Changes in Heat and Energy During Depressurization-Induced Natural Gas Hydrate Dissociation in Porous Media
Abstract
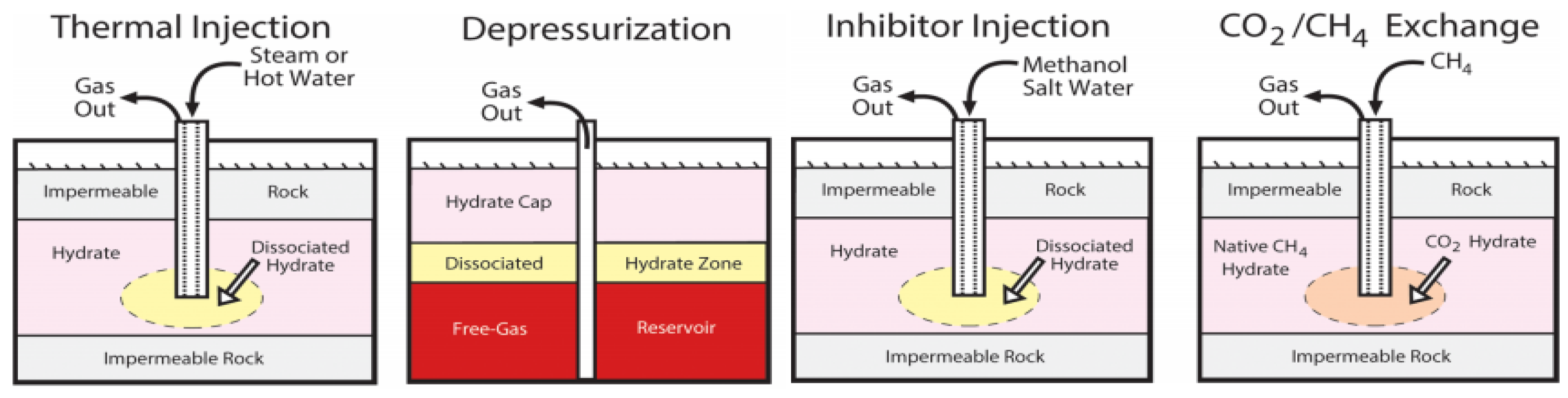
:1. Introduction
2. THMC Coupled Model for Hydrate Dissociation
2.1. Problem Definition
2.2. Modelling Framework
2.2.1. Governing Equations
