Preparation and Optimization of Steel Slag-Desulfurization Gypsum Composites Based on Interception of Arsenic-Contaminated Water at the Ground Surface
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials
2.2. Experimental Methods
2.2.1. Experimental Materials
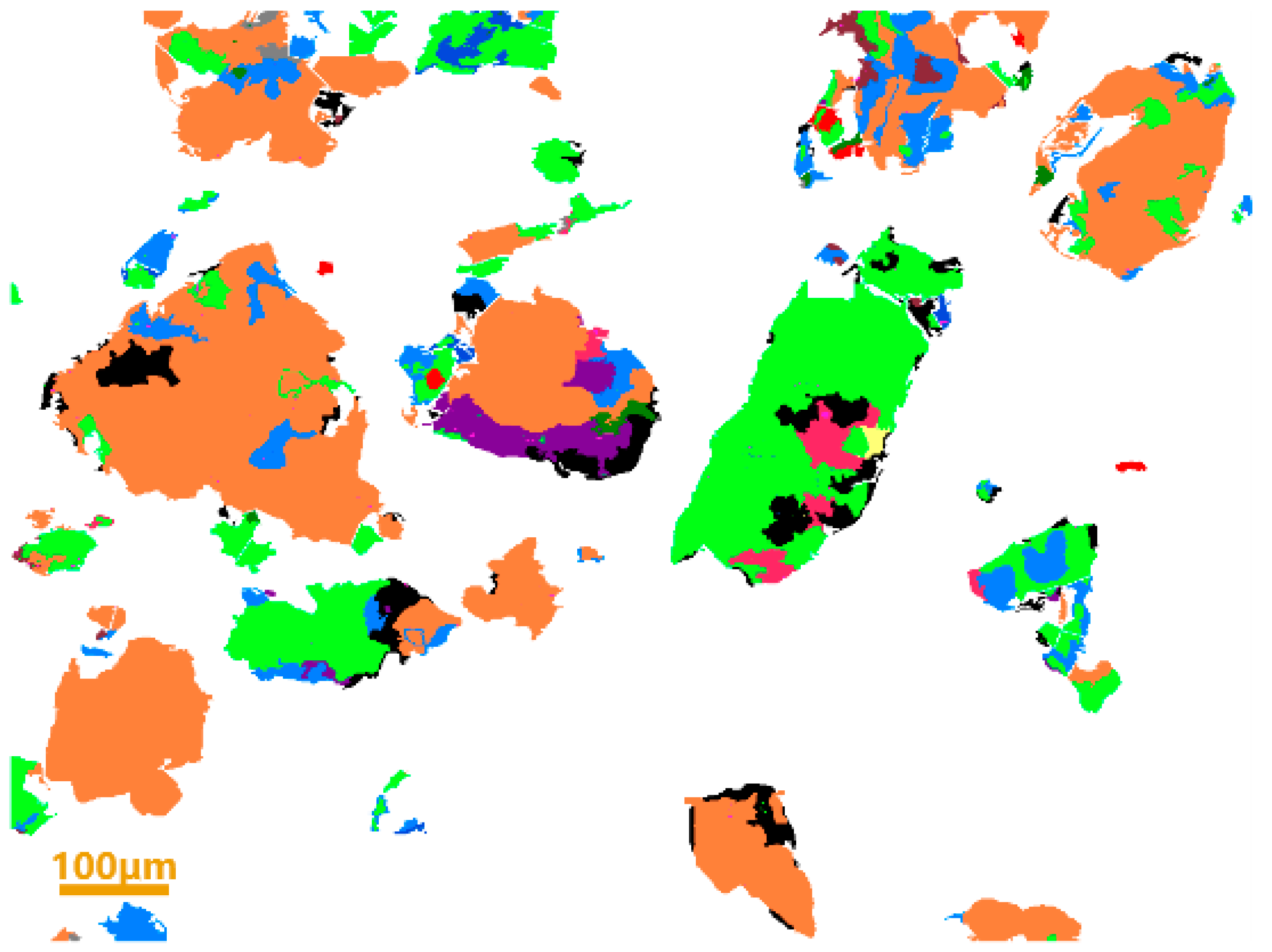
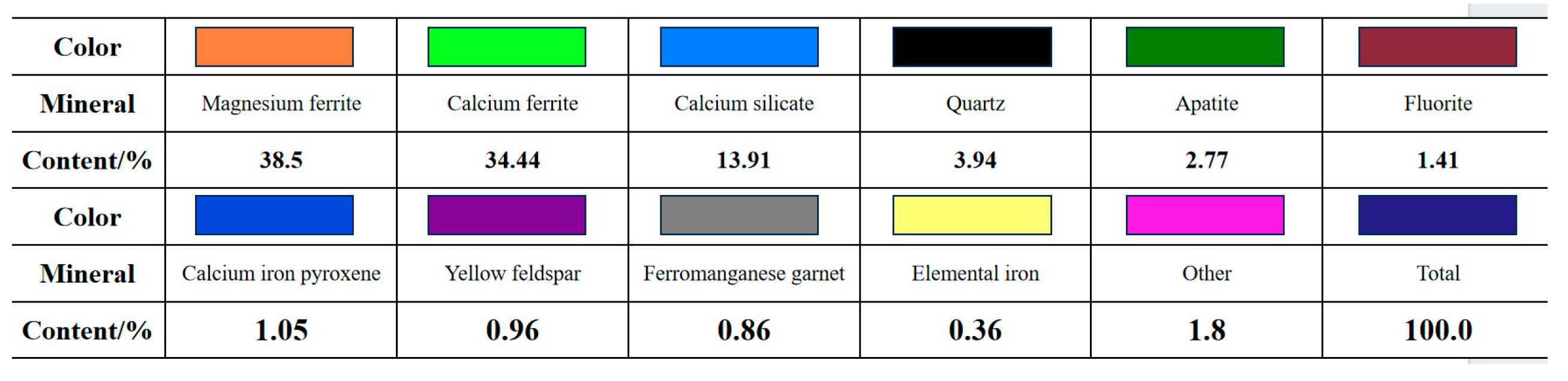
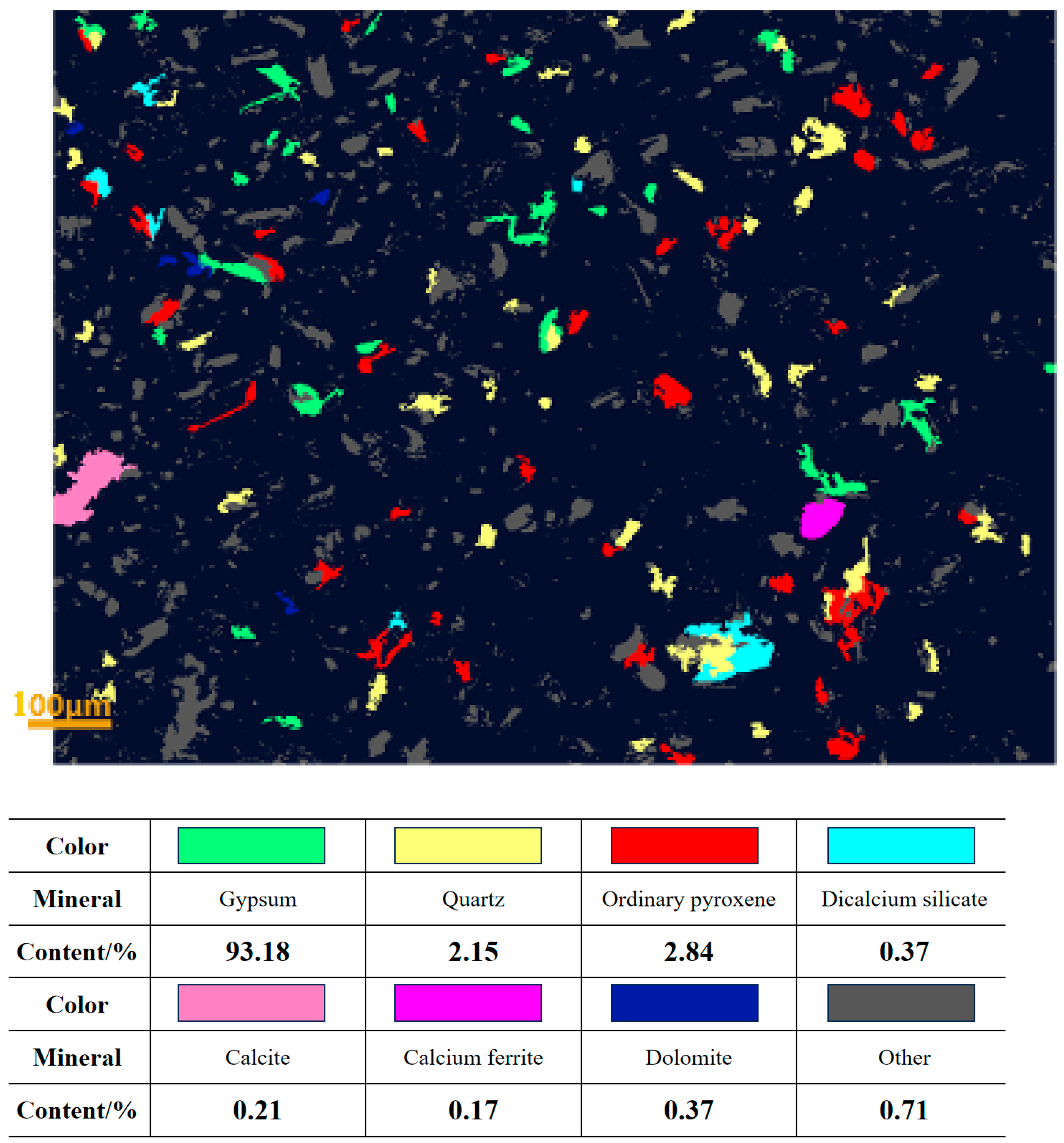
2.2.2. Physicochemical Properties of Experimental Materials
2.2.3. Permeable Reactive Barrier Simulation Experiments
