Fungi for Sustainable Pharmaceutical Remediation: Enzymatic Innovations, Challenges, and Applications—A Review
Abstract
1. Introduction
2. Pharmaceuticals in the Environment
2.1. Sources of Pharmaceuticals in the Environment
2.2. Environmental and Ecological Impacts of Pharmaceuticals
3. Mycoremediation of Pharmaceuticals
4. Fungal Enzymes in Drug Metabolisms and Biodegradation
4.1. Laccases in Fungal Biodegradation of Pharmaceuticals
4.2. Peroxidases in Fungal Biodegradation of Pharmaceuticals
4.3. Cytochrome P450 in Fungal Biodegradation of Pharmaceuticals
4.4. Enzymatic Pathways and Toxicity in Pharmaceutical Degradation
4.5. Enzyme Immobilization for the Pharmaceutical Degradation
| Immobilization Method and Material | Laccase Source | Pharmaceutical | Concentration (mg/L) | Incubation Time (h) at ~25 °C | Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Adsorption | ||||||
| Polypropylene beads | Myceliophthora thermophila | Morphine | 1 | 0.5 | 100 | [148] |
| Pinewood-derived nanobiochar | Trametes versicolor | Carbamazepine | 0.02 | 24 | 80 | [149] |
| Mesoporous cellular foam | Trametes versicolor | Tetracycline | 1 | 1 | 100 | [150] |
| Bentonite-based mesoporous material | Trametes versicolor | Tetracycline | 10 | 3 | 60 | [151] |
| Pristine few-layer graphene | Trametes versicolor | Labetalol hydrochloride | 1 | 1.5 | 100 | [152] |
| Adsorption/Entrapment | ||||||
| Graphene oxide–alginate matrix | Aspergillus niger | Cetirizine dihydrochloride | 20 | 1 | 100 | [153] |
| Covalent bonding | ||||||
| Polyamide/polyethylenimine nanofibers | Trametes versicolor | Triclosan | 10 | 20 | 74 | [154] |
| Titania nanoparticles | Pycnoporus sanguineus | Diclofenac | 10 | 4 | 50 | [144] |
| Titania nanoparticles | Pycnoporus sanguineus | Acetaminophen | 10 | 4 | 90 | [144] |
| Polyvinylidene fluoride membrane with multi-walled carbon nanotubes | Trametes hirsuta | Diclofenac | 5 | 4 | 95 | [143] |
| Polyvinylidene fluoride membrane with multi-walled carbon nanotubes | Trametes hirsuta | Carbamazepine | 5 | 48 | 27 | [143] |
| Micro-biochar from pine wood (PW) and pig manure (PM) | Trametes versicolor | Diclofenac | 0.5 | 5 (PW)/2 (PM) | 99 | [145] |
| Chitosan macro-beads | Trametes versicolor | Diclofenac | 50 | 4 | 90 | [155] |
| Polyacrylonitrile–biochar composite nanofibrous membrane | Trametes versicolor | Diclofenac | 0.2 | 8 | 73 | [156] |
| Polyacrylonitrile−biochar composite nanofibrous membrane | Trametes versicolor | Chlortetracycline | 0.2 | 8 | 63 | [156] |
| Polyimide aerogels | Trametes versicolor | Carbamazepine | 0.02 | 24 | 74 | [157] |
| Commercial silica gel particles | Trametes versicolor | Sulfamethoxazole | 20 | 0.5 | 53 | [158] |
| Commercial silica gel particles | Trametes versicolor | Amoxicillin | 20 | 4 | 80 | [158] |
| Cross-linking | ||||||
| M-CLEA | Cerrena unicolor | Tetracycline | 100 | 48 | 100 | [146] |
| CLEA | Trametes versicolor | Diclofenac | 0.001 | 24 | 90 | [147] |
| Encapsulation | ||||||
| Poly(l-lactic acid)-co-poly(ε-caprolactone) nanofibers | Trametes versicolor | Naproxen | 1 | 24 | 90 | [142] |
| Poly(l-lactic acid)-co-poly(ε-caprolactone) nanofibers | Trametes versicolor | Diclofenac | 1 | 24 | 90 | [142] |
4.6. Enzymatic Bioreactors for Pharmaceutical Degradation
5. Challenges and Potentials of Pharmaceutical Mycoremediation
5.1. The Role of Omics Technologies
5.2. Enzyme-Related Challenges in Pharmaceutical Biodegradation
