Effect of Spirulina Microalgae Powder in Gluten-Free Biscuits and Snacks Formulated with Quinoa Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Quinoa Flour
2.2.2. Preparation of Mixture
2.2.3. Rheological Properties
2.2.4. Thermal Properties
2.2.5. Preparation and Evaluation of Baking Quality and Sensory Properties of Biscuits
2.2.6. Preparation and Sensory Evaluation of Snacks
2.3. Color Determinations
2.4. Analytical Methods
2.4.1. Proximate Composition
2.4.2. Texture Analysis
2.5. Statistical Analysis
3. Results and Discussions
3.1. Thermal Properties of QF and Their Blends with SAP
3.2. Viscoamylograph Measurements
3.3. Proximate Composition of Raw Materials, Biscuits and Snacks
3.4. Mineral Contents of Raw Materials and Their Products
3.5. Amino Acid Composition
3.6. Total Phenolic Content (TPC) and Antioxidant Capacity
3.7. Color Attributes of Raw Materials, Biscuits and Snacks
3.8. Baking Quality of Biscuits
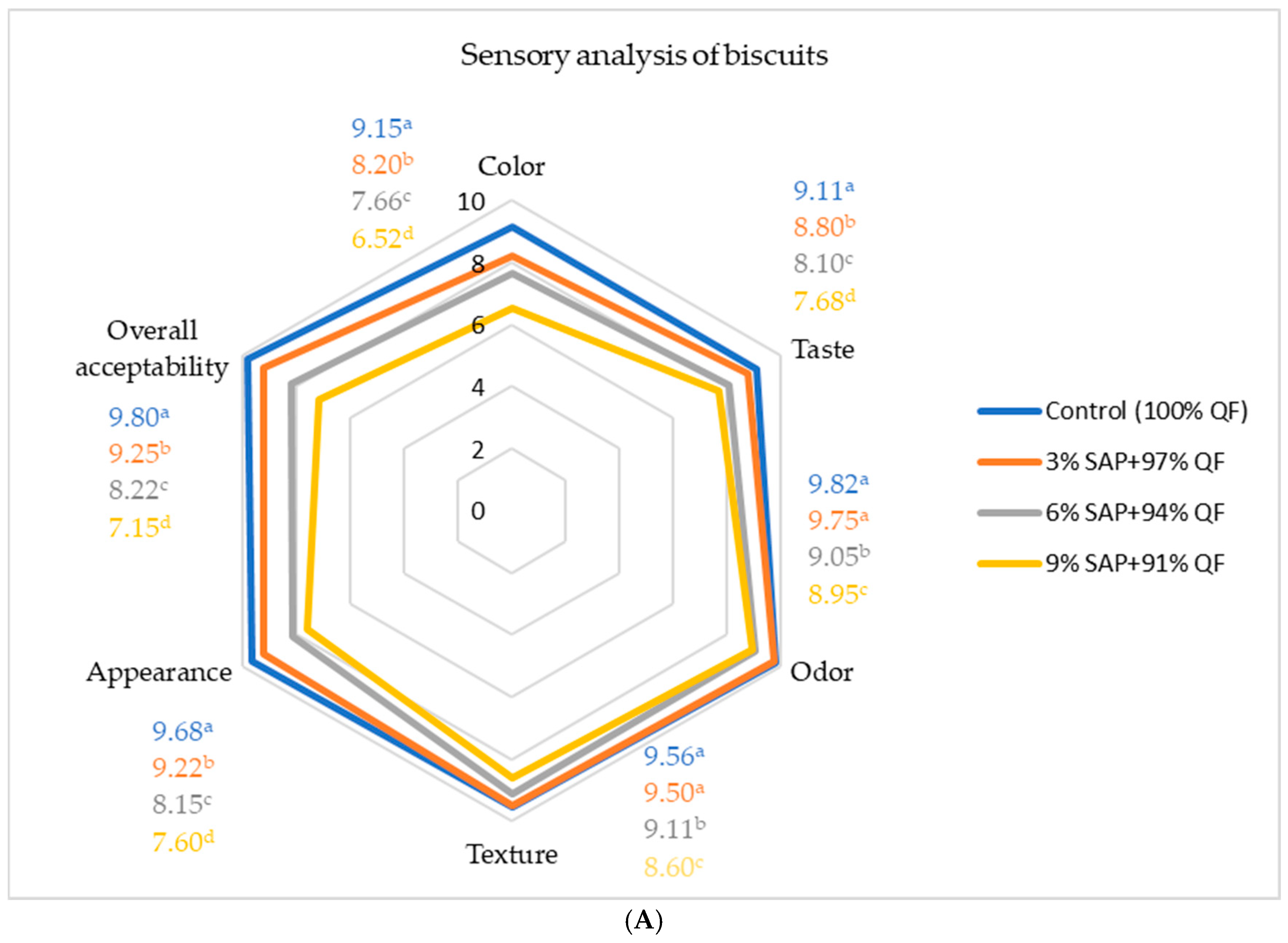
3.9. Organoleptic Properties of Biscuits and Snacks
3.10. Texture Parameters of Biscuits and Snacks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bodbodak, S.; Nejatian, M.; Ghandehari Yazdi, A.P.; Kamali Rousta, L.; Rafiee, Z.; JalaliJivan, M.; Kharazmi, M.S.; Jafari, S.M. Improving the thermal stability of natural bioactive ingredients via encapsulation technology. Crit. Rev. Food Sci. Nutr. 2024, 64, 2824–2846. [Google Scholar] [CrossRef]
- Nejatian, M.; Darabzadeh, N.; Bodbodak, S.; Saberian, H.; Rafiee, Z.; Kharazmi, M.S.; Jafari, S.M. Practical application of nanoencapsulated nutraceuticals in real food products; a systematic review. Adv. Colloid Interface Sci. 2022, 305, 102690. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.N.; Panesar, P.S.; Singh, S. Optimization of antioxidant activity, textural and sensory characteristics of gluten-free cookies made from whole indian quinoa flour. LWT 2018, 93, 573–582. [Google Scholar] [CrossRef]
- Marak, N.R.; Das, P.; Das Purkayastha, M.; Baruah, L.D. Effect of quinoa (Chenopodium quinoa W.) flour supplementation in breads on the lipid profile and glycemic index: An in vivo study. Front. Nutr. 2024, 11, 1341539. [Google Scholar] [CrossRef]
- Conte, P.; Fadda, C.; Drabinska, N.; Krupa-Kozak, U. Technological and nutritional challenges, and novelty in gluten-free breadmaking: A review. Pol. J. Food Nutr. Sci. 2019, 69, 5–21. [Google Scholar] [CrossRef]
- El-Hadidy, G.S.; Rizk, E.A.; El-Dreny, E.G. Improvement of Nutritional Value, Physical and Sensory Properties of Biscuits Using Quinoa, Naked Barley and Carrot. Egypt. J. Food. Sci. 2020, 48, 147–157. [Google Scholar]