3. Results and Discussion
3.1. Effects of Steel Slag Particle Size on the Removal of Arsenic and Antimony
3.2. Effects of Particle Size of Desulfurized Gypsum on the Removal of Arsenic and Antimony
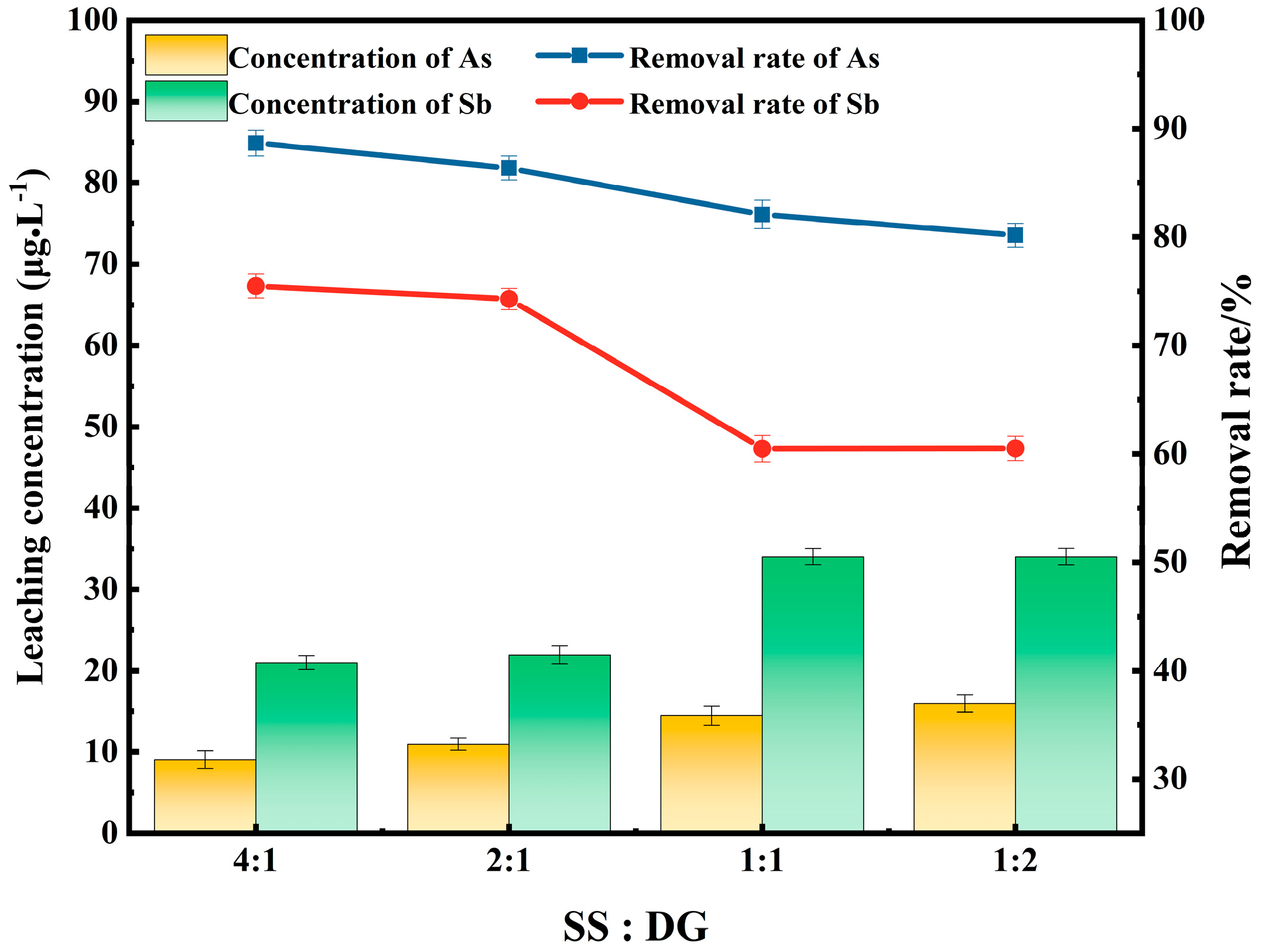
3.3. Effects of the Ratio of Steel Slag and Desulfurization Gypsum on the Removal of Arsenic and Antimony
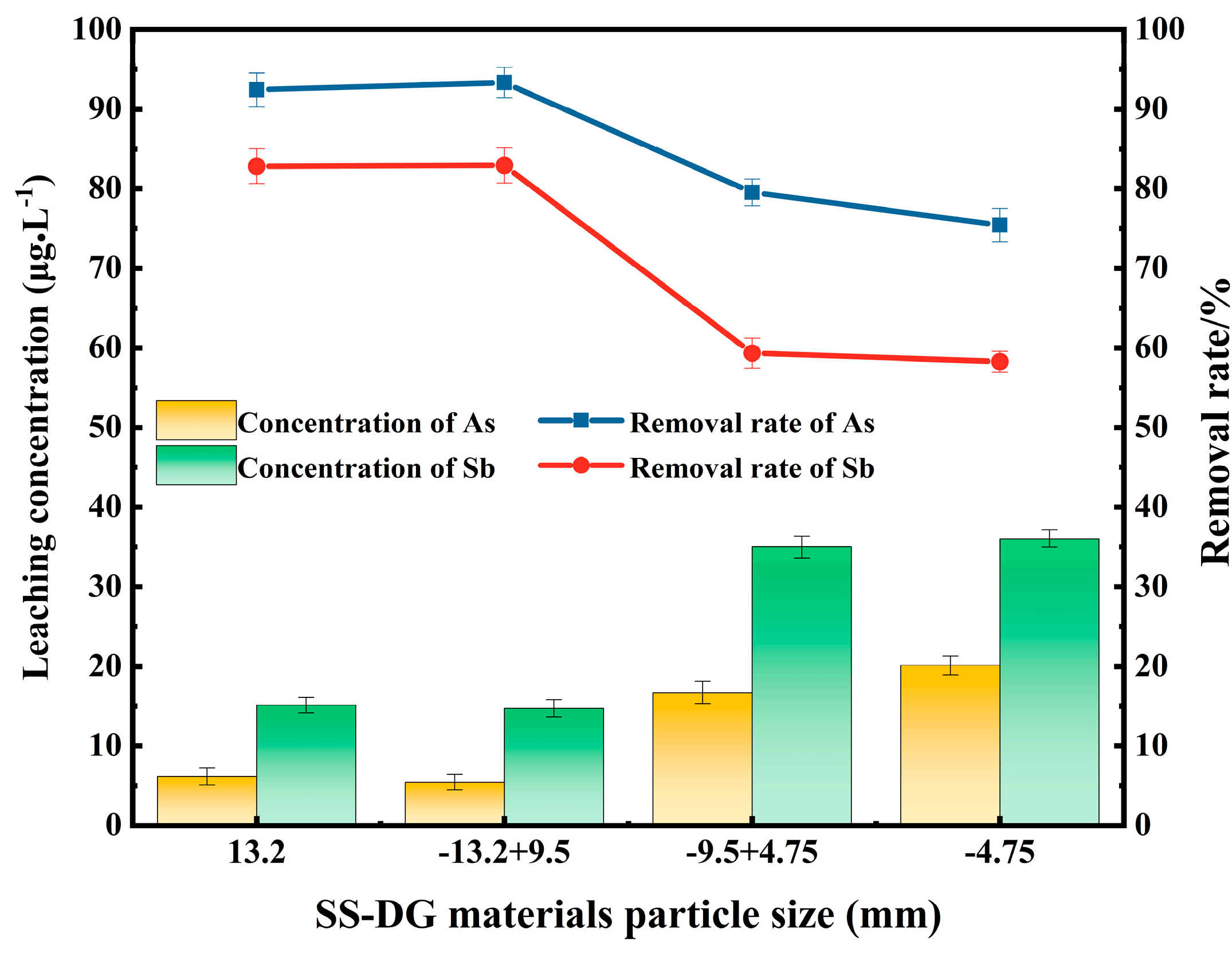
3.4. Effects of Particle Size of SS-DG Mixed Test Block on the Removal of Arsenic and Antimony