5.3. Strategies to Enhance Fungal Enzyme Efficiency in Pharmaceutical Biodegradation
5.3.1. Metabolic Engineering and Strain Optimization
5.3.2. Bioreactor Systems and Environmental Optimization
5.3.3. Recombinant Enzyme Production and Immobilization
5.3.4. Multi-Enzymatic Systems and Redox Partner Integration
5.3.5. Process Optimization for Enhanced Biodegradation
6. Conclusions and Future Outlooks
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puhlmann, N.; Vidaurre, R.; Kümmerer, K. Designing greener active pharmaceutical ingredients: Insights from pharmaceutical industry into drug discovery and development. Eur. J. Pharm. Sci. 2024, 192, 106614. [Google Scholar]
- Malmqvist, E.; Fumagalli, D.; Munthe, C.; Larsson, D.J. Pharmaceutical pollution from human use and the polluter pays principle. Public Health Ethics 2023, 16, 152–164. [Google Scholar] [PubMed]
- Zenker, A.; Cicero, M.R.; Prestinaci, F.; Bottoni, P.; Carere, M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus on the aquatic environment. J. Environ. Manag. 2014, 133, 378–387. [Google Scholar]
- Kock, A.; Glanville, H.C.; Law, A.C.; Stanton, T.; Carter, L.J.; Taylor, J.C. Emerging challenges of the impacts of pharmaceuticals on aquatic ecosystems: A diatom perspective. Sci. Total Environ. 2023, 878, 162939. [Google Scholar] [PubMed]
- Miettinen, M.; Khan, S.A. Pharmaceutical pollution: A weakly regulated global environmental risk. Rev. Eur. Comp. Int. Environ. Law 2022, 31, 75–88. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, A.; Bergmann, A.; Grüttner, G.; Carius, A. Pharmaceuticals in the environment: Global occurrence and potential cooperative action under the strategic approach to international chemicals management (SAICM). IWW Rhein.-Westfälisches Inst. Wasser 2015, 35, 823–835. [Google Scholar]
- Zhang, H.; Wang, X.C.; Zheng, Y.; Dzakpasu, M. Removal of pharmaceutical active compounds in wastewater by constructed wetlands: Performance and mechanisms. J. Environ. Manag. 2023, 325, 116478. [Google Scholar]
- Dinakarkumar, Y.; Gnanasekaran, R.; Reddy, G.K.; Vasu, V.; Balamurugan, P.; Murali, G. Fungal bioremediation: An overview of the mechanisms, applications, and future perspectives. Environ. Chem. Ecotoxicol. 2024, 6, 293–302. [Google Scholar]
- Torres-Farradá, G.; Thijs, S.; Rineau, F.; Guerra, G.; Vangronsveld, J. White rot fungi as tools for the bioremediation of xenobiotics: A review. J. Fungi 2024, 10, 167. [Google Scholar] [CrossRef]
- Khan, M.F.; Hof, C.; Niemcová, P.; Murphy, C.D. Recent advances in fungal xenobiotic metabolism: Enzymes and applications. World J. Microbiol. Biotechnol. 2023, 39, 296. [Google Scholar]
- Khan, M.F.; Murphy, C.D. Nitroreduction of flutamide by Cunninghamella elegans NADPH: Cytochrome P450 reductase. Biochem. Biophys. Rep. 2022, 29, 101209. [Google Scholar]
- Khan, M.F.; Hof, C.; Niemcova, P.; Murphy, C.D. Biotransformation of fluorinated drugs and xenobiotics by the model fungus Cunninghamella elegans. Methods Enzymol. 2024, 696, 251–285. [Google Scholar] [PubMed]
- Khan, M.F.; Murphy, C.D. Application of microbial biofilms in biocatalysis and biodegradation. In Enzymes for Pollutant Degradation; Springer Nature: Singapore, 2022; pp. 93–118. [Google Scholar]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The use of algae and fungi for removal of pharmaceuticals by bioremediation and biosorption processes: A review. Water 2019, 11, 1555. [Google Scholar] [CrossRef]