2.2.2. Auxiliary Equations
2.2.3. Physics-Based Constrained Conditions
3. Case Study
3.1. Case Introduction
3.2. Computation Parameters and Settings
3.3. Results
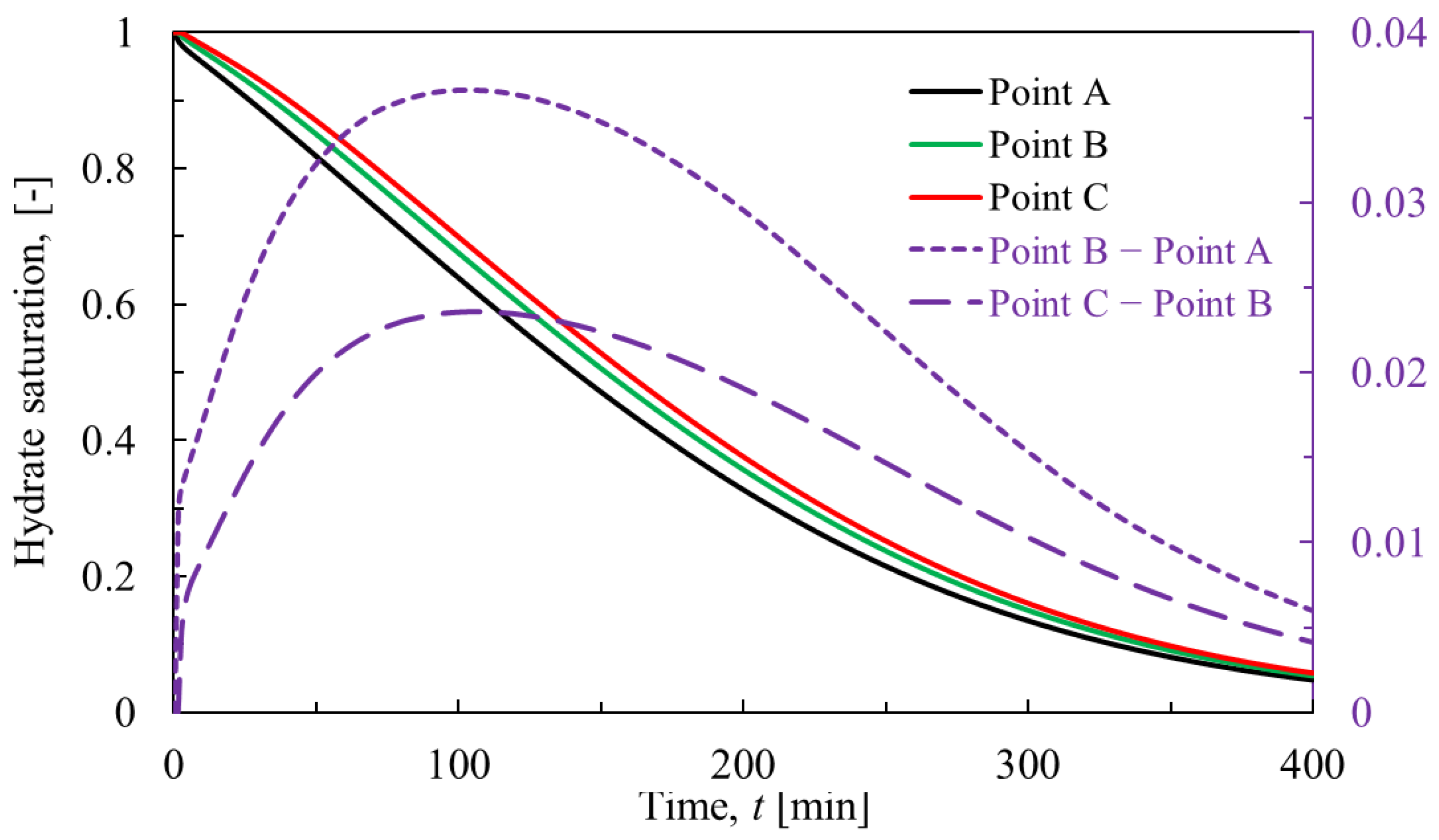
3.3.1. Hydrate Dissociation
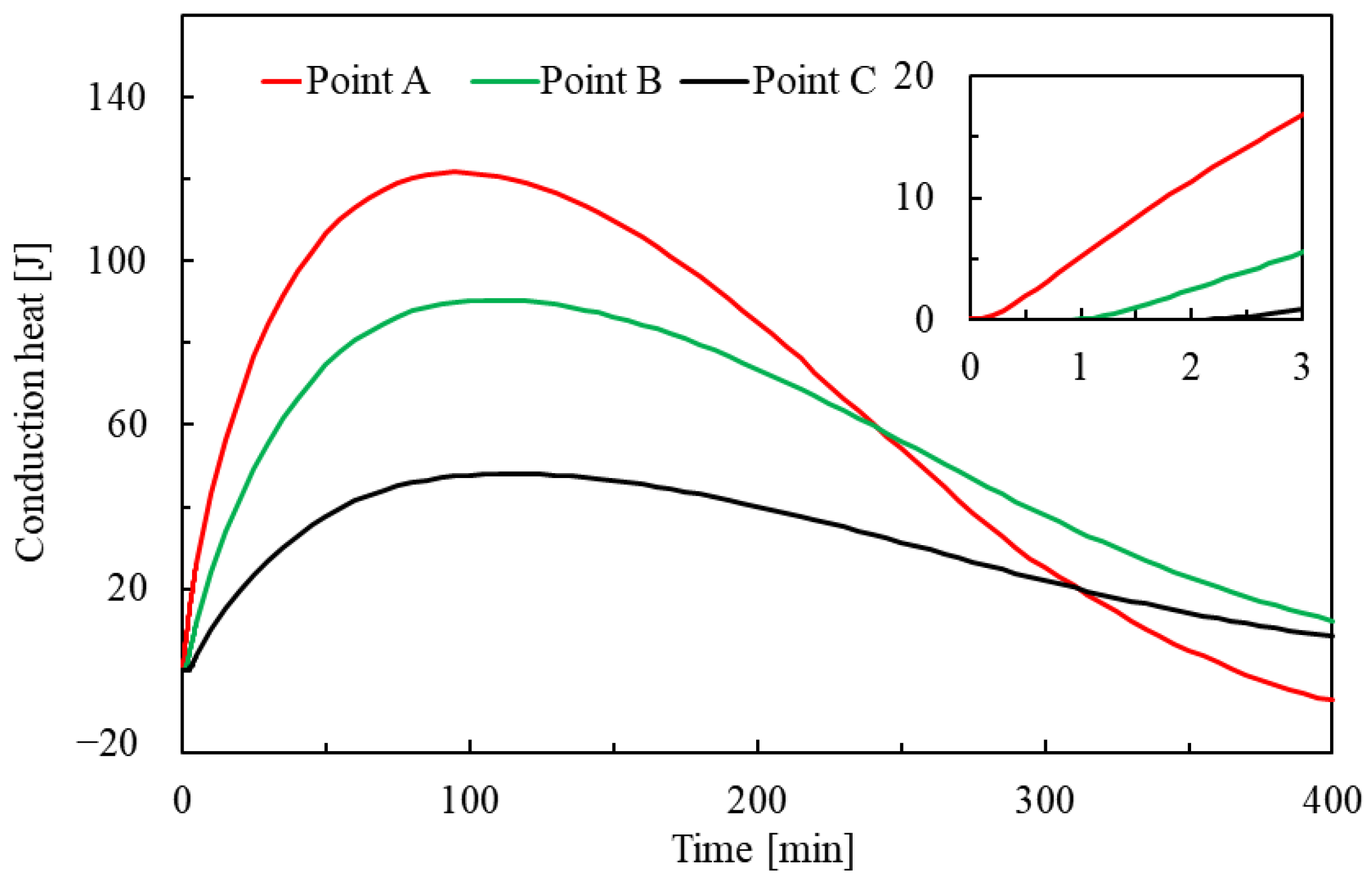
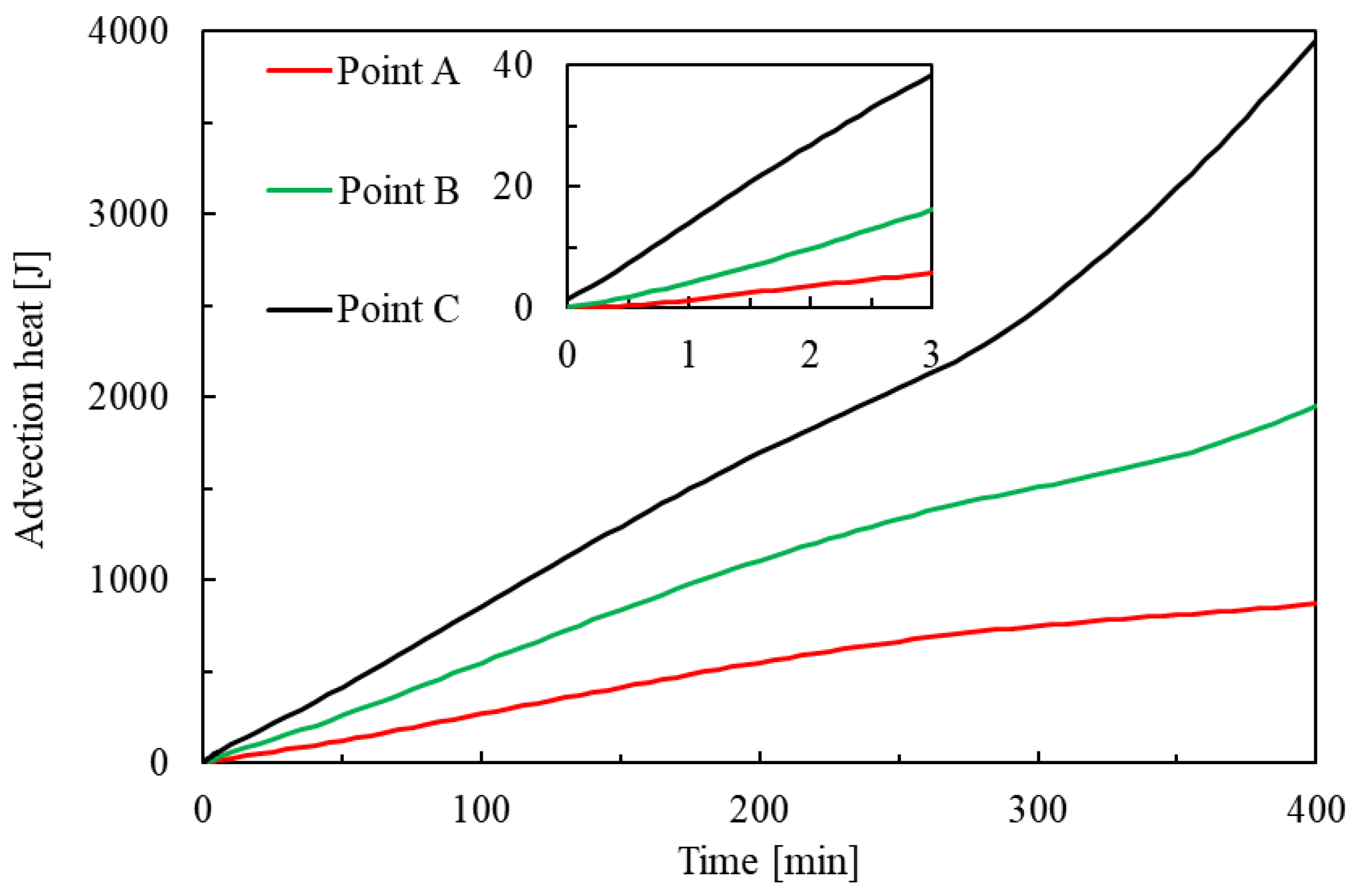
