Magnesium-Modified Biochar for Removing Phosphorus from Aquaculture Facilities: A Case Study in Idaho, USA
Abstract
:1. Introduction
2. Materials and Methods
- A total of 24 water filter bags (remove particle size down to 100 mesh);
- A total of 12 non-modified biochar bags and 12 Mg-modified biochar bags (each has 500 g of biochar);
- In total, 48 ft. of 2-inch flexible hose (2 ft per tank);
- pH meter and thermometer;
- A total of 25 zip ties and 25 Ziploc bags for collecting the samples;
- A total of 3 sample bottles for collecting water samples.
2.1. Techno-Economic Assessment
2.2. Environmental Impact Assessment
3. Case Study
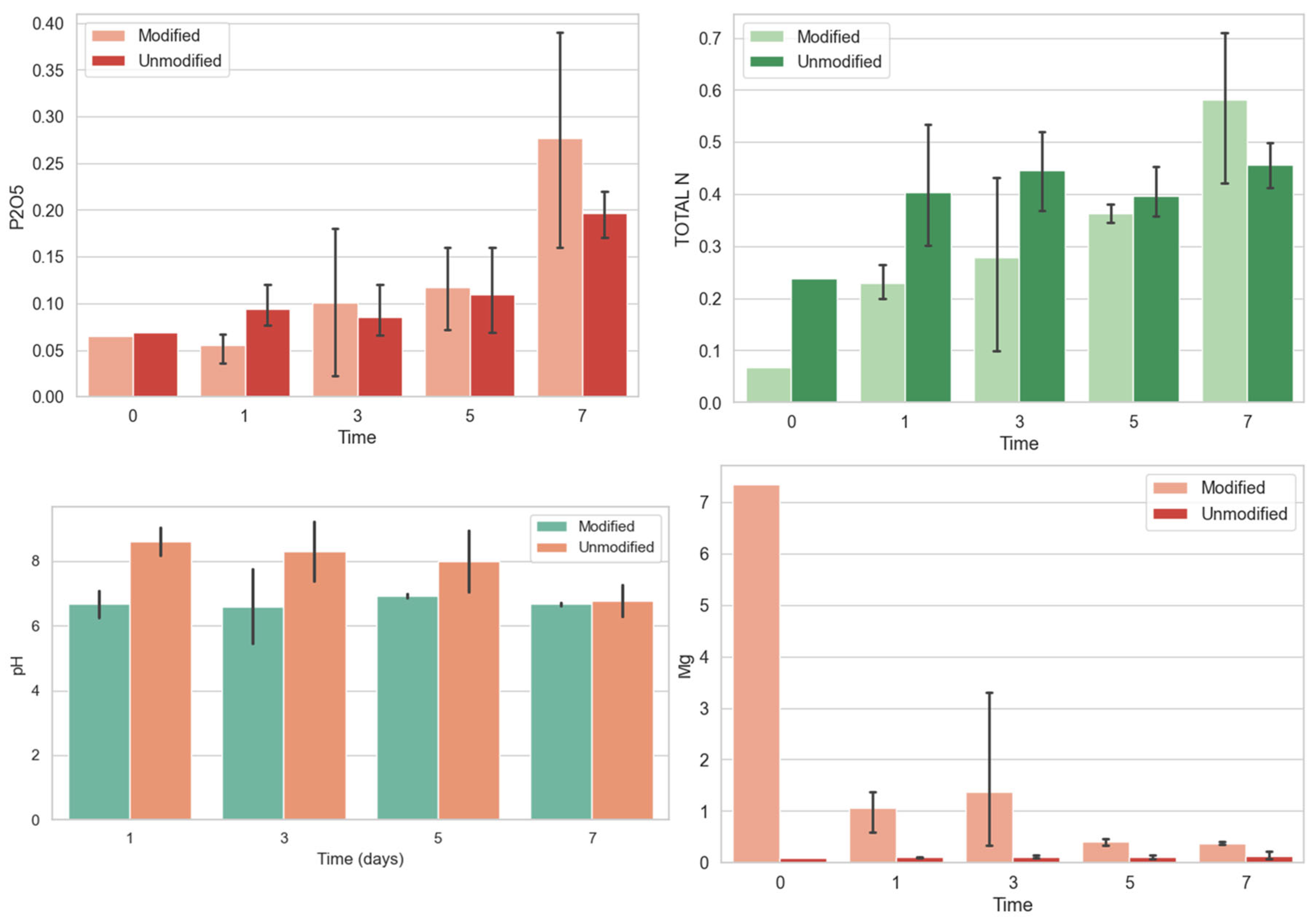
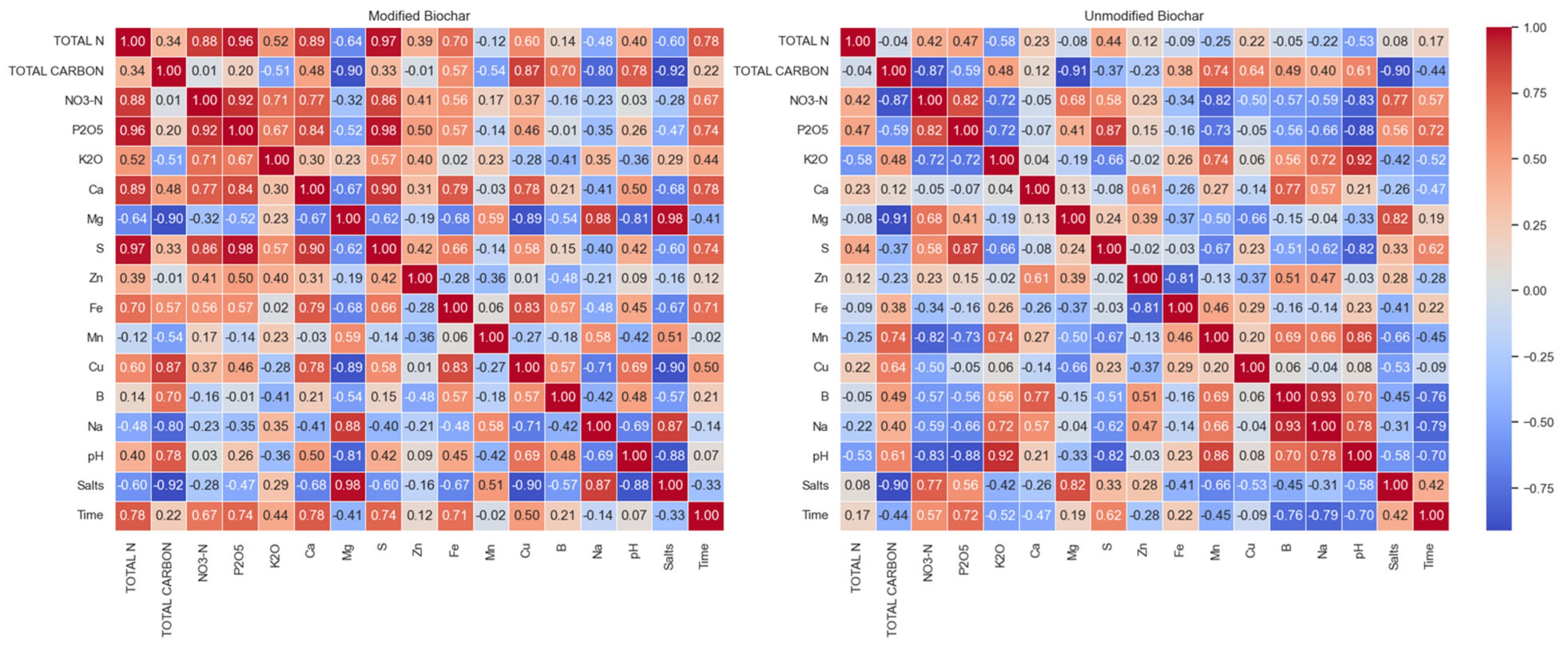
4. Results and Discussion
5. Conclusions
- Understanding the complexities of modified biochar production, reaction mechanisms, and multi-functional performance in water treatment.
- Generating valuable data and a base of knowledge to effectively assess water quality in the Thousand Springs and Hagerman areas of Idaho.
- Developing an environmentally friendly and economical method to remove the eutrophic contaminants from the farm effluents to sustain and improve the productivity of fish farming in Idaho and the nation.
- Reducing water pollution generated from aquaculture production facilities to enhance sustainability benefits to the surrounding area and aquaculture industry using engineered biochar water treatment systems.
- Increasing fish production within the EPA permit limits, and consequently stabilizing employment and increasing profitability.
- The collected nutrient-rich biochar after water treatment has the potential as a commercial slow-release fertilizer to improve soil fertility and crop health. The possible supplementary benefits could result in the recycling of excess nutrients from downstream water through repurposing as soil amendments.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Idaho Department of Environmental Aquaculture in Idaho. Available online: https://www.deq.idaho.gov/water-quality/wastewater/ (accessed on 15 April 2024).
- Low-Phosphorous Feeds Development. Available online: https://wracuw.org/products-and-publications/low-phosphorous-feeds-development (accessed on 18 March 2024).