- Garg, S.; Kim, M.; Romero-Suarez, D. Current advancements in fungal engineering technologies for Sustainable Development Goals. Trends Microbiol. 2024, 33, 285–301. [Google Scholar]
- Lakhani, S.; Acharya, D.; Sakariya, R.; Sharma, D.; Patel, P.; Shah, M.; Prajapati, M. A comprehensive study of bioremediation for pharmaceutical wastewater treatment. Clean. Chem. Eng. 2022, 4, 100073. [Google Scholar]
- Boxall, A.B.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar]
- Szymonik, A.; Lach, J.; Malińska, K. Fate and removal of pharmaceuticals and illegal drugs present in drinking water and wastewater. Ecol. Chem. Eng. S 2017, 24, 65–85. [Google Scholar]
- Amobonye, A.; Aruwa, C.E.; Aransiola, S.; Omame, J.; Alabi, T.D.; Lalung, J. The potential of fungi in the bioremediation of pharmaceutically active compounds: A comprehensive review. Front. Microbiol. 2023, 14, 1207792. [Google Scholar]
- Park, J.; Kim, C.; Hong, Y.; Lee, W.; Chung, H.; Jeong, D.H.; Kim, H. Distribution and removal of pharmaceuticals in liquid and solid phases in the unit processes of sewage treatment plants. Int. J. Environ. Res. Public Health 2020, 17, 687. [Google Scholar] [CrossRef]
- Mosharaf, M.K.; Gomes, R.L.; Cook, S.; Alam, M.S.; Rasmussen, A. Wastewater reuse and pharmaceutical pollution in agriculture: Uptake, transport, accumulation and metabolism of pharmaceutical pollutants within plants. Chemosphere 2024, 364, 143055. [Google Scholar]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical pollution in aquatic environments: A concise review of environmental impacts and bioremediation systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Yan, Z.; Liu, J.; Wang, P.; Wang, Y. Bioaccumulation and trophic transfer of pharmaceuticals in food webs from a large freshwater lake. Environ. Pollut. 2017, 222, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [PubMed]
- Grabicová, K.; Grabic, R.; Fedorova, G.; Kolářová, J.; Turek, J.; Brooks, B.W.; Randák, T. Psychoactive pharmaceuticals in aquatic systems: A comparative assessment of environmental monitoring approaches for water and fish. Environ. Pollut. 2020, 261, 114150. [Google Scholar] [CrossRef]
- Weerasinghe, C.; Akhtar, N.; Uddin, M.H.; Rachamalla, M.; Sumon, K.A.; Islam, M.J.; Bhandari, R.K.; Rashid, H. Contraceptive-Pill-Sourced Synthetic Estrogen and Progestogen in Water Causes Decrease in GSI and HSI and Alters Blood Glucose Levels in Climbing Perch (Anabas testudineus). Hydrobiology 2022, 2, 19–35. [Google Scholar] [CrossRef]
- Khan, M.F.; Murphy, C.D. Bacterial degradation of the anti-depressant drug fluoxetine produces trifluoroacetic acid and fluoride ion. Appl. Microbiol. Biotechnol. 2021, 105, 9359–9369. [Google Scholar] [PubMed]
- Ifedinezi, O.V.; Nnaji, N.D.; Anumudu, C.K.; Ekwueme, C.T.; Uhegwu, C.C.; Ihenetu, F.C.; Obioha, P.; Simon, B.O.; Ezechukwu, P.S.; Onyeaka, H. Environmental antimicrobial resistance: Implications for food safety and public health. Antibiotics 2024, 13, 1087. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Bokhari, A.; Karimian, M.; Zahra, M.M.; Sillanpää, M.; Panchal, H.; Alrubaie, A.J.; Rezakhani, Y. A comprehensive review of various approaches for treatment of tertiary wastewater with emerging contaminants: What do we know? Environ. Monit. Assess. 2022, 194, 884. [Google Scholar]
- Desai, M.; Njoku, A.; Nimo-Sefah, L. Comparing environmental policies to reduce pharmaceutical pollution and address disparities. Int. J. Environ. Res. Public Health 2022, 19, 8292. [Google Scholar] [CrossRef]