3.3.2. Change in Heat
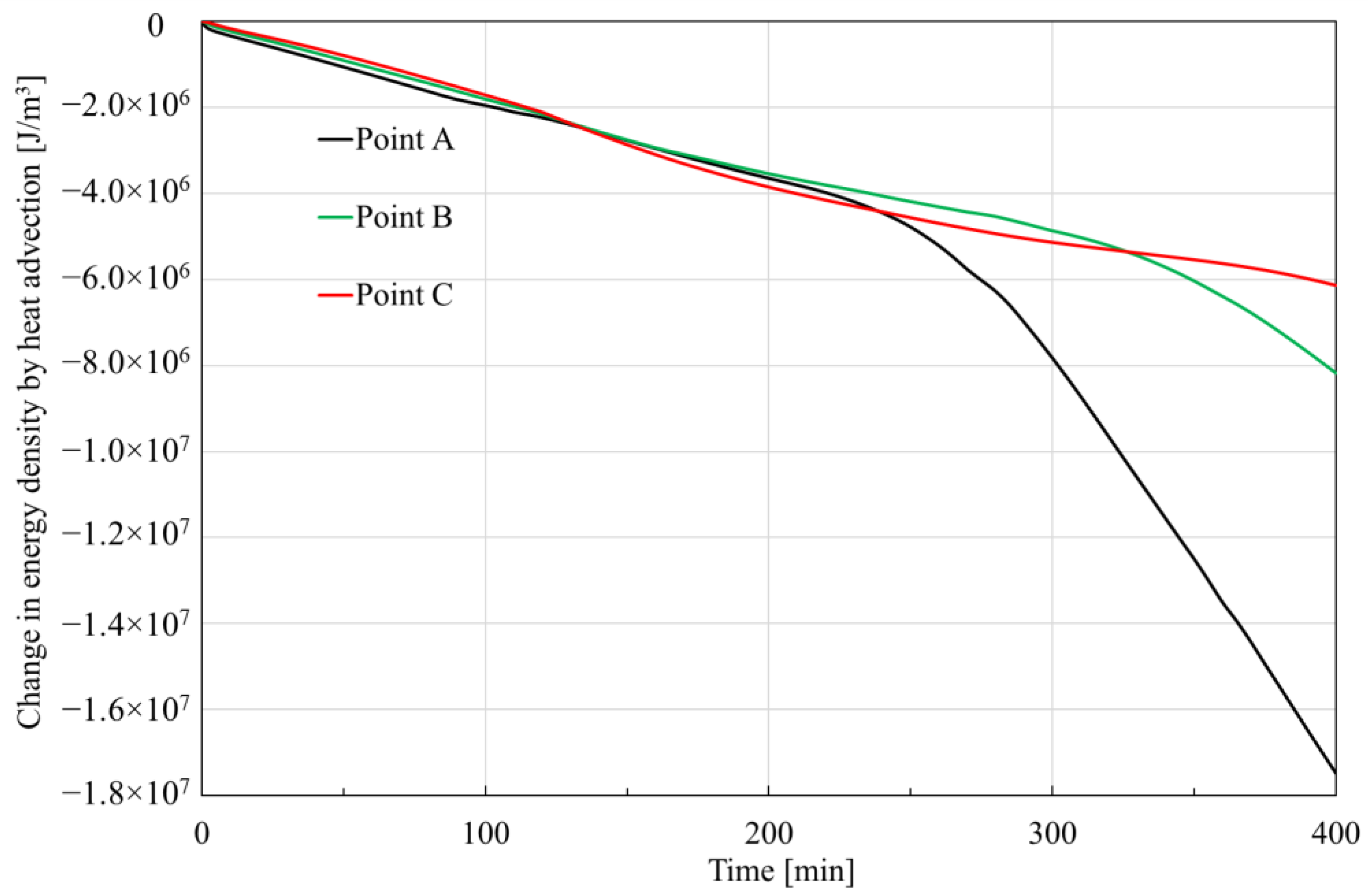
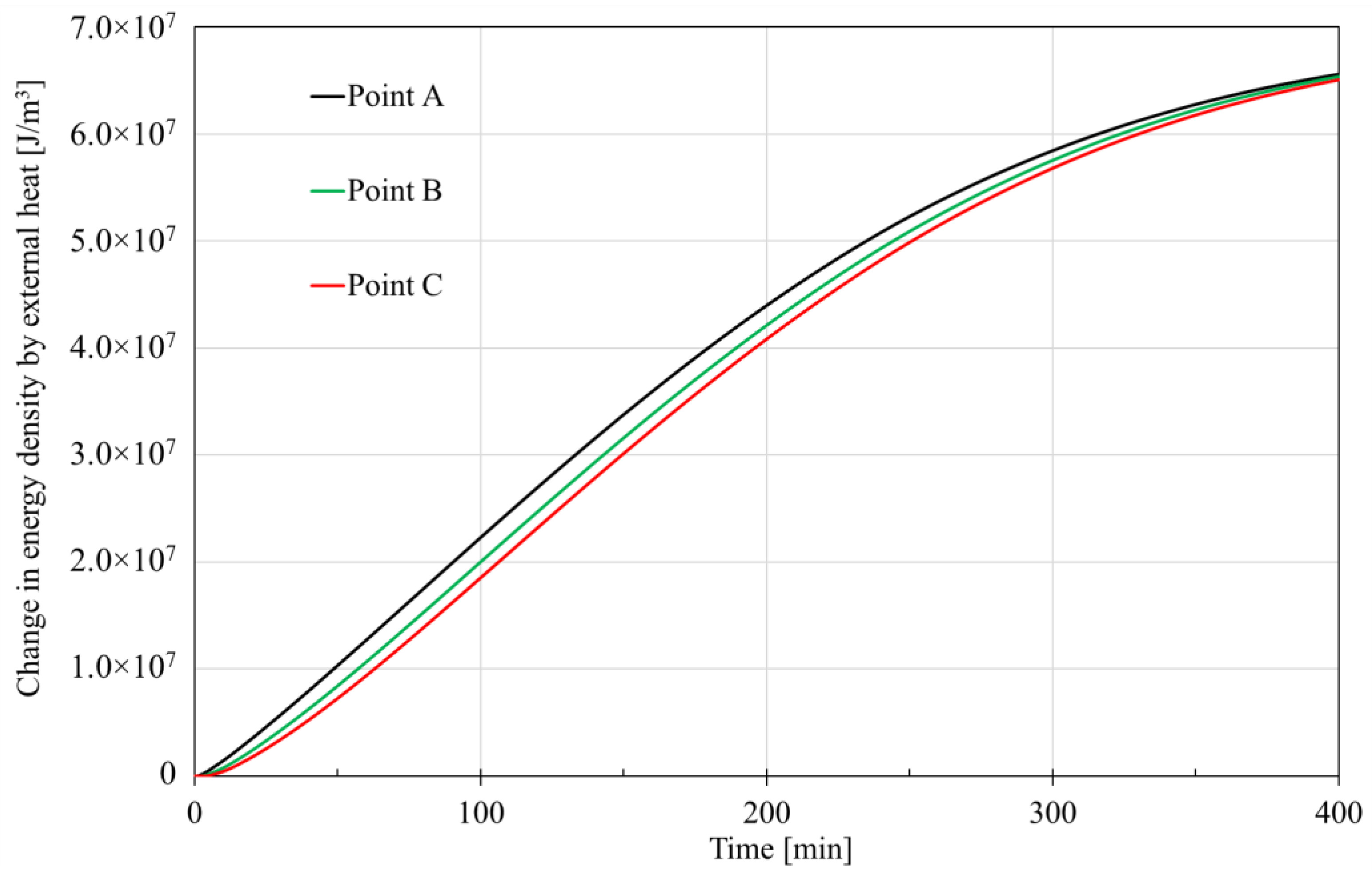
3.3.3. Change in Energy
4. Discussion
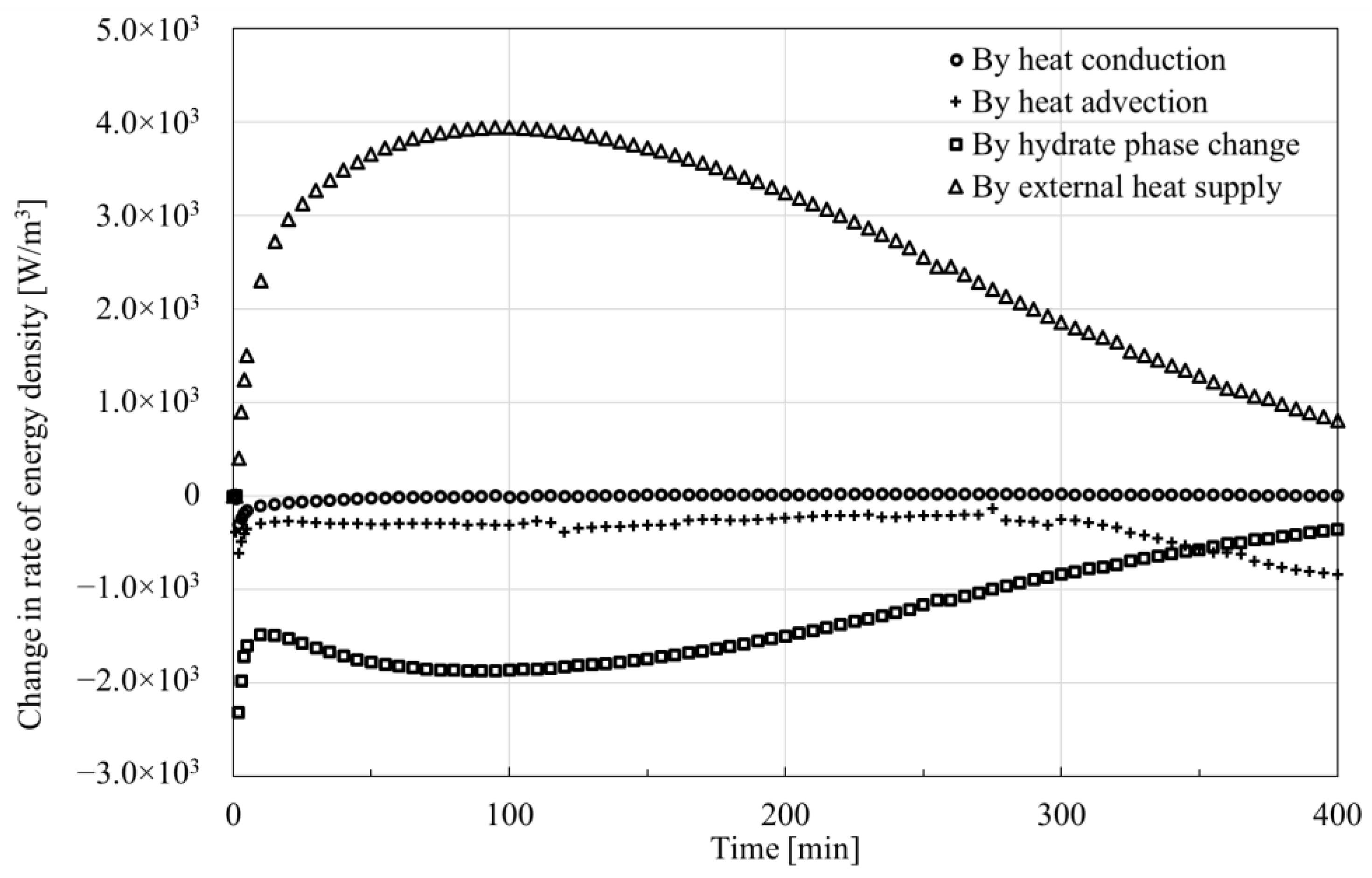
4.1. Comparison Among Contributions of Influence Factors to the Change in Energy Density
4.2. Implications for Lab-Scale Experiments and In Situ Engineering
5. Conclusions
- (1)
- The trends and values of conduction heat and advection heat show different patterns during hydrate dissociation. The cumulative conduction heat shows a trend of increasing and then decreasing in the process of hydrate dissociation, while the cumulative advection heat shows a monotonically increasing trend. Additionally, the cumulative conduction heat is much smaller than the cumulative advection heat by one order of magnitude in the investigated case, which implicates that the laboratory tests of parameters related to the advection heat transfer should be more prudent.
