Cloud Point Extraction as an Environmentally Friendly Technique for Sample Preparation
Abstract
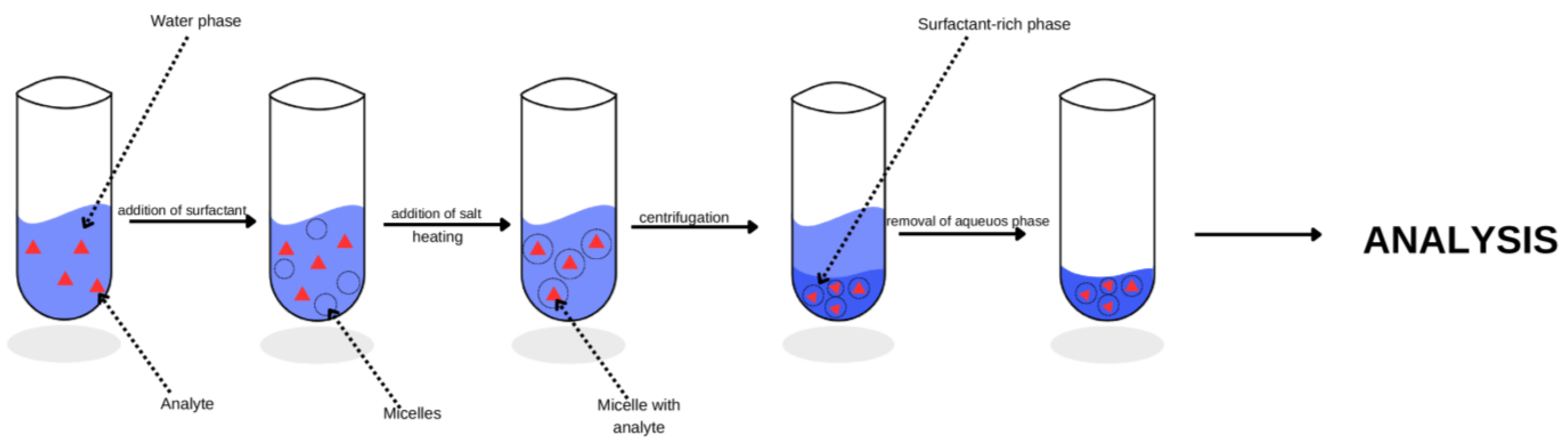
:1. Introduction
2. CPE as Liquid–Liquid Extraction Modification
3. CPE Mechanism
4. Surfactant Types and Classifications
| Surfactant | CMC [mM] | CPT [°C] |
|---|---|---|
| Brij-35 | 0.09 | >100 |
| Brij-58 | 0.077 | >100 |
| CTAB | 0.9–1.0 | 24.5 * |
| Genapol-X080 | 0.06–0.15 | >45 |
| SDS | 8.14 | >100 |
| Tergitol 15-S-7 | 0.074 | 37 |
| Triton X-100 | 0.2–0.9 | 65 |
| Triton X-114 | 0.2 | 23 |
| Tween 80 | 0.015 | 65 |
5. CPE Optimization
5.1. Effect of Surfactant Concentration
5.2. pH of the Solution—The Impact of Acidic Conditions
5.3. Temperature
5.4. Salting out Effect
6. The Application of CPE
6.1. Detection Techniques Versus CPE
6.2. Determination of Xenobiotics in Biological Matrices
6.3. Application of CPE in Environmental Samples
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | Caffeic acid |
| CLA | Chlorogenic acid |
| CMC | Critical micelle concentration |
| CPE | Cloud point extraction |
| CPT | Cloud point temperature |
| CTAB | Cetyltrimethylammonium bromide |
| DNJ | 1-deoxynojirimycin |
| ESI | Electrospray ionisation |
| ETAAS | Electrothermal atomic absorption spectrometry |
| FA | Ferulic acid |
| FAAS | Flame atomic absorption spectrometry |
| FLD | Fluorescence detector |
| GC | Gas chromatography |
| HPLC | High performance liquid chromatography |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| ICP-OES | Inductively coupled plasma–optical emission spectrometry |
| LC-ESI-MS/MS | Liquid chromatography–electrospray ionization–tandem mass spectrometry |
| LLOQ | Lower limit of quantitation |
| LLE | Liquid–liquid extraction |
| LOD | Limit of detection |
| LOQ | Limit of quantitation |
| SDS | Sodium dodecyl sulphate |
| TX-114 | Triton X-114 |
References
- Watanabe, H.; Tanaka, H. A Non-Ionic Surfactant as a New Solvent for Liquid—Liquid Extraction of Zinc(II) with 1-(2-Pyridylazo)-2-Naphthol. Talanta 1978, 25, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Kori, S. Cloud Point Extraction Coupled with Back Extraction: A Green Methodology in Analytical Chemistry. Forensic Sci. Res. 2021, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Snigur, D.; Azooz, E.A.; Zhukovetska, O.; Guzenko, O.; Mortada, W. Recent Innovations in Cloud Point Extraction towards a More Efficient and Environmentally Friendly Procedure. TrAC Trends Anal. Chem. 2023, 164, 117113. [Google Scholar] [CrossRef]
- Kojro, G.; Wroczyński, P. Cloud Point Extraction in the Determination of Drugs in Biological Matrices. J. Chromatogr. Sci. 2020, 58, 151–162. [Google Scholar] [CrossRef]
- Casero, I.; Sicilia, D.; Rubio, S.; Pérez-Bendito, D. An Acid-Induced Phase Cloud Point Separation Approach Using Anionic Surfactants for the Extraction and Preconcentration of Organic Compounds. Anal. Chem. 1999, 71, 4519–4526. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Y.; Liu, M. Vortex-Assisted Acid-Induced Cloud Point Extraction Coupled With Spectrofluorometry for the Determination of Fluoroquinolones in Environmental Water Samples. Spectrosc. Lett. 2014, 47, 206–213. [Google Scholar] [CrossRef]