- Hussein, A.S.; Mostafa, S.; Fouad, S.; Hegazy, N.A.; Zaky, A.A. Production and Evaluation of Gluten-Free Pasta and Pan Bread from Spirulina Algae Powder and Quinoa Flour. Processes 2023, 11, 2899. [Google Scholar] [CrossRef]
- Braga-Souto, R.N.; Bürck, M.; Nakamoto, M.M.; Braga, A.R.C. Cracking Spirulina flavor: Compounds, sensory evaluations, and solutions. Trends Food Sci. Technol. 2025, 156, 104847. [Google Scholar] [CrossRef]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef]
- Satpathy, L.; Dash, D.; Sahoo, P.; Anwar, T.; Parida, S. Quantitation of Total Protein Content in Some Common Edible Food Sources by Lowry Protein Assay. Lett. App. Nanobiosci. 2020, 9, 1275–1283. [Google Scholar]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Hamza, H.A.; Salem, M.L.; Kamal, S.; Alhasani, R.H.; Alsharif, I.; Mahrous, H.; Abdella, A. Synergisticn Hypolipidemic and Immunomodulatory Activity of Lactobacillus and Spirulina platensis. Fermentation 2022, 8, 220. [Google Scholar] [CrossRef]
- Subramaiam, H.; Chu, W.L.; Radhakrishnan, A.K.; Chakravarthi, S.; Selvaduray, K.R.; Kok, Y.Y. Evaluating anticancer and immunomodulatory effects of spirulina (Arthrospira) platensis and gamma-tocotrienol supplementation in a syngeneic mouse model of breast cancer. Nutrients 2021, 13, 2320. [Google Scholar] [CrossRef]
- Bohórquez-Medina, S.L.; Bohórquez-Medina, A.L.; Zapata, V.A.B.; Cconchoy, F.I.; Huamanchumo, C.J.T.; Quispe, G.B.; Hernandez, A.V. Spirulina Supplementation and Obesity Related Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Metab. Clin. Exp. 2021, 116, 154607. [Google Scholar] [CrossRef]
- Hatami, E.; Ghalishourani, S.S.; Najafgholizadeh, A.; Pourmasoumi, M.; Hadi, A.; Clark, C.C.; Assaroudi, M.; Salehi-Sahlabadi, A.; Joukar, F.; Mansour-Ghanaei, F. The effect of spirulina on type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2021, 20, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Prete, V.; Abate, A.C.; Di Pietro, P.; De Lucia, M.; Vecchione, C.; Carrizzo, A. Beneficial Effects of Spirulina Supplementation in the Management of Cardiovascular Diseases. Nutrients 2024, 16, 642. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.A.; Paterson, S.; Gómez-Cortés, P.; Hernández-Ledesma, B. Bioactive peptides released from microalgae during gastrointestinal digestion. In Protein Digestion-Derived Peptides; Academic Press: Cambridge, MA, USA, 2024; pp. 335–352. [Google Scholar]
- Fantechi, T.; Contini, C.; Casini, L. Pasta goes green: Consumer preferences for spirulina-enriched pasta in Italy. Algal Res. 2023, 75, 103275. [Google Scholar] [CrossRef]
- Letras, P.; Oliveira, S.; Varela, J.; Nunes, M.C.; Raymundo, A. 3D printed gluten-free cereal snack with incorporation of Spirulina (Arthrospira platensis) and/or Chlorella vulgaris. Algal Res. 2022, 68, 102863. [Google Scholar] [CrossRef]