- (2)
- Phase change, conduction heat, advection heat and external heat make various contributions to the change in energy. An external heat source makes a main contribution to the increase in energy, while phase change mainly governs the decrease in energy. The role of heat conduction can be almost neglected in spite of versatile patterns during hydrate dissociation, but the contribution of heat advection should still be considered.
- (3)
- This study has two implications for lab-scale experiments and in situ engineering. Future in situ designs should go further from temperature to heat and energy because temperature is the explicit phenomenon which would be measured, but heat and energy are the implicit essence directly related to the cost evaluation of real-world hydrate exploration. Additionally, refining the design of the external heat source is suggested to improve the similarity between lab-scale experiments and in situ engineering due to the dramatic role of external heat sources on the change in energy.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| THMC | Thermal–Hydraulic–Mechanical–Chemical |
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Linga, P.; Haligva, C.; Nam, S.C.; Ripmeester, J.A.; Englezos, P. Gas hydrate formation in a variable volume bed of silica sand particles. Energy Fuels 2009, 23, 5496–5507. [Google Scholar] [CrossRef]
- Walsh, M.R.; Hancock, S.H.; Wilson, S.J.; Patil, S.L.; Moridis, G.J.; Boswell, R.; Collett, T.S.; Koh, C.A.; Sloan, E.D. Preliminary report on the commercial viability of gas production from natural gas hydrates. Energy Econ. 2009, 31, 815–823. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, C.; Wu, M.; Peng, Y.; Yao, Y.; Luo, W. Review of exploration and production technology of natural gas hydrate. Adv. Geo-Energy Res. 2018, 2, 53–62. [Google Scholar] [CrossRef]
- Liu, L.; Dai, S.; Ning, F.; Cai, J.; Liu, C.; Wu, N. Fractal characteristics of unsaturated sands—Implications to relative permeability in hydrate-bearing sediments. J. Nat. Gas Sci. Eng. 2019, 66, 11–17. [Google Scholar] [CrossRef]
- Lei, L.; Gai, X.; Seol, Y. Load-bearing characteristic of methane hydrate within coarse-grained sediments—Insights from isotropic consolidation. Mar. Pet. Geol. 2020, 121, 104571. [Google Scholar] [CrossRef]
- Hyodo, M.; Wu, Y.; Nakashima, K.; Kajiyama, S.; Nakata, Y. Influence of Fines Content on the Mechanical Behavior of Methane Hydrate-Bearing Sediments. J. Geophys. Res. Solid Earth 2017, 122, 7511–7524. [Google Scholar] [CrossRef]
- Dai, S.; Cha, J.H.; Rosenbaum, E.J.; Zhang, W.; Seol, Y. Thermal conductivity measurements in unsaturated hydrate-bearing sediments. Geophys. Res. Lett. 2015, 42, 6295–6305. [Google Scholar] [CrossRef]
- Santamarina, J.; Dai, S.; Terzariol, M.; Jang, J.; Waite, W.; Winters, W.; Nagao, J.; Yoneda, J.; Konno, Y.; Fujii, T.; et al. Hydro-bio-geomechanical properties of hydrate-bearing sediments from Nankai Trough. Mar. Pet. Geol. 2014, 66, 434–450. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, X.; Bi, J.; He, G.; Guo, Z. Submarine slope stability analysis during natural gas hydrate dissociation. Mar. Georesources Geotechnol. 2019, 37, 467–476. [Google Scholar] [CrossRef]
- Uchida, S.; Klar, A.; Yamamoto, K. Sand production model in gas hydrate-bearing sediments. Int. J. Rock Mech. Min. Sci. 2016, 86, 303–316. [Google Scholar] [CrossRef]
- Nixon, M.F.; Grozic, J.L.H. Submarine slope failure due to gas hydrate dissociation: A preliminary quantification. Can. Geotech. J. 2007, 44, 314–325. [Google Scholar] [CrossRef]
- Ruppel, C.D.; Kessler, J.D. The interaction of climate change and methane hydrates. Rev. Geophys. 2017, 55, 126–168. [Google Scholar] [CrossRef]
- Nicholas, J.W.; Dieker, L.E.; Sloan, E.D.; Koh, C.A. Assessing the feasibility of hydrate deposition on pipeline walls-Adhesion force measurements of clathrate hydrate particles on carbon steel. J. Colloid Interface Sci. 2009, 331, 322–328. [Google Scholar] [CrossRef]