- Madej, K. Microwave-Assisted and Cloud-Point Extraction in Determination of Drugs and Other Bioactive Compounds. Trends Anal. Chem.—TrAC 2009, 28, 436–446. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Kojro, G.; Buś-Kwaśnik, K.; Rudzki, P.J.; Marszałek, R.; Leś, A.; Wroczyński, P. Cloud-Point Extraction Is Compatible with Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometry for the Determination of Bisoprolol in Human Plasma. J. Chromatogr. A 2015, 1423, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gniazdowska, E.; Korytowska, N.; Kłudka, G.; Giebułtowicz, J. Determination of Antidepressants in Human Plasma by Modified Cloud-Point Extraction Coupled with Mass Spectrometry. Pharmaceuticals 2020, 13, 458. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Meng, J.; Li, X.Y.; Zhou, J.; Sun, X.L.; Wen, A.D. Determination of Venlafaxine in Human Plasma by High-Performance Liquid Chromatography Using Cloud-Point Extraction and Spectrofluorimetric Detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 872, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Paleologos, E.K.; Giokas, D.L.; Karayannis, M.I. Micelle-Mediated Separation and Cloud-Point Extraction. TrAC Trends Anal. Chem. 2005, 24, 426–436. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, L.; Lv, R.; Wang, H.; Song, S.; Cao, M. Facile Cloud Point Extraction for the Separation and Determination of Phenolic Acids from Dandelion. ACS Omega 2021, 6, 13508–13515. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Jiang, Y.-W.; Kou, P.; Liu, Z.-M.; Efferth, T.; Li, Y.-Y.; Fu, Y.-J. Application of Integrative Cloud Point Extraction and Concentration for the Analysis of Polyphenols and Alkaloids in Mulberry Leaves. J. Pharm. Biomed. Anal. 2019, 167, 132–139. [Google Scholar] [CrossRef]
- Sznek, B.; Czyrski, A. The Application of Design of Experiments and Artificial Neural Networks in the Evaluation of the Impact of Acidic Conditions on Cloud Point Extraction. J. Chromatogr. A 2025, 1743, 465686. [Google Scholar] [CrossRef]
- Hammad, S.F.; Abdallah, I.A.; Bedair, A.; Mansour, F.R. Homogeneous Liquid-Liquid Extraction as an Alternative Sample Preparation Technique for Biomedical Analysis. J. Sep. Sci. 2022, 45, 185–209. [Google Scholar] [CrossRef]
- Silvestre, C.I.C.; Santos, J.L.M.; Lima, J.L.F.C.; Zagatto, E.A.G. Liquid-Liquid Extraction in Flow Analysis: A Critical Review. Anal. Chim. Acta 2009, 652, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef]
- Hagarová, I.; Urík, M. Cloud Point Extraction in Beverage Analysis: Innovations and Applications for Trace Elements. Beverages 2024, 10, 67. [Google Scholar] [CrossRef]
- Halko, R.; Hagarová, I.; Andruch, V. Innovative Approaches in Cloud-Point Extraction. J. Chromatogr. A 2023, 1701, 464053. [Google Scholar] [CrossRef]