- Rabitti, N.S.; Bayudan, S.; Laureati, M.; Neugart, S.; Schouteten, J.J.; Apelman, L.; Dahlstedt, S.; Sandvik, P. Snacks from the sea: A cross-national comparison of consumer acceptance for crackers added with algae. Eur. Food Res. Technol. 2024, 250, 2193–2209. [Google Scholar] [CrossRef]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in bread formulated with wheat flours of different alveograph strength. Foods 2023, 12, 3724. [Google Scholar] [CrossRef] [PubMed]
- Şahin, O.I. Functional and sensorial properties of cookies enriched with SPIRULINA and DUNALIELLA biomass. J. Food Sci. Technol. 2020, 57, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Oliveira, S.M.; Amado, I.R.; Fasolin, L.H.; Vicente, A.A.; Pastrana, L.M.; Fuciños, P. 3D printed functional cookies fortified with Arthrospira platensis: Evaluation of its antioxidant potential and physical-chemical characterization. Food Hydrocoll. 2020, 107, 105893. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000; ISBN 13, 9781891127120. [Google Scholar]
- Zaky, A.A.; Hussein, A.S.; Mostafa, S.; Abd El-Aty, A.M. Impact of sunflower meal protein isolate supplementation on pasta quality. Separations 2022, 9, 429. [Google Scholar] [CrossRef]
- Zaky, A.A.; Liu, Y.; Han, P.; Ma, A.; Jia, Y. Effect of flavorzyme digestion on the antioxidant capacities of ultra-filtrated rice bran protein hydrolyzates. J. Food Process. Preserv. 2020, 44, e14551. [Google Scholar] [CrossRef]
- Bertrand, R.; Holmes, W.; Orgeron, C.; McIntyre, C.; Hernandez, R.; Revellame, E.D. Rapid estimation of parameters for gelatinization of waxy corn starch. Foods 2019, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Mauro, R.R.; Vela, A.J.; Ronda, F. Impact of starch concentration on the pasting and rheological properties of gluten-free gels. Effects of amylose content and thermal and hydration properties. Foods 2023, 12, 2281. [Google Scholar] [CrossRef]
- Hedayati, S.; Shahidi, F.; Koocheki, A.; Farahnaky, A.; Majzoobi, M. Functional properties of granular cold-water swelling maize starch: Effect of sucrose and glucose. Int. J. Food Sci. Technol. 2016, 51, 2416–2423. [Google Scholar] [CrossRef]
- Svoboda, R.; Pakosta, M.; Doležel, P. How the Presence of Crystalline Phase Affects Structural Relaxation in Molecular Liquids: The Case of Amorphous Indomethacin. Int. J. Mol. Sci. 2023, 24, 16275. [Google Scholar] [CrossRef] [PubMed]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef] [PubMed]