- Litke, D. Review of Phosphorus Control Measures in the United States and Their Effects on Water Quality; U.S. Geological Survey: Reston, VA, USA, 1999.
- Silvani, L.; Vrchotova, B.; Kastanek, P.; Demnerova, K.; Pettiti, I.; Papini, M.P. Characterizing Biochar as Alternative Sorbent for Oil Spill Remediation. Sci. Rep. 2017, 7, 43912. [Google Scholar] [CrossRef]
- Silvani, L.; Di Palma, P.R.; Riccardi, C.; Eek, E.; Hale, S.E.; Viotti, P.; Papini, M.P. Use of Biochar as Alternative Sorbent for the Active Capping of Oil Contaminated Sediments. J. Environ. Chem. Eng. 2017, 5, 5241–5249. [Google Scholar]
- Moller, G.; Strawn, D. Biochar Water Treatment. U.S. Patent 10,351,455, 16 July 2019. [Google Scholar]
- DeMessie, B.; Sahle-Demessie, E.; Sorial, G.A. Cleaning Water Contaminated with Heavy Metal Ions Using Pyrolyzed Biochar Adsorbents. Sep. Sci. Technol. 2015, 50, 2448–2457. [Google Scholar] [CrossRef]
- Cai, R.; Wang, X.; Ji, X.; Peng, B.; Tan, C.; Huang, X. Phosphate Reclaim from Simulated and Real Eutrophic Water by Magnetic Biochar Derived from Water Hyacinth. J. Environ. Manag. 2017, 187, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Ngatia, L.W.; Hsieh, Y.P.; Nemours, D.; Fu, R.; Taylor, R.W. Potential Phosphorus Eutrophication Mitigation Strategy: Biochar Carbon Composition, Thermal Stability and pH Influence Phosphorus Sorption. Chemosphere 2017, 180, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-Modified Biochar for Simultaneous Removal of Ammonium, Nitrate, and Phosphate from Eutrophic Water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar]
- Novais, S.V.; Zenero, M.D.O.; Barreto, M.S.C.; Montes, C.R.; Cerri, C.E.P. Phosphorus Removal from Eutrophic Water Using Modified Biochar. Sci. Total Environ. 2018, 633, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Liu, M.; Ren, H. Removal of Ammonium and Phosphate from Water by Mg-Modified Biochar: Influence of Mg Pretreatment and Pyrolysis Temperature. BioResources 2019, 14, 6203–6218. [Google Scholar] [CrossRef]
- Liu, X.; Gao, M.; Qiu, W.; Khan, Z.H.; Liu, N.; Lin, L.; Song, Z. Fe–Mn–Ce Oxide-Modified Biochar Composites as Efficient Adsorbents for Removing As (III) from Water: Adsorption Performance and Mechanisms. Environ. Sci. Pollut. Res. 2019, 26, 17373–17382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tang, W.; Jin, X.; Shan, B. Using Biochar Capping to Reduce Nitrogen Release from Sediments in Eutrophic Lakes. Sci. Total Environ. 2019, 646, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, Y.; Wang, K.; Ding, K.; Sha, M.; Cao, Y.; Kong, F.; Wang, S. Recovery of Phosphorus from Eutrophic Water Using Nano Zero-Valent Iron-Modified Biochar and Its Utilization. Chemosphere 2021, 284, 131391. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, Y.; Nan, H.; Pei, L.; Huang, Y.; Cao, X.; Xu, X.; Zhao, L. Metal Chloride-Loaded Biochar for Phosphorus Recovery: Noteworthy Roles of Inherent Minerals in Precursor. Chemosphere 2021, 266, 128991. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hou, S.; Wang, J.; Zhu, H.; Shutes, B.; Yan, B. Biochar-Amended Constructed Wetlands for Eutrophication Control and Microcystin (MC-LR) Removal. Chemosphere 2022, 295, 133830. [Google Scholar] [CrossRef] [PubMed]