- Lin, A.Y.; Tsai, Y.T. Occurrence of pharmaceuticals in Taiwan’s surface waters: Impact of waste streams from hospitals and pharmaceutical production facilities. Sci. Total Environ. 2009, 407, 3793–3802. [Google Scholar]
- Li, D.; Yang, M.; Hu, J.; Ren, L.; Zhang, Y.; Li, K. Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ. Toxicol. Chem. 2008, 27, 80–86. [Google Scholar]
- Babić, S.; Mutavdžić, D.; Ašperger, D.; Horvat, A.J.; Kaštelan-Macan, M. Determination of veterinary pharmaceuticals in production wastewater by HPTLC-videodensitometry. Chromatographia 2007, 65, 105–110. [Google Scholar]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.J. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [PubMed]
- Sim, W.J.; Lee, J.W.; Lee, E.S.; Shin, S.K.; Hwang, S.R.; Oh, J.E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufacturers. Chemosphere 2011, 82, 179–186. [Google Scholar] [PubMed]
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.E.; Fick, J. Occurrence and abundance of antibiotics and resistance genes in rivers, canal and near drug formulation facilities—A study in Pakistan. PLoS ONE 2013, 8, e62712. [Google Scholar]
- Rutgersson, C.; Fick, J.; Marathe, N.; Kristiansson, E.; Janzon, A.; Angelin, M.; Johansson, A.; Shouche, Y.; Flach, C.F.; Larsson, D.J. Fluoroquinolones and qnr genes in sediment, water, soil, and human fecal flora in an environment polluted by manufacturing discharges. Environ. Sci. Technol. 2014, 48, 7825–7832. [Google Scholar]
- Klosterhaus, S.L.; Grace, R.; Hamilton, M.C.; Yee, D. Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ. Int. 2013, 54, 92–99. [Google Scholar]
- Yan, C.; Yang, Y.; Zhou, J.; Liu, M.; Nie, M.; Shi, H.; Gu, L. Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar]
- Liang, X.; Chen, B.; Nie, X.; Shi, Z.; Huang, X.; Li, X. The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 2013, 92, 1410–1416. [Google Scholar]
- Yang, Y.; Fu, J.; Peng, H.; Hou, L.; Liu, M.; Zhou, J.L. Occurrence and phase distribution of selected pharmaceuticals in the Yangtze Estuary and its coastal zone. J. Hazard. Mater. 2011, 190, 588–596. [Google Scholar]
- McEneff, G.; Barron, L.; Kelleher, B.; Paull, B.; Quinn, B. A year-long study of the spatial occurrence and relative distribution of pharmaceutical residues in sewage effluent, receiving marine waters and marine bivalves. Sci. Total Environ. 2014, 476, 317–326. [Google Scholar]
- Li, Y.W.; Wu, X.L.; Mo, C.H.; Tai, Y.P.; Huang, X.P.; Xiang, L. Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearl River Delta area, southern China. J. Agric. Food Chem. 2011, 59, 7268–7276. [Google Scholar]
- Gibson, R.; Durán-Álvarez, J.C.; Estrada, K.L.; Chávez, A.; Cisneros, B.J. Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 2010, 81, 1437–1445. [Google Scholar] [PubMed]
- Spongberg, A.L.; Witter, J.D.; Acuña, J.; Vargas, J.; Murillo, M.; Umaña, G.; Gómez, E.; Perez, G. Reconnaissance of selected PPCP compounds in Costa Rican surface waters. Water Res. 2011, 45, 6709–6717. [Google Scholar]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. Environmentally friendly analysis of emerging contaminants by pressurized hot water extraction–stir bar sorptive extraction–derivatization and gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 401–411. [Google Scholar] [PubMed]
- Lester, Y.; Mamane, H.; Zucker, I.; Avisar, D. Treating wastewater from a pharmaceutical formulation facility by biological process and ozone. Water Res. 2013, 47, 4349–4356. [Google Scholar]