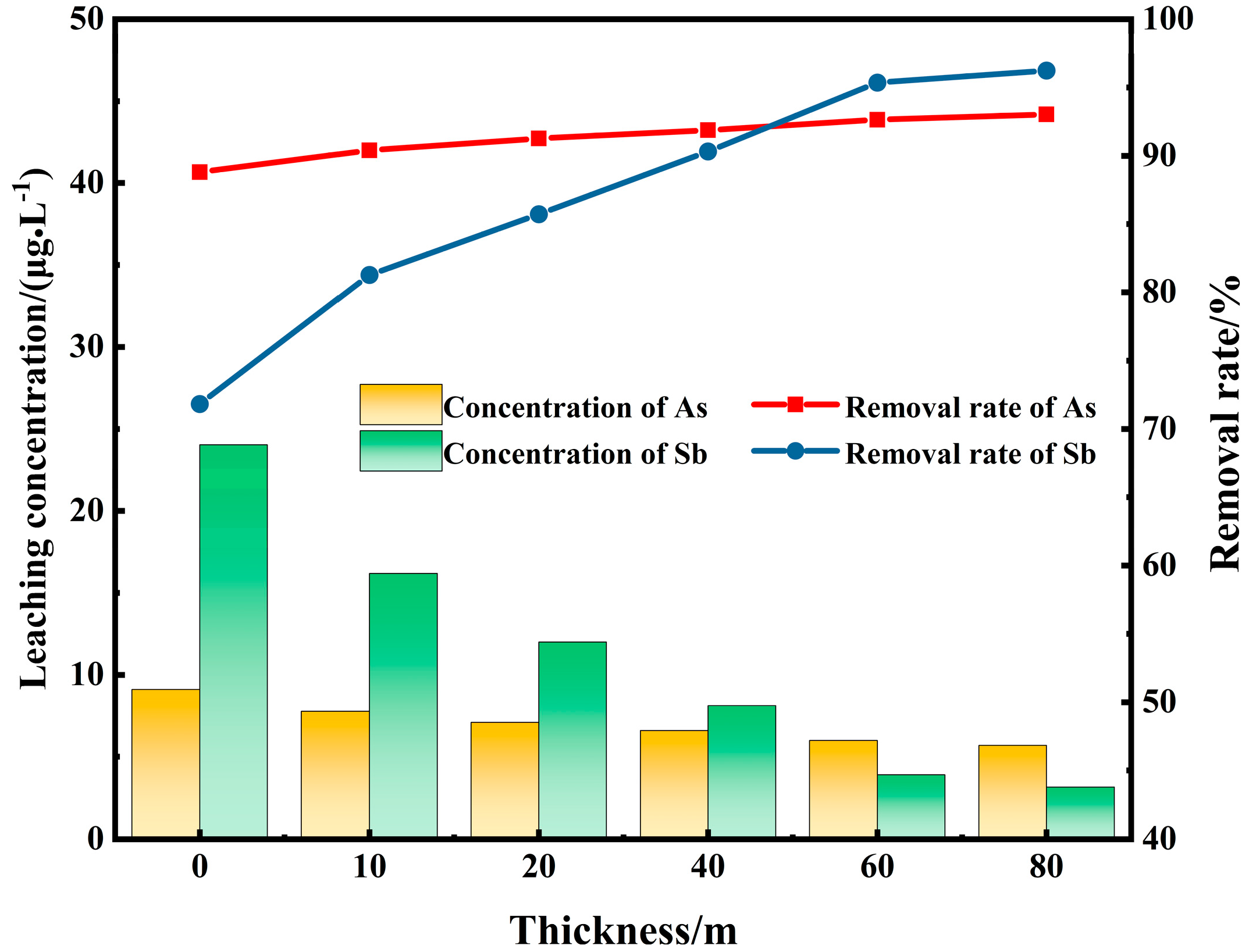
3.5. Permeable Reactive Wall Thickness Simulation Test
3.6. EDS Analysis of Arsenic Enrichment Area
4. Conclusions
- (1)
- The effects of the particle sizes of steel slag and desulfurization gypsum, the ratio of steel slag to desulfurization gypsum, and the particle size of the SS-DG mixed test block on the removal of arsenic and antimony by the permeable reactive wall were investigated. Results indicated that the steel slag with a particle size of −4.75 + 1.18 mm and the desulfurization gypsum block with a particle size of −13.2 + 9.5 mm mixed evenly at a ratio of 4:1 make a 40 m permeable reactive wall. After treatment, the As content in the polluted water was 6 μg/L, and the Sb content was 3.9 μg/L, which was lower than the standard of drinking water. The removal rates of As and Sb were 91.85% and 90.58%, respectively. The purpose of using steel slag and desulfurization gypsum to intercept heavy metals and toxic ions in surface runoff was achieved.
- (2)
- The formation of C-S-H gel and ettringite in the dielectric material is the main way to stabilize As. This stabilization occurs through several processes, including the adsorption, physical encapsulation, and lattice solidification of C-S-H gel, as well as the ion exchange reaction of ettringite with arsenic ions and arsenate. Together, these mechanisms can synergistically improve the stabilization effect of As.