- Mortada, W.I. Recent Developments and Applications of Cloud Point Extraction: A Critical Review. Microchem. J. 2020, 157, 105055. [Google Scholar] [CrossRef]
- Hagarová, I.; Nemček, L. Reliable Quantification of Ultratrace Selenium in Food, Beverages, and Water Samples by Cloud Point Extraction and Spectrometric Analysis. Nutrients 2022, 14, 3530. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.; Mandal, A.B. The Clouding Phenomena of Mixed Surfactant (Non-Ionic Triton X-114 + Cationic Gemini 16-5-16) Solutions: Influence of Inorganic and Organic Additives on the Cloud Point. J. Mol. Liq. 2015, 212, 237–244. [Google Scholar] [CrossRef]
- Manzoori, J.; Abdolmohammad-Zadeh, H. Extraction and Preconcentration of Lead Using Cloud Point Methodology: Application to Its Determination in Real Samples by Flame Atomic Absorption Spectrometry. Acta Chim. Slov. 2007, 54, 378–384. [Google Scholar]
- Mudawadkar, A.D.; Sonawane, G.H.; Patil, T.J. Micellization of Anionic Surfactant-Sodium Dodecyl Sulfate in Presence of Additive Urea and Acetamide in Aqueous Medium Using Clouding Phenomenon. J. Chem. Pharm. Res. 2015, 7, 331–338. [Google Scholar]
- Kumar, S.; Sharma, D.; Khan, Z.A. Kabir-ud-Din Occurrence of Cloud Points in Sodium Dodecyl Sulfate−Tetra-n-Butylammonium Bromide System. Langmuir 2001, 17, 5813–5816. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, D.; Khan, Z.A. Kabir-ud-Din Salt−Induced Cloud Point in Anionic Surfactant Solutions: Role of the Headgroup and Additives. Langmuir 2002, 18, 4205–4209. [Google Scholar] [CrossRef]
- Singh, V.; Tyagi, R. Investigations of Mixed Surfactant Systems of Lauryl Alcohol Based Bissulfosuccinate Anionic Gemini Surfactant with Conventional Surfactants: A Fluorometric Study. J. Taibah Univ. Sci. 2015, 9, 477–489. [Google Scholar] [CrossRef]
- Pytlakowska, K.; Kozik, V.; Dabioch, M. Complex-Forming Organic Ligands in Cloud-Point Extraction of Metal Ions: A Review. Talanta 2013, 110, 202–228. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Kojro, G.; Piotrowski, R.; Kułakowski, P.; Wroczyński, P. Cloud-point extraction is compatible with liquid chromatography coupled to electrospray ionization mass spectrometry for determination of antazoline in human plasma. J. Pharm. Biomed. Anal. 2016, 128, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.N.; Nies, E. Effect of End Groups on the Cloud Point Temperature of Aqueous Solutions of Thermoresponsive Polymers: An Inside View by Flory–Huggins Theory. Polymers 2024, 16, 563. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S. The Effect of Surfactant Concentration, Salinity, Temperature, and pH on Surfactant Adsorption for Chemical Enhanced Oil Recovery: A Review. J. Pet. Explor. Prod. Technol. 2020, 10, 125–137. [Google Scholar] [CrossRef]
- Sicilia, D.; Rubio, S.; Pérez-Bendito, D. Evaluation of the Factors Affecting Extraction of Organic Compounds Based on the Acid-Induced Phase Cloud Point Approach. Anal. Chim. Acta 2002, 460, 13–22. [Google Scholar] [CrossRef]