- Collado, N.; Rodriguez-Mozaz, S.; Gros, M.; Rubirola, A.; Barceló, D.; Comas, J.; Rodriguez-Roda, I.; Buttiglieri, G. Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ. Pollut. 2014, 185, 202–212. [Google Scholar]
- Lin, A.Y.; Yu, T.H.; Lin, C.F. Pharmaceutical contamination in residential, industrial, and agricultural waste streams: Risk to aqueous environments in Taiwan. Chemosphere 2008, 74, 131–141. [Google Scholar]
- Wille, K.; Noppe, H.; Verheyden, K.; Vanden Bussche, J.; De Wulf, E.; Van Caeter, P.; Janssen, C.R.; De Brabander, H.F.; Vanhaecke, L. Validation and application of an LC-MS/MS method for the simultaneous quantification of 13 pharmaceuticals in seawater. Anal. Bioanal. Chem. 2010, 397, 1797–1808. [Google Scholar]
- Hu, P.; Guo, C.; Zhang, Y.; Lv, J.; Zhang, Y.; Xu, J. Occurrence, distribution and risk assessment of abused drugs and their metabolites in a typical urban river in north China. Front. Environ. Sci. Eng. 2019, 13, 1. [Google Scholar]
- Prasse, C.; Schlüsener, M.P.; Schulz, R.; Ternes, T.A. Antiviral drugs in wastewater and surface waters: A new pharmaceutical class of environmental relevance? Environ. Sci. Technol. 2010, 44, 1728–1735. [Google Scholar]
- Phillips, P.J.; Smith, S.G.; Kolpin, D.W.; Zaugg, S.D.; Buxton, H.T.; Furlong, E.T.; Esposito, K.; Stinson, B. Pharmaceutical formulation facilities as sources of opioids and other pharmaceuticals to wastewater treatment plant effluents. Environ. Sci. Technol. 2010, 44, 4910–4916. [Google Scholar]
- Yang, Q.; Zhang, H.; Li, X.L.; Wang, Z.; Xu, Y.; Ren, S.; Chen, X.; Xu, Y.; Hao, H.; Wang, H. Extracellular enzyme production and phylogenetic distribution of yeasts in wastewater treatment systems. Bioresour. Technol. 2013, 140, 264–273. [Google Scholar]
- Khan, M.F.; Murphy, C.D. Cytochrome P450 5208A3 is a promiscuous xenobiotic biotransforming enzyme in Cunninghamella elegans. Enzyme Microb. Technol. 2022, 161, 110102. [Google Scholar]
- Kayal, A.; Mandal, S. Microbial degradation of antibiotic: Future possibility of mitigating antibiotic pollution. Environ. Monit. Assess. 2022, 194, 639. [Google Scholar] [PubMed]
- Chen, J.; Liu, S.S.; Wu, Q.; Huang, W.J.; Yang, F.; Wang, Y.J.; He, L.X.; Ying, G.G.; Chen, W.L.; Chen, C.E. Removal, fate, and bioavailability of fluoroquinolone antibiotics in a phytoremediation system with four wetland plants: Combing dynamic DGT and traditional methods. Sci. Total Environ. 2023, 881, 163464. [Google Scholar]
- Kim, D.W.; Heinze, T.M.; Kim, B.S.; Schnackenberg, L.K.; Woodling, K.A.; Sutherland, J.B. Modification of norfloxacin by a Microbacterium sp. strain isolated from a wastewater treatment plant. Appl. Environ. Microbiol. 2011, 77, 6100–6108. [Google Scholar]
- Amorim, C.L.; Moreira, I.S.; Maia, A.S.; Tiritan, M.E.; Castro, P.M. Biodegradation of ofloxacin, norfloxacin, and ciprofloxacin as single and mixed substrates by Labrys portucalensis F11. Appl. Microbiol. Biotechnol. 2014, 98, 3181–3190. [Google Scholar]
- Gros, M.; Cruz-Morato, C.; Marco-Urrea, E.; Longrée, P.; Singer, H.; Sarrà, M.; Hollender, J.; Vicent, T.; Rodriguez-Mozaz, S.; Barceló, D. Biodegradation of the X-ray contrast agent iopromide and the fluoroquinolone antibiotic ofloxacin by the white rot fungus Trametes versicolor in hospital wastewaters and identification of degradation products. Water Res. 2014, 60, 228–241. [Google Scholar]