- Konno, Y.; Fujii, T.; Sato, A.; Akamine, K.; Naiki, M.; Masuda, Y.; Yamamoto, K.; Nagao, J. Key Findings of the World’s First Offshore Methane Hydrate Production Test off the Coast of Japan: Toward Future Commercial Production. Energy Fuels 2017, 31, 2607–2616. [Google Scholar] [CrossRef]
- Minshull, T.A.; Marín-Moreno, H.; Betlem, P.; Bialas, J.; Bünz, S.; Burwicz, E.; Cameselle, A.L.; Cifci, G.; Giustiniani, M.; Hillman, J.I.; et al. Hydrate occurrence in Europe: A review of available evidence. Mar. Pet. Geol. 2020, 111, 735–764. [Google Scholar] [CrossRef]
- White, M.D.; Kneafsey, T.; Seol, Y.; Waite, W.; Uchida, S.; Lin, J.; Myshakin, E.; Gai, X.; Gupta, S.; Reagan, M.; et al. An international code comparison study on coupled thermal, hydrologic and geomechanical processes of natural gas hydrate-bearing sediments. Mar. Pet. Geol. 2020, 120, 104566. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, B.; Luo, X.; Tang, M.; Zhang, X.; Yang, L.; Nie, Y.; Zhou, J.; Zhang, L.; Li, G. Multiphysical evolution and dynamic competition involved in natural gas hydrate dissociation in porous media and its implications for engineering. Energy 2024 2024, 289, 130032. [Google Scholar] [CrossRef]
- Yousif, M.H.; Abass, H.H.; Selim, M.S.; Sloan, E.D. Experimental and theoretical investigation of methane-gas-hydrate dissociation in porous media. SPE Reserv. Eng. Soc. Pet. Eng. 1991, 6, 69–76. [Google Scholar] [CrossRef]
- Masuda, Y.; Fujinaga, Y.; Naganawa, S.; Fujita, K.; Sato, K.; Hayashi, Y. Modeling and experimental studies on dissociation of methane gas hydrates in Berea sandstone cores. In Proceedings of the 3rd International Conference on Gas Hydrates, Salt Lake City, UT, USA, 18–22 July 1999. [Google Scholar]
- Goel, N.; Wiggins, M.; Shah, S. Analytical modeling of gas recovery from in situ hydrates dissociation. J. Pet. Sci. Eng. 2001, 29, 115–127. [Google Scholar] [CrossRef]
- Kwon, T.H.; Cho, G.C.; Santamarina, J.C. Gas hydrate dissociation in sediments: Pressure-temperature evolution. Geochem. Geophys. Geosystems 2008, 9, 1–14. [Google Scholar] [CrossRef]
- Sánchez, M.; Santamarina, C.; Teymouri, M.; Gai, X. Coupled Numerical Modeling of Gas Hydrate-Bearing Sediments: From Laboratory to Field-Scale Analyses. J. Geophys. Res. Solid Earth 2018, 123, 10326–10348. [Google Scholar] [CrossRef]
- Yun, T.S.; Fratta, D.; Santamarina, J.C. Hydrate-Bearing sediments from the krishna-godavari basin: Physical characterization, pressure core testing, and scaled production monitoring. Energy Fuels 2010, 24, 5972–5983. [Google Scholar] [CrossRef]
- Song, R.; Feng, X.; Wang, Y.; Sun, S.; Liu, J. Dissociation and transport modeling of methane hydrate in core-scale sandy sediments: A comparative study. Energy 2021, 221, 119890. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Chen, J.; Yin, Z.; Rossi, F.; Tronconi, E.; Mei, S. Experimental study on the competition between carbon dioxide hydrate and ice below the freezing point. Chem. Eng. Sci. 2023, 268, 118426. [Google Scholar]
- Wan, Y.; Wu, N.; Chen, Q.; Li, W.; Hu, G.; Huang, L.; Ouyang, W. Coupled thermal-hydrodynamic-mechanical–chemical numerical simulation for gas production from hydrate-bearing sediments based on hybrid finite volume and finite element method. Comput. Geotech. 2022, 145, 104692. [Google Scholar] [CrossRef]
- Yin, F.; Gao, Y.; Chen, Y.; Sun, B.; Li, S.; Zhao, D. Numerical investigation on the long-term production behavior of horizontal well at the gas hydrate production site in South China Sea. Appl. Energy 2022, 311, 118603. [Google Scholar]
- Li, Q.; Liu, J.; Wang, S.; Guo, Y.; Han, X.; Li, Q.; Cheng, Y.; Dong, Z.; Li, X.; Zhang, X. Numerical insights into factors affecting collapse behavior of horizontal wellbore in clayey silt hydrate-bearing sediments and the accompanying control strategy. Ocean. Eng. 2024, 297, 117029. [Google Scholar]
- Dong, L.; Wu, N.; Leonenko, Y.; Wan, Y.; Liao, H.; Hu, G.; Li, Y. A coupled thermal-hydraulic-mechanical model for drilling fluid invasion into hydrate-bearing sediments. Energy 2023, 278, 127785. [Google Scholar]