- (3)
- Although this study confirmed the efficient removal of arsenic and antimony by SS-DG composites, the following limitations and research gaps still exist: the experiments were based on laboratory simulation conditions without considering the effects of the dynamic flow of groundwater and the interfering effects of competing ions in practical applications; the long-term stability of the materials and the adsorption mechanism still need to be verified; in addition, the engineering feasibility of large-scale applications and the assessment of the environmental benefits of the whole life cycle. In addition, the engineering feasibility of large-scale application and the assessment of the environmental benefits of the whole life cycle have not been carried out. In the future, it is necessary to combine dynamic flow field experiments and field pilot tests to improve the practicality and sustainability of the technology.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sultan, M.W.; Qureshi, F.; Ahmed, S.; Kamyab, H.; Rajendran, S.; Ibrahim, H.; Yusuf, M. A comprehensive review on arsenic contamination in groundwater: Sources, detection, mitigation strategies and cost analysis. Environ. Res. 2025, 265, 120457. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, W.; Meng, D.; Ma, C.; Li, H. Investigation of arsenic contamination in soil and plants along the river of Xinzhou abandoned gold mine in Qingyuan, China. Chemosphere 2024, 359, 142350. [Google Scholar] [CrossRef] [PubMed]
- Strawn, D.G. Review of interactions between phosphorus and arsenic in soils from four case studies. Geochem. Trans. 1028, 19, 10. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, W.; Li, J.; Cui, D.; Zhang, Z.; Sun, G.; Zhu, Y.; Yang, H.; Zhang, X. Arsenic pollution concerning surface water and sediment of Jie River: A pilot area where gold smelting enterprises are concentrated. Environ. Res. 2024, 249, 118384. [Google Scholar] [CrossRef]
- Tian, S.; Liu, Z.; Mao, Q.; Ye, H.; Tian, C.; Zhu, Y.; Zhang, L. Leaching characteristics and environmental impact of heavy metals in tailings under rainfall conditions: A case study of an ion-adsorption rare earth mining area. Ecotoxicol. Environ. Saf. 2024, 281, 116642. [Google Scholar] [CrossRef]
- Rinklebe, J.; Antoniadis, V.; Shaheen, S.M.; Rosche, O.; Altermann, M. Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ. Int. 2019, 126, 76–88. [Google Scholar] [CrossRef]
- Sevak, P.; Pushkar, B. Arsenic pollution cycle, toxicity and sustainable remediation technologies: A comprehensive review and bibliometric analysis. J. Environ. Manag. 2024, 349, 119504. [Google Scholar] [CrossRef]
- Choudhury, T.R.; Alam, S.; Alam, M.N.E.; Maksud, M.A.; Khan, S.R.; Habib, M.A. Innovative metal–phenolic nanocomposite sorbent: A groundbreaking solution for arsenic-free drinking water—Synthesis and characterization approaches. Desalin. Water Treat. 2024, 320, 100764. [Google Scholar] [CrossRef]
- Bauer, M.; Blodau, C. Arsenic distribution in the dissolved, colloidal and particulate size fraction of experimental solutions rich in dissolved organic matter and ferric iron. Geochim. Cosmochim. Acta 2009, 73, 529–542. [Google Scholar] [CrossRef]
- Ghosh, S.; Debsarkar, A.; Dutta, A. Technology alternatives for decontamination of arsenic-rich groundwater—A critical review. Environ. Technol. Innov. 2019, 13, 277–303. [Google Scholar] [CrossRef]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A review on decontamination of arsenic-contained water by electrocoagulation: Reactor configurations and operating cost along with removal mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Bahar, M.M.; Mahbub, K.R.; Naidu, R.; Megharaj, M. As(V) removal from aqueous solution using a low-cost adsorbent coir pith ash: Equilibrium and kinetic study. Environ. Technol. Innov. 2018, 9, 198–209. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Ghosal, P.S.; Mukherjee, A.; Gupta, A.K. A review on the management of arsenic-laden spent adsorbent: Insights of global practices, process criticality, and sustainable solutions. Environ. Technol. Innov. 2022, 27, 102500. [Google Scholar] [CrossRef]