- Ghosh, S.; Rusyn, I.; Dmytruk, O.V.; Dmytruk, K.V.; Onyeaka, H.; Gryzenhout, M.; Gafforov, Y. Filamentous fungi for sustainable remediation of pharmaceutical compounds, heavy metal and oil hydrocarbons. Front. Bioeng. Biotechnol. 2023, 11, 1106973. [Google Scholar]
- Dhiman, N.; Chaudhary, S.; Singh, A.; Chauhan, A.; Kumar, R. Sustainable degradation of pharmaceutical waste using different fungal strains: Enzyme induction, kinetics and isotherm studies. Environ. Technol. Innov. 2022, 25, 102156. [Google Scholar]
- Akrout, I.; Staita, K.; Zouari-Mechichi, H.; Ghariani, B.; Khmaissa, M.; Navarro, D.; Doan, A.; Albert, Q.; Faulds, C.; Sciara, G.; et al. Valorizing fungal diversity for the degradation of fluoroquinolones. Heliyon 2024, 10, e30611. [Google Scholar]
- Wetzstein, H.G.; Schneider, J.; Karl, W. Metabolite proving fungal cleavage of the aromatic core part of a fluoroquinolone antibiotic. AMB Express 2012, 2, 3. [Google Scholar] [PubMed]
- Parshikov, I.A.; Freeman, J.P.; Lay, J.O., Jr.; Beger, R.D.; Williams, A.J.; Sutherland, J.B. Microbiological transformation of enrofloxacin by the fungus Mucor ramannianus. Appl. Environ. Microbiol. 2000, 66, 2664–2667. [Google Scholar] [PubMed]
- Wetzstein, H.G.; Schmeer, N.; Karl, W. Degradation of the fluoroquinolone enrofloxacin by the brown rot fungus Gloeophyllum striatum: Identification of metabolites. Appl. Environ. Microbiol. 1997, 63, 4272–4281. [Google Scholar]
- Marengo, J.R.; Kok, R.A.; Burrows, L.A.; Velagaleti, R.R.; Stamm, J.M. Biodegradation of 14C-sarafloxacin hydrochloride, a fluoroquinolone antimicrobial by Phanerochaete chrysosporium. J. Sci. Ind. Res. 2001, 60, 121–130. [Google Scholar]
- Parshikov, I.A.; Freeman, J.P.; Lay, J.O., Jr.; Moody, J.D.; Williams, A.J.; Beger, R.D.; Sutherland, J.B. Metabolism of the veterinary fluoroquinolone sarafloxacin by the fungus Mucor ramannianus. J. Ind. Microbiol. Biotechnol. 2001, 26, 140–144. [Google Scholar]
- Rusch, M.; Kauschat, A.; Spielmeyer, A.; Römpp, A.; Hausmann, H.; Zorn, H.; Hamscher, G. Biotransformation of the antibiotic danofloxacin by Xylaria longipes leads to an efficient reduction of its antibacterial activity. J. Agric. Food Chem. 2015, 63, 6897–6904. [Google Scholar]
- Bodin, H.; Daneshvar, A.; Gros, M.; Hultberg, M. Effects of biopellets composed of microalgae and fungi on pharmaceuticals present at environmentally relevant levels in water. Ecol. Eng. 2016, 91, 169–172. [Google Scholar]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of selected pharmaceutical and personal care products (PPCPs) by white-rot fungi. World J. Microbiol. Biotechnol. 2011, 27, 1839–1846. [Google Scholar] [CrossRef]
- Buchicchio, A.; Bianco, G.; Sofo, A.; Masi, S.; Caniani, D. Biodegradation of carbamazepine and clarithromycin by Trichoderma harzianum and Pleurotus ostreatus investigated by liquid chromatography–high-resolution tandem mass spectrometry (FTICR MS-IRMPD). Sci. Total Environ. 2016, 557, 733–739. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, H.; Ren, L.; Ou, Y.; Jiang, S.; Chai, Y.; Chen, A.; Yan, B.; Zhang, J.; Yan, Z. Treatment of amoxicillin-containing wastewater by Trichoderma strains selected from activated sludge. Sci. Total Environ. 2023, 867, 161565. [Google Scholar] [PubMed]
- Tormo-Budowski, R.; Cambronero-Heinrichs, J.C.; Durán, J.E.; Masís-Mora, M.; Ramírez-Morales, D.; Quirós-Fournier, J.P.; Rodríguez-Rodríguez, C.E. Removal of pharmaceuticals and ecotoxicological changes in wastewater using Trametes versicolor: A comparison of fungal stirred tank and trickle-bed bioreactors. Chem. Eng. J. 2021, 410, 128210. [Google Scholar] [CrossRef]