- Chemin, M.; Paurd, O.; Villaceque, L.; Riaublanc, A.; Le-Bail, P. The Changes in Starch Gelatinization Behavior under the Influence of Acetic Acid in Vegetable Sponge Cake Batter in Order to Obtain New Snacks. Polymers 2022, 14, 4053. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, N.; Khatkar, B.S. Effects of A- and B-type starch granules on composition, structural, thermal, morphological, and pasting properties of starches from diverse wheat varieties. Food Bioeng. 2023, 2, 373–383. [Google Scholar] [CrossRef]
- Frauenlob, J.; Hetebrij, E.; D’Amico, S.; Schoenlechner, R. Role of α-Amylase in the pasting behavior of wheat flours upon storage. Starch Stärke 2018, 70, 1700123. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, K.; Zheng, K.; Zhu, W.; Ding, W.; Lyv, Q.; Chen, L.; Wang, G.; Zhuang, K.; Chen, X. Pasting Properties of Various Waxy Rice Flours: Effect of α-Amylase Activity, Protein, and Amylopectin. J. Food Qual. 2022, 2022, 1636819. [Google Scholar] [CrossRef]
- Al-Attar, H.; Ahmed, J.; Thomas, L. Rheological, pasting and textural properties of corn flour as influenced by the addition of rice and lentil flour. LWT 2022, 160, 113231. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Farg, H.; El-Makhzangy, A.; El-Shawaf, A.G. Chemical and technological studies on micro algae extracts and its utilization in some food products. Chemical, biochemical characteristics and nutritional value of spirulina algae. J. Product. Dev. 2021, 26, 107–117. [Google Scholar] [CrossRef]
- Jancurová, M.; Minarovicová, L.; Dandár, A. Rheological properties of doughs with buckwheat and quinoa additives. Chem. Pap. 2009, 63, 738–741. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L. Nutrition facts and functional potential of quinoa (Chenopodium quinoa Willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- El Sohaimy, S.A.; Refaay, T.M.; Zaytoun, M.A.M. Physicochemical and functional properties of quinoa protein isolate. Ann. Agric. Sci. 2015, 60, 297–305. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, D.; Guo, L.; Yao, Y.; Yao, X.; Wang, Z.; Wu, K.; Cao, X.; Gao, X. Effects of Quinoa Flour on Wheat Dough Quality, Baking Quality, and in vitro Starch Digestibility of the Crispy Biscuits. Front. Nutr. 2022, 9, 846808. [Google Scholar] [CrossRef]
- Pabon, K.S.M.; Acosta, D.F.R.; Bravo, J.E. Second-generation snacks prepared from quinoa with probiotic. Physicochemical properties, in vitro digestibility, antioxidant activity and consumer acceptability. Heliyon 2024, 10, e36525. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, R.; Nieto, G. Developing a functional gluten-free sourdough bread by incorporating quinoa, amaranth, rice and spirulina. LWT 2024, 201, 116162. [Google Scholar] [CrossRef]
- Culetu, A.; Susman, I.E.; Duta, D.E.; Belc, N. Nutritional and functional properties of gluten-free flours. Appl. Sci. 2021, 11, 6283. [Google Scholar] [CrossRef]