- Kim, H.C.; Bishnoi, P.R.; Heidemann, R.A.; Rizvi, S.S.H. Kinetics of methane hydrate dissociation. Chem. Eng. Sci. 1987, 42, 1645–1653. [Google Scholar] [CrossRef]
- Clarke, M.; Snobbish, P.R. Determination of the activation energy and intrinsic rate constant of methane gas hydrate dissociation. Can. J. Chem. Eng. 2001, 79, 143–147. [Google Scholar] [CrossRef]
- Carman, P.C. Permeability of saturated sands, soils and cays. J. Agric. Sci. 1939, 29, 262–273. [Google Scholar] [CrossRef]
- Hardwick, J.S.; Mathias, S.A. Masuda’s sandstone core hydrate dissociation experiment revisited. Chem. Eng. Sci. 2018, 175, 98–109. [Google Scholar] [CrossRef]
- Nazridoust, K.; Ahmadi, G. Computational modeling of methane hydrate dissociation in a sandstone core. Chem. Eng. Sci. 2007, 62, 6155–6177. [Google Scholar] [CrossRef]
- Sun, X.; Luo, H.; Soga, K. A coupled thermal–hydraulic–mechanical–chemical (THMC) model for methane hydrate bearing sediments. J. Zhejiang Univ.-Sci. A Appl. Phys. Eng. 2018, 19, 600–623. [Google Scholar]
- Song, Y.C.; Liang, H.F. 2-D numerical simulation of natural gas hydrate dissociation through depressurization by fully implicit method. China Ocean. Eng. 2009, 23, 529–542. [Google Scholar]
- Tanaka, M.; Girard, G.; Davis, R.; Peuto, A.; Bignell, N. Recommended table for the density of water between 0 °C and 40 °C based on recent experimental reports. Metrologia 2001, 38, 301–309. [Google Scholar] [CrossRef]
- Younglove, B.A.; Ely, J.F. Thermophysical properties of fluids. II. Methane, ethane, propane, isobutane, and normal butane. J. Phys. Chem. Ref. Data 1987, 16, 577–798. [Google Scholar] [CrossRef]
- Likhachev, E.R. Dependence of water viscosity on temperature and pressure. Tech. Phys. 2003, 48, 514–515. [Google Scholar] [CrossRef]




| Phenomena | Governing Laws | Auxiliary Relations |
|---|---|---|
| Phase change | Mass conservation | Kinetic model for hydrate dissociation rate |
| Water flow | Mass conservation | Darcy’s law for seepage |
| Gas flow | Mass conservation | Darcy’s law for seepage |
| Stress redistribution | Momentum conservation | Elasticity theory for force equilibrium |
| Heat transfer | Energy conservation | Heat conduction and advection theory |
| Parameters | Materials | Symbols | Values |
|---|---|---|---|
| Molar mass [kg/mol] | Hydrate | Mh | 0.1195 |
| Water | Mw | 0.018 | |
| Methane | Mg | 0.016 | |
| Heat capacity [kg/m3] | Matrix | Cs | 800 |
| Hydrate | Ch | 2010 | |
| Water | Cg | 2180 | |
| Methane | Cw | 4200 | |
| Thermal conductivity [W/m/K] | Matrix | λs | 8.8 |
| Hydrate | λh | 0.57 | |
| Water | λg | 0.0335 | |
| Methane | λw | 0.6 | |
| Density [kg/m3] | Matrix | ρs | 2650 |
| Hydrate | ρh | 913 | |
| Water a | ρw | Check notes | |
| Methane b | ρg | Check notes | |
| Poisson’s ratio [-] | Matrix | νb | 0.2 |
| Hydrate | νh | 0.32 | |
| Young’s modulus [GPa] | Matrix | Eb | 20 |
| Hydrate | Eh | 9.06 | |
| Dynamic viscosity [Pa] | Water c | μg | Check notes |
| Methane b | μw | Check notes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Zhang, H.; Shi, Y.; Zhou, J.; Fu, L. Changes in Heat and Energy During Depressurization-Induced Natural Gas Hydrate Dissociation in Porous Media. Processes 2025, 13, 1023. https://doi.org/10.3390/pr13041023
Zhu M, Zhang H, Shi Y, Zhou J, Fu L. Changes in Heat and Energy During Depressurization-Induced Natural Gas Hydrate Dissociation in Porous Media. Processes. 2025; 13(4):1023. https://doi.org/10.3390/pr13041023
Chicago/Turabian StyleZhu, Mengchen, Haitao Zhang, Yunwei Shi, Jiaxing Zhou, and Liang Fu. 2025. "Changes in Heat and Energy During Depressurization-Induced Natural Gas Hydrate Dissociation in Porous Media" Processes 13, no. 4: 1023. https://doi.org/10.3390/pr13041023
APA StyleZhu, M., Zhang, H., Shi, Y., Zhou, J., & Fu, L. (2025). Changes in Heat and Energy During Depressurization-Induced Natural Gas Hydrate Dissociation in Porous Media. Processes, 13(4), 1023. https://doi.org/10.3390/pr13041023