- Palli, L.; Castellet-Rovira, F.; Pérez-Trujillo, M.; Caniani, D.; Sarrà-Adroguer, M.; Gori, R. Preliminary evaluation of Pleurotus ostreatus for the removal of selected pharmaceuticals from hospital wastewater. Biotechnol. Prog. 2017, 33, 1529–1537. [Google Scholar] [CrossRef]
- Badia-Fabregat, M.; Lucas, D.; Pereira, M.A.; Alves, M.; Pennanen, T.; Fritze, H.; Rodríguez-Mozaz, S.; Barceló, D.; Vicent, T.; Caminal, G. Continuous fungal treatment of non-sterile veterinary hospital effluent: Pharmaceuticals removal and microbial community assessment. Appl. Microbiol. Biotechnol. 2016, 100, 2401–2415. [Google Scholar]
- Cruz-Morató, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barceló, D.; Vicent, T.; Sarrà, M.; Marco-Urrea, E. Hospital wastewater treatment by fungal bioreactor: Removal efficiency for pharmaceuticals and endocrine disruptor compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar]
- Del Álamo, A.C.; Pariente, M.I.; Vasiliadou, I.; Padrino, B.; Puyol, D.; Molina, R.; Martínez, F. Removal of pharmaceutical compounds from urban wastewater by an advanced bio-oxidation process based on Trametes versicolor immobilised in a continuous RBC system. Environ. Sci. Pollut. Res. 2018, 25, 34884–34892. [Google Scholar]
- Cruz-Morató, C.; Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D.; Marco-Urrea, E.; Vicent, T.; Sarrà, M. Degradation of pharmaceuticals in non-sterile urban wastewater by Trametes versicolor in a fluidised bed bioreactor. Water Res. 2013, 47, 5200–5210. [Google Scholar] [PubMed]
- Marco-Urrea, E.; Pérez-Trujillo, M.; Cruz-Morató, C.; Caminal, G.; Vicent, T. Degradation of the drug sodium diclofenac by Trametes versicolor pellets and identification of some intermediates by NMR. J. Hazard. Mater. 2010, 176, 836–842. [Google Scholar]
- Tran, N.H.; Urase, T.; Kusakabe, O. Biodegradation characteristics of pharmaceutical substances by whole fungal culture Trametes versicolor and its laccase. J. Water Environ. Technol. 2010, 8, 125–140. [Google Scholar]
- Pezzella, C.; Macellaro, G.; Sannia, G.; Raganati, F.; Olivieri, G.; Marzocchella, A.; Schlosser, D.; Piscitelli, A. Exploitation of Trametes versicolor for bioremediation of endocrine disrupting chemicals in bioreactors. PLoS ONE 2017, 12, e0178758. [Google Scholar]
- Becker, D.; Rodriguez-Mozaz, S.; Insa, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; Misovic, A.; Oehlmann, J.; et al. Removal of endocrine disrupting chemicals in wastewater by enzymatic treatment with fungal laccases. Org. Process Res. Dev. 2017, 21, 480–491. [Google Scholar]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef]
- Jones, S.M.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883. [Google Scholar]
- Singh, D.; Gupta, N. Microbial laccase: A robust enzyme and its industrial applications. Biologia 2020, 75, 1183–1193. [Google Scholar]
- Al-Sareji, O.J.; Meiczinger, M.; Salman, J.M.; Al-Juboori, R.A.; Hashim, K.S.; Somogyi, V.; Jakab, M. Ketoprofen and aspirin removal by laccase immobilized on date stones. Chemosphere 2023, 311, 137133. [Google Scholar] [PubMed]
- Zhang, J.; Cai, Q.; Chen, J.; Lu, Y.; Ren, X.; Liu, Q.; Wen, L.; Mateen, M. Enhanced removal of ibuprofen in water using dynamic dialysis of laccase catalysis. J. Water Process Eng. 2022, 47, 102791. [Google Scholar]