- Sicilia, D.; Rubio, S.; Pérez-Bendito, D.; Maniasso, N.; Zagatto, E.A.G. Anionic Surfactants in Acid Media: A New Cloud Point Extraction Approach for the Determination of Polycyclic Aromatic Hydrocarbons in Environmental Samples. Anal. Chim. Acta 1999, 392, 29–38. [Google Scholar] [CrossRef]
- Herrmann, K.W. Micellar Properties of Some Zwitterionic Surfactants. J. Colloid Interface Sci. 1966, 22, 352–359. [Google Scholar] [CrossRef]
- Michałowska, A.; Kupczyk, O.; Czyrski, A. The Chemometric Evaluation of the Factors Influencing Cloud Point Extraction for Fluoroquinolones. Pharmaceutics 2023, 15, 1774. [Google Scholar] [CrossRef]
- Czyrski, A.; Jarzębski, H. Response Surface Methodology as a Useful Tool for Evaluation of the Recovery of the Fluoroquinolones from Plasma—The Study on Applicability of Box-Behnken Design, Central Composite Design and Doehlert Design. Processes 2020, 8, 473. [Google Scholar] [CrossRef]
- Nekouei, S.; Nekouei, F. Cloud Point Extraction and Spectrophotometric Determination of Arsenic (III) Using Amaranth as an Extraction Agent in Water Samples. Orient. J. Chem. 2014, 30, 873–878. [Google Scholar] [CrossRef]
- Mortada, W.I.; Awad, A.A.; El-Defrawy, M.M.; Khalifa, M.E. Air-Assisted Cloud Point Extraction Coupled with Inductively Coupled Plasma Optical Emission Spectroscopy for Determination of Samarium in Environmental Samples. Anal. Sci. 2022, 38, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Hadri, H.E.; Hackley, V.A. Investigation of Cloud Point Extraction for the Analysis of Metallic Nanoparticles in a Soil Matrix. Environ. Sci. Nano 2017, 4, 105–116. [Google Scholar] [CrossRef]
- Yang, R.; Li, Q.; Zhou, W.; Yu, S.; Liu, J. Speciation Analysis of Selenium Nanoparticles and Inorganic Selenium Species by Dual-Cloud Point Extraction and ICP-MS Determination. Anal. Chem. 2022, 94, 16328–16336. [Google Scholar] [CrossRef] [PubMed]
- Blanchet-Chouinard, G.; Larivière, D. Determination of Polonium-210 in Environmental Samples Using Diglycolamide-Based Cloud Point Extraction Coupled to Alpha Spectrometry Analysis. Appl. Radiat. Isot. 2021, 168, 109549. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, A.; Ogiwara, M.; Ikeda, R.; Okada, H.; Ohashi, K. Cloud Point Extraction and Preconcentration for the Gas Chromatography of Phenothiazine Tranquilizers in Spiked Human Serum. Anal. Sci. 2004, 20, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Kartal Temel, N.; Gürkan, R. An Indirect Method for the Analysis of Bisphenol A, as a Mn(III)-Chelate Complex, in Milk Samples by Ultrasound Assisted-Cloud Point Extraction/Flame Atomic Absorption Spectrometry. Anal. Methods 2022, 14, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- Llaver, M.; Wuilloud, R.G. Studying the Effect of an Ionic Liquid on Cloud Point Extraction Technique for Highly Efficient Preconcentration and Speciation Analysis of Tellurium in Water, Soil and Sediment Samples. Talanta 2020, 212, 120802. [Google Scholar] [CrossRef] [PubMed]