- Das, T.K.; Bezbaruah, A.N. Comparative study of arsenic removal by iron-based nanomaterials: Potential candidates for field applications. Sci. Total Environ. 2021, 764, 142914. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Natural attenuation processes for remediation of arsenic contaminated soils and groundwater. J. Hazard. Mater. 2006, 138, 459–470. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Murciego, A. Stabilization methods for the treatment of weathered arsenopyrite mine wastes: Arsenic immobilization under selective leaching conditions. J. Clean. Prod. 2021, 283, 125265. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Tran, H.N.; Vu, H.A.; Trinh, M.V.; Nguyen, T.V.; Loganathan, P.; Vigneswaran, S.; Nguyen, T.M.; Trinh, V.T.; Vu, D.L.; et al. Laterite as a low-cost adsorbent in a sustainable decentralized filtration system to remove arsenic from groundwater in Vietnam. Sci. Total Environ. 2020, 699, 134267. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, J.; Gu, J.; Liu, S.; Huang, Y.; Sadeghi, H. Influence of biopolymer-vegetation interaction on soil hydro-mechanical properties under climate change: A review. Sci. Total Environ. 2024, 954, 176535. [Google Scholar] [CrossRef]
- Ghadir, P.; Zamanian, M.; Mahbubi-Motlagh, N.; Saberian, M.; Li, J.; Ranjbar, N. Shear strength and life cycle assessment of volcanic ash-based geopolymer and cement stabilized soil: A comparative study. Transp. Geotech. 2021, 31, 100639. [Google Scholar] [CrossRef]
- Wang, H.; Ju, C.; Zhou, M.; Chen, J.; Dong, Y.; Hou, H. Sustainable and efficient stabilization/solidification of Pb, Cr, and Cd in lead-zinc tailings by using highly reactive pozzolanic solid waste. J. Environ. Manag. 2022, 306, 114473. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Mao, L.; Hashmi, M.Z.; Xu, F.; Tang, X. Stabilization/solidification of chromium-bearing electroplating sludge with alkali-activated slag binders. Chemosphere 2020, 240, 124885. [Google Scholar] [CrossRef] [PubMed]
- Khadka, S.D.; Jayawickrama, P.W.; Senadheera, S.; Segvic, B. Stabilization of highly expansive soils containing sulfate using metakaolin and fly ash based geopolymer modified with lime and gypsum. Transp. Geotech. 2020, 23, 100327. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.-F.; Yang, H.; Bao, C.-J.; Wang, J.-S.; Sun, Y.-H.; Liu, D.-W.; Shen, P.-L.; Su, C. Red mud-metakaolin based cementitious material for remediation of arsenic pollution: Stabilization mechanism and leaching behavior of arsenic in lollingite. J. Environ. Manag. 2021, 300, 113715. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Cheng, T.-W.; Ding, Y.-C.; Lin, K.-L.; Tsao, S.-W.; Huang, C.-P. Geopolymer technology for the solidification of simulated ion exchange resins with radionuclides. J. Environ. Manag. 2019, 235, 19–27. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Osorio-López, C.; Seco-Reigosa, N.; Garrido-Rodríguez, B.; Cutillas-Barreiro, L.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. As(V) adsorption on forest and vineyard soils and pyritic material with or without mussel shell: Kinetics and fractionation. J. Taiwan Inst. Chem. Eng. 2014, 45, 1007–1014. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, S.; Li, X.; Wang, X.; Jia, Y. Simultaneous removal and oxidation of arsenic from water by δ-MnO2 modified activated carbon. J. Environ. Sci. 2020, 94, 147–160. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. Industrial waste materials as adsorbents for the removal of As and other toxic elements from an abandoned mine spoil heap leachate: A case study in Asturias. J. Hazard. Mater. 2020, 384, 121446. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Alam, M.A.; Alam, M.O.; Chakraborty, S.; Owens, G.; Bhattacharya, T.; Mondal, N.K. Enhanced aqueous phase arsenic removal by a biochar based iron nanocomposite. Environ. Technol. Innov. 2020, 19, 100936. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Ho, T.-B.-C.; Huang, C.P.; Chen, C.-W.; Chen, W.-H.; Hsieh, S.; Hsieh, S.-L.; Dong, C.-D. Adsorption of lead(II) onto PE microplastics as a function of particle size: Influencing factors and adsorption mechanism. Chemosphere 2022, 304, 135276. [Google Scholar] [CrossRef]
- Vannier, S.; Gossard, A.; Magnier, L.; Proust, V.; David, T.; Grandjean, A. Effects of adding zeolite particles on the hierarchical microstructure of zeolite-geopolymer composites and their Sr2+ adsorption properties. Mater. Des. 2024, 244, 113233. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Y.; Chen, X.; Yan, H. Fulvic acid removal from landfill contaminated groundwater by a permeable reactive barrier: From laboratory to field-scale analyses. J. Environ. Chem. Eng. 2024, 12, 114752. [Google Scholar] [CrossRef]