- Kończak, M.; Huber, M. Application of the Engineered Sewage Sludge-Derived Biochar to Minimize Water Eutrophication by Removal of Ammonium and Phosphate Ions from Water. J. Clean. Prod. 2022, 331, 129994. [Google Scholar] [CrossRef]
- Bare, R.; Struhs, E.; Mirkouei, A.; Overturf, K.; Small, B. Engineered Biomaterials for Reducing Phosphorus and Nitrogen Levels from Downstream Water of Aquaculture Facilities. Processes 2023, 11, 1029. [Google Scholar] [CrossRef]
- Bare, W.F.R.; Struhs, E.; Mirkouei, A.; Overturf, K.; Chacón-Patiño, M.L.; McKenna, A.M.; Chen, H.; Raja, K.S. Controlling Eutrophication of Aquaculture Production Water Using Biochar: Correlation of Molecular Composition with Adsorption Characteristics as Revealed by FT-ICR Mass Spectrometry. Processes 2023, 11, 2883. [Google Scholar] [CrossRef]
- Struhs, E.; Mirkouei, A.; You, Y.; Mohajeri, A. Techno-Economic and Environmental Assessments for Nutrient-Rich Biochar Production from Cattle Manure: A Case Study in Idaho, USA. Appl. Energy 2020, 279, 115782. [Google Scholar] [CrossRef]
- Takeshita, S.; Farzaneh, H.; Dashti, M. Life-Cycle Assessment of the Wastewater Treatment Technologies in Indonesia’s Fish-Processing Industry. Energies 2020, 13, 6591. [Google Scholar] [CrossRef]
- Colomb, V.; Colsaet, A.; Ait-Amar, S.; Basset-Mens, C.; Mevel, G.; To, V.; Gac, A.; Koch, P.; Mousset, J.; Salou, T. AGRIBALYSE: The French Public LCI Database for Agricultural Products. OCL 2015, 22, D104. Available online: https://agritrop.cirad.fr/591319/1/ocl140047-s(1).pdf (accessed on 19 March 2025). [CrossRef]
- Frischknecht, R.; Jungbluth, N.; Althaus, H.-J.; Doka, G.; Dones, R.; Heck, T.; Hellweg, S.; Hischier, R.; Nemecek, T.; Rebitzer, G.; et al. The Ecoinvent Database: Overview and Methodological Framework (7 pp). Int. J. Life Cycle Assess. 2005, 10, 3–9. [Google Scholar] [CrossRef]
- Adams, P.; James, C.; Speas, C. Rainbow Trout (Oncorhynchus Mykiss) Species and Conservation Assessment; U.S. Forest Service 2008. p. 25. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5202738.pdf (accessed on 19 March 2025).
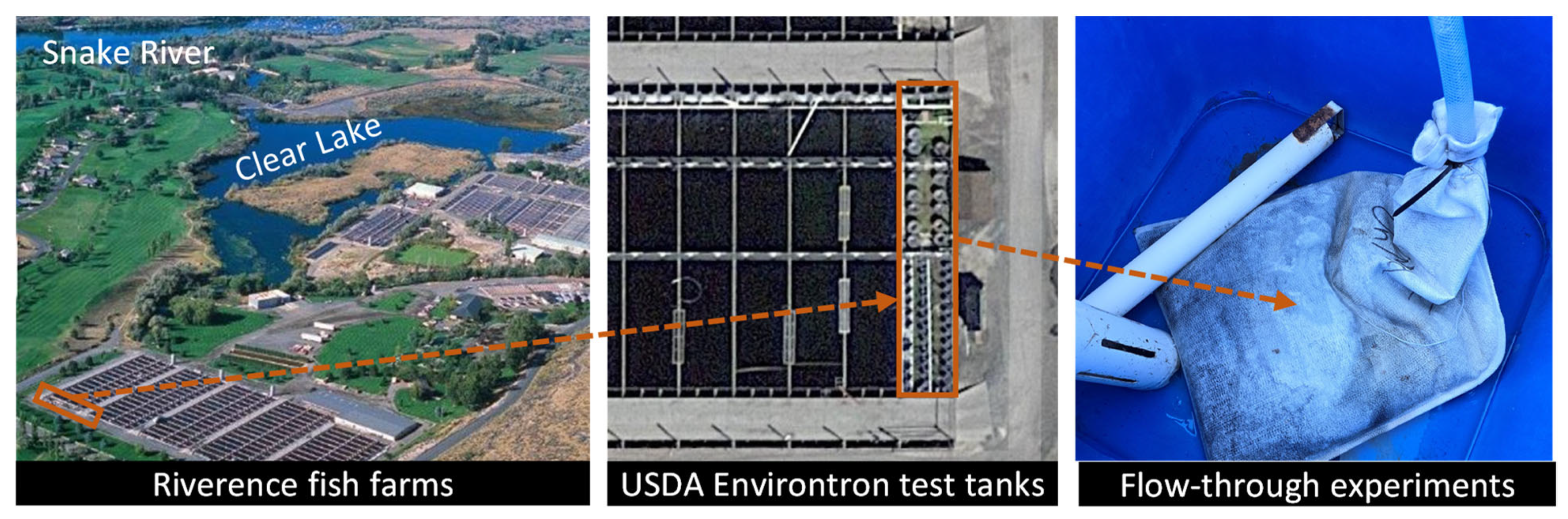
| Authors | Biochar Modification | Eutrophic Target | Biomass Type | Ref. |
|---|---|---|---|---|
| Cai et al. (2017) | Fe-oxide | Water hyacinth | [8] | |
| Ngatia et al. (2017) | - | Switchgrass, kudzu, and Chinese tallow | [9] | |
| Yin et al. (2018) | Al and Mg | Soybean straw | [10] | |
| Novais et al. (2018) | Al | Poultry manure and sugarcane straw | [11] | |
| Yin et al. (2019) | Mg | Poplar chips | [12] | |
| Liu et al. (2019) | Fe-Mn-Ce | - | [13] | |
| Zhu et al. (2019) | - | Phyllostachys pubescens | [14] | |