- Filik, H.; Sener, I.; Cekiç, S.D.; Kiliç, E.; Apak, R. Spectrophotometric Determination of Paracetamol in Urine with Tetrahydroxycalix[4]Arene as a Coupling Reagent and Preconcentration with Triton X-114 Using Cloud Point Extraction. Chem. Pharm. Bull. 2006, 54, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Taha, S.S.; Ali, D.S.; Hassan, R.O.; Omar Othman, H.; Smaoui, S. Colorimetric and Fluorescent Dual Mode Detection of Fe (III) Ion in Blood Samples in Combination with Cloud Point Extraction. Microchem. J. 2023, 195, 109390. [Google Scholar] [CrossRef]
- Taha, S.S.; Ali, D.S. Simple Cloud Point Microextraction Based on Indophenol Dye Formation for Mesalazine Determination in Pharmaceutical and Biological Samples. Microchem. J. 2023, 191, 108862. [Google Scholar] [CrossRef]
- Rihana-Abdallah, A.; Li, Z.; Lanigan, K. Using Cloud Point Extraction for Preconcentration and Determination of Iron, Lead, and Cadmium in Drinking Water by Flame Atomic Absorption Spectrometry. Anal. Lett. 2021, 55, 1296–1305. [Google Scholar] [CrossRef]
- Wei, W.-J.; Yang, Y.; Li, X.-Y.; Huang, P.; Wang, Q.; Yang, P.-J. Cloud Point Extraction (CPE) Combined with Single Particle -Inductively Coupled Plasma-Mass Spectrometry (SP-ICP-MS) to Analyze and Characterize Nano-Silver Sulfide in Water Environment. Talanta 2022, 239, 123117. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Dong, L.; Li, Q.; Li, P.; Hao, Z.; Yu, S.; Liu, J. Counting Nanoplastics in Environmental Waters by Single Particle Inductively Coupled Plasma Mass Spectroscopy after Cloud-Point Extraction and In Situ Labeling of Gold Nanoparticles. Environ. Sci. Technol. 2021, 55, 4783–4791. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Karabelas, A.J. Lycopene Recovery from Tomato Peel under Mild Conditions Assisted by Enzymatic Pre-Treatment and Non-Ionic Surfactants. Acta Biochim. Pol. 2012, 59, 71–74. [Google Scholar] [CrossRef]
- Katsoyannos, E.; Gortzi, O.; Chatzilazarou, A.; Athanasiadis, V.; Tsaknis, J.; Lalas, S. Evaluation of the Suitability of Low Hazard Surfactants for the Separation of Phenols and Carotenoids from Red-Flesh Orange Juice and Olive Mill Wastewater Using Cloud Point Extraction. J. Sep. Sci. 2012, 35, 2665–2670. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Cloud Point Extraction of Phenolic Compounds from Pretreated Olive Mill Wastewater. J. Environ. Chem. Eng. 2014, 2, 1480–1486. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Honarvar, M.; Zarei, A.R.; Mashhadi Akbar Boojar, M.; Bakhoda, H. A New Approach for Separation and Recovery of Betaine from Beet Molasses Based on Cloud Point Extraction Technique. J. Food Sci. Technol. 2018, 55, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Jain, A.; De, S. Effect of Different Operating Conditions in Cloud Point Assisted Extraction of Thymol from Ajwain (Trachyspermum ammi L.) Seeds and Recovery Using Solvent. J. Food Sci. Technol. 2017, 54, 4353–4361. [Google Scholar] [CrossRef]
