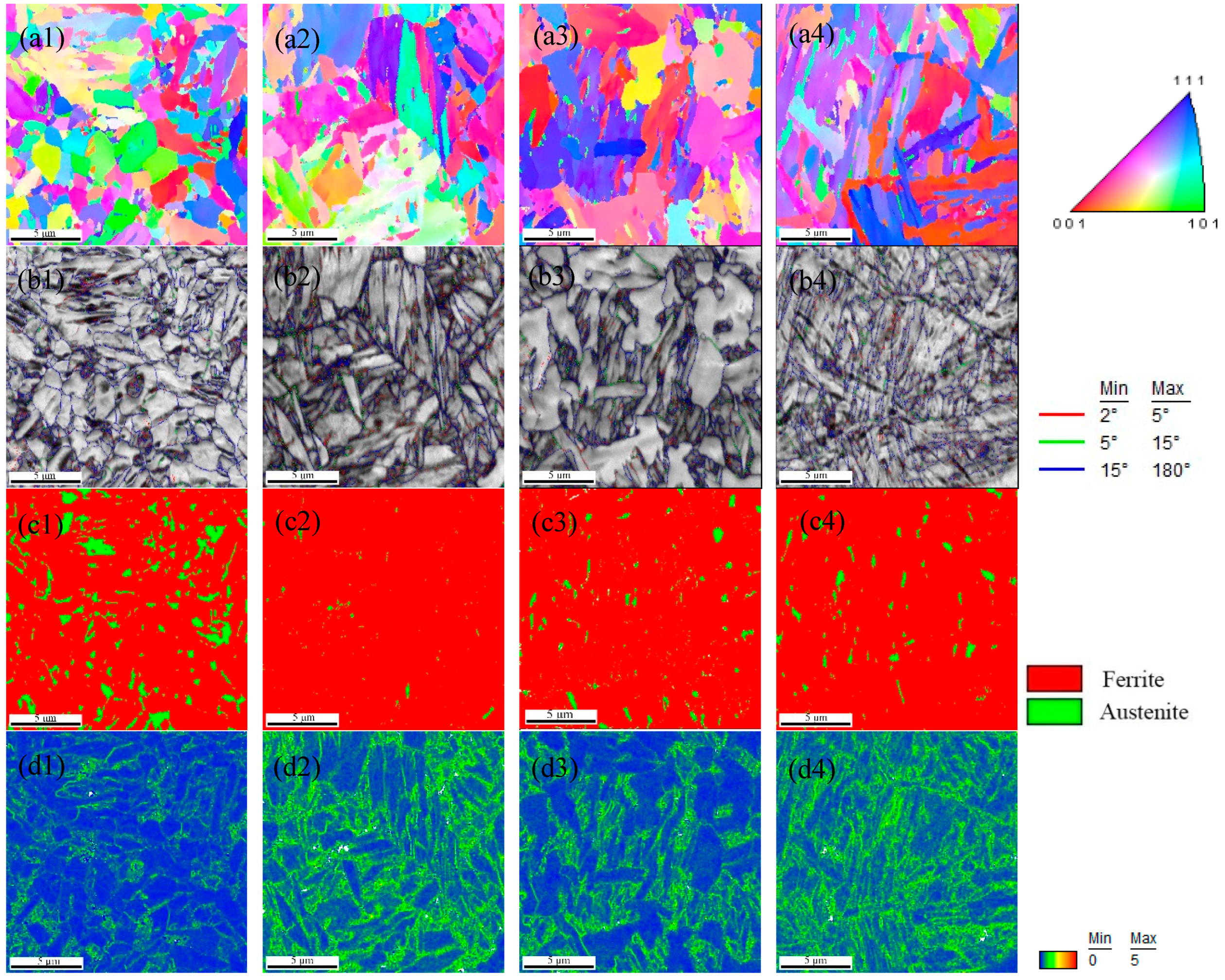

| C | Si | Mn | P | S | O | N | Al | Ni | Cr | Cu | Mo | V | Ti | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.24 | 0.62 | 2.14 | 0.014 | 0.0014 | 0.0023 | 0.0017 | 0.85 | 0.0089 | 0.0317 | 0.034 | 0.0173 | 0.005 | 0.018 | 0.014 |
| HAGBs (>15°) | LAGBs (<15°) | AGS (μm) | |
|---|---|---|---|
| As-annealed | 78.1 | 21.9 | 1.8 |
| Q | 60.9 | 39.1 | 3.5 |
| Q&T | 74.3 | 25.7 | 2.8 |
| Q&P | 73 | 27 | 2.3 |
| Volume Fraction of RA(%) | As-Annealed | Q | Q&T | Q&P |
|---|---|---|---|---|
| EBSD | 11.9 | 0.7 | 3.4 | 3.3 |
| XRD | 14.4 | 7.1 | 5.3 | 6.4 |
| Parameters | As-Annealed | Q | Q&T | Q&P |
|---|---|---|---|---|
| aγ (Å) | 3.60 ± 0.034 | 3.63 ± 0.032 | 3.62 ± 0.028 | 3.63 ± 0.044 |
| Cγ (wt.%) | 1.22 ± 0.066 | 1.82 ± 0.068 | 1.60 ± 0.080 | 1.77 ± 0.022 |
| HV0.5 | UTS (MPA) | YS (MPA) | Elongation (%) | |
|---|---|---|---|---|
| As-annealed | 299.5 ± 2.6 | 1060 ± 39 | 678 ± 23 | 26.5 ± 0.7 |
| Quenched | 569.3 ± 8.8 | 1318 ± 26 | 714 ± 21 | 12.0 ± 0.4 |
| Q&T | 439.0 ± 3.6 | 1069 ± 4 | 957 ± 19 | 11.8 ± 0.9 |
| Q&P | 499.0 ± 6.4 | 1298 ± 30 | 1010 ± 10 | 10.4 ± 1.5 |
| Sample | Average Crystallite Size, d (nm) | Lattice Microstrain, e | Dislocation Density, ρ (m−2) |
|---|---|---|---|
| 1 | 24.18 | 0.00071 | 4.12 × 1014 |
| 2 | 20.21 | 0.00093 | 6.42 × 1014 |
| 3 | 15.29 | 0.00049 | 4.43 × 1014 |
| 4 | 17.04 | 0.0014 | 1.15 × 1015 |
| Sample | Number (10 μm−2) | Volume Fraction (%) | Average Size (nm) |
|---|---|---|---|
| As-annealed | 84 ± 12 | 1.08 ± 0.03 | 21.7 ± 9.1 |
| Quenched | 75 ± 20 | 1.90 ± 0.04 | 30.4 ± 8.4 |
| Q&T | 339 ± 50 | 6.98 ± 0.04 | 27.4 ± 5.4 |
| Q&P | 330 ± 43 | 5.04 ± 0.07 | 23.6 ± 7.5 |