- Masten Rutar, J.; Jagodic Hudobivnik, M.; Nečemer, M.; Mikuš, K.V.; Arčon, I.; Ogrinc, N. Nutritional quality and safety of the spirulina dietary supplements sold on the Slovenian market. Foods 2022, 11, 849. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Uribe-Wandurraga, Z.N.; Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Effect of microalgae addition on mineral content, colour and mechanical properties of breadsticks. Food Funct. 2019, 10, 4685–4692. [Google Scholar] [CrossRef]
- de Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxidative Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.E.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Fradinho, P.; Niccolai, A.; Soares, R.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Effect of Arthrospira platensis (spirulina) incorporation on the rheological and bioactive properties of gluten-free fresh pasta. Algal Res. 2020, 45, 101743. [Google Scholar] [CrossRef]
- Aune, D. Plant foods, antioxidant biomarkers, and the risk of cardiovascular disease, cancer, and mortality: A review of the evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional Composition, Nutritional Properties, and Biological Activities of Moroccan Spirulina Microalga. J. Food Qual. 2019, 2019, 3707219. [Google Scholar] [CrossRef]
- Aktas, A.B. Spirulina: Exploring Its Promising Role in Food Industry and Overcoming Challenges as a Food Analogue. In Food Analogues: Emerging Methods and Challenges; Springer Nature: Cham, Switzerland, 2024; pp. 165–182. [Google Scholar]
- Alam, S.; Singh, B.; Khaira, H.; Kaur, J.; Singh, S.K. Optimization of extrusion process variables for development of pulse-carrot pomace incorporated rice based snacks. J. Food Legumes 2013, 26, 103–114. [Google Scholar]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Raja, W.H.; Kumar, S.; Bhat, Z.F.; Kumar, P. Effect of ambient storage on the quality characteristics of aerobically packaged fish curls incorporated with different flours. SpringerPlus 2014, 3, 106. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A.; Majewska, B. Effect of Spirulina (Spirulina platensis) addition on textural and quality properties of cookies. Ital. J. Food Sci. 2018, 30, 1–12. [Google Scholar]
- Su, K.; Fan, Z.; Usman, M.; Zhao, A.; Dong, H.; Duan, X.; Weng, L.; Shahid, A.; Xu, J. Effect of Spirulina platensis on the structure and aggregation of gluten proteins to improve texture and physiochemical properties of wheat noodles. Food Hydrocoll. 2025, 162, 110959. [Google Scholar] [CrossRef]



| Samples | To (°C) | Tp (°C) | Te (°C) | ΔT (°C) | ΔH gel J/g |
|---|---|---|---|---|---|
| Control (100% QF) | 59.55 | 102.56 | 144.60 | 85.05 | 82.89 |