| Ren et al. (2021) | Fe | Reed straw | [15] | |
| Yang et al. (2021) | Fe and Mg | Sawdust and sediment | [16] | |
| Cheng et al. (2022) | - | - | [17] | |
| Konczak and Huber (2022) | - | Sewage sludge | [18] | |
| Bare et al. (2023) | - | Pine | [19] | |
| Bare et al. (2023) | - | Pine | [20] | |
| This project * | Mg | Pine | - |
| Day | Experimental Tasks |
|---|---|
| 0 | Collect 3 untreated water samples and check water pH, temp, and Magic Valley Lab for N, P, and C (3 times). Set up the water filter bags with 500 g biochar—lay them flat for better absorption. Duration: 1, 3, 5, and 7 days (24 water filter bags). |
| 1 | Collect 3 modified biochar bags and 3 non-modified biochar bags. |
| 3 | Collect 3 modified biochar bags and 3 non-modified biochar bags. |
| 5 | Collect 3 modified biochar bags and 3 non-modified biochar bags. |
| 7 | Collect 3 modified biochar bags and 3 non-modified biochar bags. |
| Parameter | Sample | Method | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Total organic carbon (mg/L) | 1.15 | 1.22 | 1.08 | SM 5310B |
| Total P (mg/L) | 0.09 | 0.09 | 0.09 | EPA 36.1 |
| Nitrate/N (mg/L) | 2.58 | 2.61 | 2.59 | EPA 300 |
| Nitrite/N (mg/L) | <0.40 | <0.40 | <0.40 | EPA 300 |
| Process | Capital (USD/yr) | Variable (USD/yr) |
|---|---|---|
| Collection (Wood, MgCl2) | 4445 | 4361 |
| Grinding | 10,912 | 11,653 |
| Drying | 13,976 | 17,254 |
| Portable refinery | 59,976 | 4954 |
| Biomaterials storage | 6076 | 2116 |
| Transportation | 9076 | 6914 |
| Fish farm | - | 4457 |
| Total | 104,461 | 51,709 |
| Impact Category | Per Metric Ton Biochar | Unit |
|---|---|---|
| Global Warming Potential (GWP100) | 692 | kg CO2-eq |
| Acidification Potential | 2.4 | kg SO2-eq |
| Ecotoxicity Potential | 0.18 | kg 1,4-DCB-eq |
| Human Toxicity Potential | 0.36 | kg 1,4-DCB-eq |
| Photochemical Oxidation Potential | 1.44 | kg C2H4-eq |
| Eutrophication | −1124 | kg PO4-eq |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Struhs, E.; Bare, W.F.R.; Mirkouei, A.; Overturf, K. Magnesium-Modified Biochar for Removing Phosphorus from Aquaculture Facilities: A Case Study in Idaho, USA. Processes 2025, 13, 1021. https://doi.org/10.3390/pr13041021
Struhs E, Bare WFR, Mirkouei A, Overturf K. Magnesium-Modified Biochar for Removing Phosphorus from Aquaculture Facilities: A Case Study in Idaho, USA. Processes. 2025; 13(4):1021. https://doi.org/10.3390/pr13041021
Chicago/Turabian StyleStruhs, Ethan, William F. Rance Bare, Amin Mirkouei, and Kenneth Overturf. 2025. "Magnesium-Modified Biochar for Removing Phosphorus from Aquaculture Facilities: A Case Study in Idaho, USA" Processes 13, no. 4: 1021. https://doi.org/10.3390/pr13041021
APA StyleStruhs, E., Bare, W. F. R., Mirkouei, A., & Overturf, K. (2025). Magnesium-Modified Biochar for Removing Phosphorus from Aquaculture Facilities: A Case Study in Idaho, USA. Processes, 13(4), 1021. https://doi.org/10.3390/pr13041021