- Chatzilazarou, A.; Katsoyannos, E.; Gortzi, O.; Lalas, S.; Paraskevopoulos, Y.; Dourtoglou, E.; Tsaknis, J. Removal of Polyphenols from Wine Sludge Using Cloud Point Extraction. J. Air Waste Manag. Assoc. 2010, 60, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Świątek, S.; Czyrski, A. Analytical Methods for Determining Psychoactive Substances in Various Matrices: A Review. Crit. Rev. Anal. Chem. 2024. [Google Scholar] [CrossRef] [PubMed]

| Substance Determined | Type of Sample | Detection Technique | Analysis Conditions | LOD/LOQ | Reference |
|---|---|---|---|---|---|
| Sm | Rock samples, wastewater | ICP-OES | Rf generator power—1200 W Plasma gas flow rate—12 L/min Auxiliary gas flow rate—1.0 L/min Nebulizer gas flow rate — 0.7 L/min Delay time—15 s Integration time—3 s Wavelength—358.160 nm. | LOD 0.06 μg/L, LLOQ 0.20 μg/L | [38] |
| Pb2+ | Tap water, rain water, waste water, urine, liver, hair | FAAS | Pb2+ determined as complex with 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone, radiation at λ = 283.3 nm. | LOD 1.49 μg/L | [23] |
| Ferulic acid (FA), chlorogenic acid (CLA), caffeic acid (CA) | Herb of dandelion | HPLC-DAD | C18 column (4.6 mm × 150 mm, 5 µm) at 30 °C. Mobile phase: MeOH:ACN:1% acetic acid (12.5:12.5:75, v/v/v), flow: 0.8 mL/min, detection at λ = 320 nm. | CLA: LOD 0.008 mg/L, CA: LOD 0.005 mg/L, FA: LOD 0.007 mg/L | [12] |
| 1-deoxynojirimycin (DNJ), rutin, isoquercitrin, CLA, astragalin | Mulberry leaves | HPLC-UV | CuroSil-PFP RP column (250 mm × 4.6 mm, 5 µm), colum temperature 30 °C. Mobile phase: gradient elution: eluent A: 0.1% aqueous formic acid, eluent B: acetonitrile. For DNJ: acetonitrile: 0.1% aqueous acetic acid (45:55, v/v). Detection at λ = 360 nm, and λ = 254 nm (for DNJ); Flow rate: 1 mL/min. | LOD < 0.58 μg/mL, LOQ < 1.87 μg/mL | [13] |
| Au | Agricultural soil | ICP-MS | Au was collected at m/z 197. Pt m/z 195 was internal standard. Dwell time 10 ms. | LLOQ (calibration curve): 0.05 µg/kg | [39] |
| Amitriptyline, citalopram, clomipramine, desipramine, doxepin, fluoxetine, fluvoxamine, imipramine, maprotline, mianserin, mirtazapine, moclobemide, nortriptyline, opipramol, paroxetine, protriptyline, sertraline, tianeptine, trazodone, trimipramine, venlafaxine | Human plasma | HPLC-ESI-MS/MS | Kinetex C18 (100 mm × 4.6 mm, 5 µm); Mobile phase: gradient elution: Eluent A: 0.1% (v/v) solution of formic acid, Eluent B: methanol with 0.1% (v/v) formic acid. Flow rate—0.75 mL/min. | LLOQ 10 ng/mL | [9] |
| Venlafaxine | Human plasma | RP—HPLC-FLD | Diamonsil C18 RP (250 mm × 4.6 mm, 5 µm) at 25 °C; Mobile phase—acetonitrile:phosphate buffer solution (pH 3.0):triethylamine (33.5:66.5:0.4) λex = 276 nm, λem = 596 nm. Flow rate: 1 mL/min. | LOD 2 ng/mL, LOQ 10 ng/mL | [10] |
| Bisoprolol | Human plasma | LC- ESI-MS/MS | Symmetry C18 column (150 mm × 4.6 mm, 3.5 µm) at 40 °C; Mobile phase—gradient elution: eluent A: 0.1% formic acid in grade water, eluent B methanol with 0.1% formic acid. Flow rate: 0.5 mL/min. | LLOQ 0.3 ng/mL | [8] |