- Sakr, M.; El Agamawi, H.; Klammler, H.; Mohamed, M.M. A review on the use of permeable reactive barriers as an effective technique for groundwater remediation. Groundw. Sustain. Dev. 2023, 21, 100914. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Li, X.; Liu, Y.; Yu, H.; Ma, M. Porous geopolymer with controllable interconnected pores—A viable permeable reactive barrier filler for lead pollutant removal. Chemosphere 2022, 307, 136128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, F.; Yao, X.; Cao, H.; Duan, W.; Dong, X. The synergistic action mechanisms of ternary industrial waste stabilized lead ion contaminated soil. Constr. Build. Mater. 2023, 409, 133827. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Kurwadkar, S.; Wilkin, R.T. Long-term performance evaluation of zero-valent iron amended permeable reactive barriers for groundwater remediation—A mechanistic approach. Geosci. Front. 2023, 14, 101494. [Google Scholar] [CrossRef]
- Ma, M.; Ha, Z.; Lv, C.; Xu, X.; Li, C.; Du, D.; Zhang, T.C.; Chi, R. A novel passivator based on electrolytic manganese residues and calcite for arsenic sorption and heavy metal passivation of contaminated soil. J. Clean. Prod. 2023, 414, 137544. [Google Scholar] [CrossRef]
- Mei, Y.; Zhuang, S.; Wang, J. Adsorption of heavy metals by biochar in aqueous solution: A review. Sci. Total Environ. 2025, 968, 178898. [Google Scholar] [CrossRef]







| Elements | As | Sb | Cr | Pb | Cd | Cu | Hg | Zn |
|---|---|---|---|---|---|---|---|---|
| WRJL | 75 | 85 | 7.4 | 12 | 42 | 1580 | <0.01 | 1070 |
| TLY | 81 | 86 | 7.1 | 15 | 51 | 1360 | <0.01 | 1550 |
| Standard | 10 | / | 50 | 10 | 5 | 1000 | 1 | 1000 |
| Elements | Ca | Fe | Mg | Mn | Si | Al | As |
|---|---|---|---|---|---|---|---|
| Steel slag (SS) | 30.35 | 18.96 | 4.08 | 2.14 | 6.83 | 1.58 | 0 |
| Desulfurization gypsum (DG) | 21.8 | 0.251 | 0 | 0 | 0.876 | 0.328 | 0 |
| Elements | Cd | Cr | Cu | Hg | Pb | Sb | Zn |
| Steel slag (SS) | 0 | 0.123 | 0.001 | 0 | 0 | 0 | 0.012 |
| Desulfurization gypsum (DG) | 0 | 0.001 | 0 | 0 | 0 | 0 | 0.003 |
| No. | Parameters | The Particle Size of SS/mm | The Particle Size of DG/mm | SS: DG | The Particle Size of SS-DG Materials/mm | Residence Time/s |
|---|---|---|---|---|---|---|
| A | The particle size of SS | +9.5, −9.5 + 4.75, −4.75 + 1.18, and −1.18 | Unbroken | 1:1 | ― | 20 |
| B | The particle size of DG | −4.75 + 1.18 | +13.2, −13.2 + 9.5, −9.5 + 4.75, −4.75 | 1:1 | ― | 20 |
| C | SS: DG | −4.75 + 1.18 | −13.2 + 9.5 | 4:1, 2:1, 1:1, 1:2 | ― | 20 |
| D | The particle size of SS-DG materials | −0.074 | −0.074 | 4:1 | 13.2, +9.5, −9.5 + 4.74, −4.75 | 20 |
| F | Residence time | −4.75 + 1.18 | −13.2 + 9.5 | 4:1 | ― | 20, 60, 100, 200, 400, 800 |
| Filter Material Composition | As Content (μg/L) | As Removal Rate (%) | Sb Content (μg/L) | Sb Removal Rate (%) | |
|---|---|---|---|---|---|
| +4.75 mm − 1.18 mm steel slag, −13.2 mm + 9.5 mm desulfurization gypsum mixed with 4:1. | 9.1 | 88.77 | 21 | 75.58 | |
| Mixed test block | Unbroken | 6.1 | 92.47 | 15 | 82.56 |
| +9.5 mm | 5.4 | 93.33 | 14.7 | 82.91 | |
| Compounds | Ca | O | Al | Si | S | Fe | As | |
|---|---|---|---|---|---|---|---|---|
| Point 1 | Ettringite | 63.31 | 8.51 | 4.89 | 5.99 | 16.02 | 0.81 | 0.47 |
| Point 2 | C-S-H gel | 17.89 | 43.36 | 2.42 | 29.25 | 0.61 | 6.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, Y.; Hu, W.; Wang, D.; Wu, D.; Ni, W.; Yang, S. Preparation and Optimization of Steel Slag-Desulfurization Gypsum Composites Based on Interception of Arsenic-Contaminated Water at the Ground Surface. Processes 2025, 13, 1033. https://doi.org/10.3390/pr13041033
Li Y, Sun Y, Hu W, Wang D, Wu D, Ni W, Yang S. Preparation and Optimization of Steel Slag-Desulfurization Gypsum Composites Based on Interception of Arsenic-Contaminated Water at the Ground Surface. Processes. 2025; 13(4):1033. https://doi.org/10.3390/pr13041033
Chicago/Turabian StyleLi, Yunyun, Yubo Sun, Wentao Hu, Dongfang Wang, Dongxu Wu, Wen Ni, and Shanshan Yang. 2025. "Preparation and Optimization of Steel Slag-Desulfurization Gypsum Composites Based on Interception of Arsenic-Contaminated Water at the Ground Surface" Processes 13, no. 4: 1033. https://doi.org/10.3390/pr13041033
APA StyleLi, Y., Sun, Y., Hu, W., Wang, D., Wu, D., Ni, W., & Yang, S. (2025). Preparation and Optimization of Steel Slag-Desulfurization Gypsum Composites Based on Interception of Arsenic-Contaminated Water at the Ground Surface. Processes, 13(4), 1033. https://doi.org/10.3390/pr13041033