| 3% SAP + 97% QF | 30.21 | 72.70 | 121.65 | 91.44 | 91.43 |
| 6% SAP + 94% QF | 33.86 | 79.14 | 131.76 | 97.09 | 114.60 |
| 9% SAP + 91% QF | 69.73 | 62.13 | 73.85 | 4.12 | 21.66 |
| Samples | Transmission Temperature (°C) | Temperature of Peak Viscosity (°C) | Peak Viscosity (BU) | Break Down (BU) | Set Back (BU) |
|---|---|---|---|---|---|
| Control (100 QF) | 63 | 110 | 1180 | 1400 | 1360 |
| 3% SAP + 97% QF | 61.5 | 105 | 1140 | 1200 | 1420 |
| 6% SAP + 94% QF | 49 | 70.5 | 1060 | 1200 | 1860 |
| 9% SAP + 91% QF | 48 | 76.5 | 1580 | 1600 | 2540 |
| Samples | Moisture | Protein | Fat | Ash | Fiber | Carbohydrates * |
|---|---|---|---|---|---|---|
| Biscuits | ||||||
| 100% QF | 4.25 d ± 0.06 | 15.00 d ± 0.07 | 31.97 c ± 0.07 | 2.09 d ± 0.04 | 1.93 d ± 0.20 | 49.01 |
| 3% SAP + 97% QF | 4.95 c ± 0.15 | 17.10 c ± 0.08 | 32.04 b ± 0.07 | 2.64 c ± 0.01 | 2.03 c ± 0.12 | 46.19 |
| 6% SAP + 94% QF | 5.15 b ± 0.07 | 18.45 b ± 0.23 | 32.14 a ± 0.10 | 3.16 b ± 0.06 | 2.45 b ± 0.08 | 43.80 |
| 9% SAP + 91% QF | 5.65 a ± 0.02 | 20.67 a ± 0.09 | 32.25 a ± 0.08 | 3.68 a ± 0.07 | 2.60 a ± 0.8 | 40.80 |
| LSD at 0.05 | 0.451 | 0.335 | 1.098 | 1.652 | 0.10 | 2.350 |
| Snacks | ||||||
| 100% QF | 2.63 d ± 0.21 | 15.74 d ± 0.04 | 13.01 c ± 0.06 | 2.21 d ± 0.08 | 2.03 a ± 0.05 | 67.01 |
| 3% SAP + 97% QF | 2.80 c ± 0.06 | 17.01 c ± 0.09 | 13.54 b ± 0.02 | 2.72 c ± 0.01 | 2.02 a ± 0.07 | 64.71 |
| 6% SAP + 94% QF | 2.95 b ± 0.03 | 18.21 b ± 0.01 | 13.88 b ± 0.05 | 3.24 b ± 0.04 | 2.04 a ± 0.03 | 62.63 |
| 9% SAP + 91% QF | 3.15 a ± 0.12 | 19.46 a ± 0.03 | 14.01 a ± 0.04 | 3.75 a ± 0.21 | 2.04 a ± 0.02 | 60.74 |
| LSD at 0.05 | 0.156 | 1.201 | 0.986 | 0.324 | 0.102 | 2.501 |
| Samples | Calcium | Phosphorus | Potassium | Sodium | Iron | Zinc |
|---|---|---|---|---|---|---|
| Biscuits | ||||||
| 100% QF | 85 d ± 0.24 | 517 a ± 2.36 | 683 a ± 0.51 | 1430.60 a ± 3.17 | 30.57 d ± 1.12 | 6.5 a ± 0.42 |
| 3% SAP + 97% QF | 92 c ± 0.60 | 153 d ± 1.13 | 652 b ± 0.24 | 1362 b ± 3.01 | 36.0 c ± 0.74 | 6.2 a ± 0.27 |
| 6% SAP + 94% QF | 109 b ± 0.05 | 159 c ± 1.62 | 621 c ± 1.55 | 1312 c ± 2.42 | 42.5 b ± 0.46 | 5.67 b ± 0.45 |
| 9% SAP + 91% QF | 122 a ± 2.14 | 164 b ± 0.55 | 584 d ± 2.54 | 1275 d ± 1.74 | 46.2 a ± 1.42 | 5.51 b ± 0.26 |
| LSD at 0.05 | 7.61866 | 11.75172 | 15.78183 | 16.11435 | 8.22791 | 1.43473 |
| Snacks | ||||||
| 100% QF | 85 d ± 1.21 | 517 a ± 0.54 | 683 a ± 0.80 | 1430 a ± 2.61 | 30.66 d ± 0.18 | 6.31 c ± 0.24 |
| 3% SAP + 97% QF | 93 c ± 2.63 | 134 d ± 2.63 | 668 b ± 1.20 | 1373 b ± 2.28 | 37.0 c ± 0.19 | 6.23 d ± 0.12 |
| 6% SAP + 94% QF | 109 b ± 0.61 | 140 c ± 0.21 | 642 c ± 2.10 | 1316 c ± 2.11 | 42.0 b ± 0.27 | 5.78 a ± 0.35 |