| Sample | |||||||
|---|---|---|---|---|---|---|---|
| As-annealed | 1.070 | 0.555 | 0.002 | 0.013 | 0.015 | 0.001 | 0.026 |
| Quenched | 2.135 | 0.618 | 0.005 | 0.017 | 0.032 | 0.009 | 0.034 |
| Q&T | 0.736 | 0.621 | 0.0002 | 0.009 | 0.005 | 0.009 | 0.034 |
| Q&P | 0.270 | 0.621 | 1.38 × 10−5 | 7.46 × 10−5 | 0.001 | 0.008 | 0.007 |
| Sample | (Calculation) | (Experiment) | |||||
|---|---|---|---|---|---|---|---|
| As-annealed | 54 | 179 | 157 | 149 | 197 | 736 | 678 ± 23 |
| Quenched | 54 | 219 | 112 | 186 | 203 | 774 | 714 ± 21 |
| Q&T | 54 | 175 | 125 | 155 | 421 | 930 | 957 ± 19 |
| Q&P | 54 | 157 | 138 | 249 | 400 | 998 | 1010 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, A.; Gao, R.; Yue, S.; Skszek, T. The Microstructure, Mechanical Properties, and Precipitation Behavior of 1000 MPa Grade GEN3 Steel after Various Quenching Processes. Processes 2024, 12, 2039. https://doi.org/10.3390/pr12092039
Ning A, Gao R, Yue S, Skszek T. The Microstructure, Mechanical Properties, and Precipitation Behavior of 1000 MPa Grade GEN3 Steel after Various Quenching Processes. Processes. 2024; 12(9):2039. https://doi.org/10.3390/pr12092039
Chicago/Turabian StyleNing, Angang, Rui Gao, Stephen Yue, and Timothy Skszek. 2024. "The Microstructure, Mechanical Properties, and Precipitation Behavior of 1000 MPa Grade GEN3 Steel after Various Quenching Processes" Processes 12, no. 9: 2039. https://doi.org/10.3390/pr12092039
APA StyleNing, A., Gao, R., Yue, S., & Skszek, T. (2024). The Microstructure, Mechanical Properties, and Precipitation Behavior of 1000 MPa Grade GEN3 Steel after Various Quenching Processes. Processes, 12(9), 2039. https://doi.org/10.3390/pr12092039