| Vitamin E | Aqueous solution | HPLC-DAD | Waters Nova-Pack column C18 (150 mm × 3.9 mm) Mobile phase: 100% acetonitrile at λ = 220 nm. Flow rate: 1 mL/min. | LLOQ 0.1 µg/mL | [5] |
| Pericyazine Chlorpromazine Fluphenazine | Human serum | GC | Non-polar fused-silica wide-bore capillary column (15 m × 0.53 mm, film thickness 1.5 μm; column temperature: 280 °C, injector and detector temperature: 290 °C. | LOD 1.5 × 10−6 mol/L | [42] |
| Paracetamol | Human urine | Spectrophotometry | λ = 580 nm (for direct spectorphotomeric determination); λ = 590 nm (for preconcentration procedure). | LOQ 0.13 μg/mL | [45] |
| Norfloxacin, ciprofloxacin, sarafloxacin, gatifloxacin | Water (lake, river, wastewater) | HPLC-DAD Fluorescence analysis | Chromatographic analysis: Agilent TC-C18 column (150 mm × 4.6 mm, 5 µm). Fluorescent spectra: the wavelengths for excitation/emission were (λexc/λem): 276/444 nm for norfloxacin, 278/449 nm for ciprofloxacin, 291/496 nm for sarafloxacin, and 288/486 nm for gatifloxacin. | LOD 0.007 µg/mL (norfloxacin), 0.010 µg/mL (ciprofloxacin), 0.009 µg/mL (sarafloxacin), and 0.013 µg/mL (gatifloxacin) | [6] |
| Se | Water (drinking, river, well, surface runoff, and spring) | ICP-MS | The surfactant-rich phase was mixed with concentrated HNO3 and H2O2 and irradiated at 120 °C (1600 W) for 5 min and heated to 190 °C. 78Se16O+ was monitored with ICP-MS (triple quadrupole). | LOD 0.03 μg/L, LOQ 0.1 μg/L | [40] |
| 231Po | Urine, water, digested samples | α-spectrometry | α-spectrometer—231Po was deposited on 1.0 mm silver disks. | LOD 3.5 mBq/L, LOQ 12 mBq/L | [41] |
| Te | Soil, water, sediment samples | ETAAS | The extractant phase was diluted with 50 µL of absolute ethanol and injected into the graphite furnace for analysis. | Te (IV) LOD 1.1 ng/L Te(VI) 1.7 ng/L | [44] |
| Bisphenol A | Milk | FAAS | Bisphenol A—Mn3+ complex was extracted to the mixture of CTAB and TX-114. The extract was diluted with methanol and analysed. | LOD 0.23 μg/L LOQ 0.76 μg/L | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sznek, B.; Kupczyk, O.; Czyrski, A. Cloud Point Extraction as an Environmentally Friendly Technique for Sample Preparation. Processes 2025, 13, 430. https://doi.org/10.3390/pr13020430
Sznek B, Kupczyk O, Czyrski A. Cloud Point Extraction as an Environmentally Friendly Technique for Sample Preparation. Processes. 2025; 13(2):430. https://doi.org/10.3390/pr13020430
Chicago/Turabian StyleSznek, Bartosz, Olga Kupczyk, and Andrzej Czyrski. 2025. "Cloud Point Extraction as an Environmentally Friendly Technique for Sample Preparation" Processes 13, no. 2: 430. https://doi.org/10.3390/pr13020430
APA StyleSznek, B., Kupczyk, O., & Czyrski, A. (2025). Cloud Point Extraction as an Environmentally Friendly Technique for Sample Preparation. Processes, 13(2), 430. https://doi.org/10.3390/pr13020430