| 9% SAP + 91% QF | 120 a ± 0.93 | 143 b ± 0.67 | 615 d ± 1.22 | 1283 d ± 2.13 | 47.0 a ± 0.12 | 5.44 b ± 0.48 |
| LSD at 0.05 | 5.31176 | 7.53121 | 16.74526 | 18.89338 | 3.38872 | 1.31408 |
| Amino Acids (g/100 g Protein) | Biscuits | Snacks | ||||||
|---|---|---|---|---|---|---|---|---|
| 100% QF | 3% SAP + 97% QF | 6% SAP + 94% QF | 9% SAP + 91% QF | 100% QF | 3% SAP + 97% QF | 6% SAP + 94% QF | 9% SAP + 91% QF | |
| Essential amino acids | ||||||||
| Isoleucine | 0.90 | 1.20 | 1.70 | 2.10 | 0.86 | 1.15 | 1.65 | 2.05 |
| Leucine | 2.80 | 2.92 | 3.12 | 3.47 | 2.61 | 2.89 | 3.02 | 3.42 |
| Lysine | 2.50 | 2.90 | 2.30 | 2.75 | 2.42 | 2.85 | 2.25 | 2.70 |
| Methionine | 0.40 | 0.90 | 1.35 | 1.70 | 0.35 | 0.85 | 1.25 | 1.65 |
| Phenylalanine | 1.65 | 1.60 | 1.53 | 1.50 | 1.60 | 1.55 | 1.50 | 1.48 |
| Threonine | 5.92 | 5.60 | 5.15 | 4.80 | 5.80 | 5.50 | 5.05 | 4.75 |
| Valine | 3.81 | 3.95 | 4.17 | 4.45 | 3.75 | 3.90 | 4.10 | 4.40 |
| Nonessential amino acids | ||||||||
| Histidine | 2.28 | 2.15 | 2.06 | 1.96 | 2.20 | 2.10 | 2.02 | 1.92 |
| Tyrosine | 1.28 | 2.00 | 2.70 | 3.25 | 1.25 | 1.95 | 2.60 | 3.20 |
| Arginine | 3.10 | 2.95 | 2.75 | 2.55 | 3.05 | 2.90 | 2.70 | 2.50 |
| Alanine | 2.30 | 2.25 | 2.10 | 2.08 | 2.25 | 2.20 | 2.15 | 2.05 |
| Aspartic | 3.80 | 3.65 | 3.55 | 3.50 | 3.75 | 3.60 | 3.50 | 3.45 |
| Cysteine | 0.19 | 0.21 | 0.25 | 0.29 | 0.15 | 0.19 | 0.23 | 0.27 |
| Glutamic acid | 8.92 | 8.65 | 8.40 | 8.00 | 8.80 | 8.50 | 8.30 | 7.95 |
| Glycine | 3.10 | 3.00 | 2.85 | 2.70 | 3.05 | 2.95 | 2.80 | 2.65 |
| Proline | 1.90 | 2.00 | 2.09 | 2.20 | 1.87 | 1.95 | 2.05 | 2.15 |
| Serine | 1.78 | 1.70 | 1.60 | 1.50 | 1.72 | 1.65 | 1.56 | 1.45 |
| Samples | TPC (mg/g) | Antioxidant Capacity | |
|---|---|---|---|
| DPPH (µmol/g) | Reducing Power (µmol/g) | ||
| Biscuits | |||
| 100% QF | 1.45 ± 0.19 d | 36.15 ± 0.13 d | 33.10 ± 0.38 d |
| 3% SAP + 97% QF | 2.15 ± 0.25 c | 38.40 ± 0.21 c | 35.5 ± 0.22 c |
| 6% SAP + 94% QF | 2.85 ± 0.16 b | 44.7 ± 0.18 b | 38.2 ± 0.45 b |
| 9% SAP + 91% QF | 3.25 ± 0.42 a | 50.18 ± 0.20 a | 43.6 ± 0.14 a |
| Snacks | |||
| 100% QF | 1.42 ± 0.21 d | 20.29 ± 0.31 d | 15.45 ± 0.16 d |
| 3% SAP + 97% QF | 1.50 ± 0.15 c | 30.35 ± 0.25 c | 20.27 ± 0.45 c |
| 6% SAP + 94% QF | 2.10 ± 0.24 b | 37.12 ± 0.67 b | 30.65 ± 0.39 b |
| 9% SAP + 91% QF | 2.90 ± 0.11 a | 41.49 ± 0.15 a | 36.58 ± 0.42 a |
| Parameters | Mixtures | |||
|---|---|---|---|---|
| Control (100 QF) | 3% SAP + 97% QF | 6% SAP + 94% QF | 9% SAP + 91% QF | |
| L* | 90.76 a ± 0.23 | 83.83 b ± 0.08 | 79.78 c ± 0.08 | 72.55 d ± 0.53 |
| a* | 0.19 c ± 0.34 | 2.86 b ± 0.52 | 3.10 a ± 0.28 | 3.35 a ± 0.42 |
| b* | 9.96 c ± 0.15 | 11.56 b ± 0.07 | 11.98 b ± 0.02 | 12.50 a ± 0.05 |
| Biscuits | ||||
| L* | 82.65 a ± 0.09 | 71.23 b ± 0.35 | 70.38 b ± 0.33 | 62.21 c ± 0.13 |
| a* | 5.65 a ± 0.42 | 2.08 c ± 0.03 | 1.67 d ± 0.18 | 2.21 b ± 0.31 |
| b* | 33.36 c ± 0.25 | 33.85 b ± 0.17 | 33.98 b ± 0.03 | 35.99 a ± 0.06 |
| Snacks | ||||
| L* | 78.31 a ± 0.02 | 44.87 b ± 0.26 | 42.39 b ± 0.24 | 40.69 c ± 0.09 |
| a* | 0.09 d ± 0.3 | 1.58 c ± 0.08 | 2.35 b ± 0.16 | 3.49 a ± 0.04 |
| b* | 9.49 c ± 0.17 | 22.62 a ± 0.11 | 22.61 a ± 0.032 | 22.33 b ± 0.28 |
| Samples | Weight (g) | Volume (cm3) | Specific Volume (v/w) | Diameter (cm) | Thickness (cm) | Spread Ratio |
|---|---|---|---|---|---|---|
| Control (100% QF) | 12.00 | 15.33 | 1.28 | 5.11 | 0.61 | 8.38 |
| 3% SAP + 97% QF | 11.33 | 14.00 | 1.24 | 5.1 | 0.58 | 8.79 |
| 6% SAP + 94% QF | 9.33 | 10.00 | 1.07 | 4.85 | 0.56 | 8.66 |
| 9% SAP + 91% QF | 9.66 | 9.33 | 0.97 | 4.75 | 0.57 | 8.33 |
| Texture Profile Parameters | Biscuits | |||
|---|---|---|---|---|
| Control (100% QF) | 3% SAP + 97% QF | 6% SAP + 94% QF | 9% SAP + 91% QF | |
| Hardness (N) | 100.99 | 100.92 | 99.67 | 21.32 |
| Deformation at hardness (mm) | 26.37 | 27.18 | 25.31 | 0.77 |
| Deformation at hardness (%) | 329.60 | 271.80 | 361.60 | 11.00 |
| Hardness work (mJ) | 76.00 | 69.90 | 129.30 | 12.90 |
| Fracturability with 1% of load sensitivity (N) | 20.12 | 23.15 | 17.66 | 21.32 |
| Snacks | ||||
| Hardness (N) | 98.45 | 110.41 | 117.33 | 107.80 |
| Deformation at hardness (mm) | 2.65 | 12.03 | 11.50 | 15.98 |
| Deformation at hardness (%) | 53.00 | 100.30 | 95.80 | 84.10 |
| Hardness work (mJ) | 138.50 | 178.70 | 122.30 | 250.10 |
| Fracturability with 1% of load sensitivity (N) | 98.45 | 9.57 | 61.07 | 13.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, A.M.S.; Mostafa, S.; Ata, S.M.; Hegazy, N.A.; Abu-Reidah, I.M.; Zaky, A.A. Effect of Spirulina Microalgae Powder in Gluten-Free Biscuits and Snacks Formulated with Quinoa Flour. Processes 2025, 13, 625. https://doi.org/10.3390/pr13030625
Hussein AMS, Mostafa S, Ata SM, Hegazy NA, Abu-Reidah IM, Zaky AA. Effect of Spirulina Microalgae Powder in Gluten-Free Biscuits and Snacks Formulated with Quinoa Flour. Processes. 2025; 13(3):625. https://doi.org/10.3390/pr13030625
Chicago/Turabian StyleHussein, Ahmed M. S., Sayed Mostafa, Shymaa M. Ata, Nefisa A. Hegazy, Ibrahim M. Abu-Reidah, and Ahmed A. Zaky. 2025. "Effect of Spirulina Microalgae Powder in Gluten-Free Biscuits and Snacks Formulated with Quinoa Flour" Processes 13, no. 3: 625. https://doi.org/10.3390/pr13030625
APA StyleHussein, A. M. S., Mostafa, S., Ata, S. M., Hegazy, N. A., Abu-Reidah, I. M., & Zaky, A. A. (2025). Effect of Spirulina Microalgae Powder in Gluten-Free Biscuits and Snacks Formulated with Quinoa Flour. Processes, 13(3), 625. https://doi.org/10.3390/pr13030625