- Sun, K.; Li, S.; Yu, J.; Gong, R.; Si, Y.; Liu, X.; Chu, G. Cu2+-assisted laccase from Trametes versicolor enhanced self-polyreaction of triclosan. Chemosphere 2019, 225, 745–754. [Google Scholar]
- Schwarz, J.; Aust, M.O.; Thiele-Bruhn, S. Metabolites from fungal laccase-catalysed transformation of sulfonamides. Chemosphere 2010, 81, 1469–1476. [Google Scholar]
- Yang, L.H.; Qiao, B.; Xu, Q.M.; Liu, S.; Yuan, Y.; Cheng, J.S. Biodegradation of sulfonamide antibiotics through the heterologous expression of laccases from bacteria and investigation of their potential degradation pathways. J. Hazard. Mater. 2021, 416, 125815. [Google Scholar] [PubMed]
- Guo, X.L.; Zhu, Z.W.; Li, H.L. Biodegradation of sulfamethoxazole by Phanerochaete chrysosporium. J. Mol. Liq. 2014, 198, 169–172. [Google Scholar]
- Chen, Z.; Li, N.; Lan, Q.; Zhang, X.; Wu, L.; Liu, J.; Yang, R. Laccase inducer Mn2+ inhibited the intracellular degradation of norfloxacin by Phanerochaete chrysosporium. Int. Biodeterior. Biodegrad. 2021, 164, 105300. [Google Scholar]
- Lloret, L.; Eibes, G.; Lú-Chau, T.A.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Laccase-catalysed degradation of anti-inflammatories and estrogens. Biochem. Eng. J. 2010, 51, 124–131. [Google Scholar]
- Chmelová, D.; Ondrejovič, M.; Miertuš, S. Laccases as effective tools in the removal of pharmaceutical products from aquatic systems. Life 2024, 14, 230. [Google Scholar] [CrossRef]
- Bronikowski, A.; Hagedoorn, P.L.; Koschorreck, K.; Urlacher, V.B. Expression of a new laccase from Moniliophthora roreri at high levels in Pichia pastoris and its potential application in micropollutant degradation. AMB Express 2017, 7, 73. [Google Scholar]
- Ostadhadi-Dehkordi, S.; Tabatabaei-Sameni, M.; Forootanfar, H.; Kolahdouz, S.; Ghazi-Khansari, M.; Faramarzi, M.A. Degradation of some benzodiazepines by a laccase-mediated system in aqueous solution. Bioresour. Technol. 2012, 125, 344–347. [Google Scholar] [PubMed]
- Adamo, M.; Comtet-Marre, S.; Büttner, E.; Kellner, H.; Luis, P.; Vallon, L.; Prego, R.; Hofrichter, M.; Girlanda, M.; Peyret, P.; et al. Fungal dye-decolorizing peroxidase diversity: Roles in either intra-or extracellular processes. Appl. Microbiol. Biotechnol. 2022, 106, 2993–3007. [Google Scholar]
- Conesa, A.; Punt, P.J.; van den Hondel, C.A. Fungal peroxidases: Molecular aspects and applications. J. Biotechnol. 2002, 93, 143–158. [Google Scholar]
- Sung, H.J.; Khan, M.F.; Kim, Y.H. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int. J. Biol. Macromol. 2019, 136, 20–26. [Google Scholar]
- Son, H.; Seo, H.; Han, S.; Kim, S.M.; Khan, M.F.; Sung, H.J.; Kang, S.H.; Kim, K.J.; Kim, Y.H. Extra disulfide and ionic salt bridge improves the thermostability of lignin peroxidase H8 under acidic condition. Enzyme Microb. Technol. 2021, 148, 109803. [Google Scholar] [CrossRef] [PubMed]
- Auriol, M.; Filali-Meknassi, Y.; Adams, C.D.; Tyagi, R.D. Natural and synthetic hormone removal using the horseradish peroxidase enzyme: Temperature and pH effects. Water Res. 2006, 40, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.U. In vitro degradation of carbamazepine and diclofenac by crude lignin peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092. [Google Scholar] [PubMed]
- Wen, X.; Jia, Y.; Li, J. Degradation of tetracycline and oxytetracycline by crude lignin peroxidase prepared from Phanerochaete chrysosporium—A white rot fungus. Chemosphere 2009, 75, 1003–1007. [Google Scholar]
- Wen, X.; Jia, Y.; Li, J. Enzymatic degradation of tetracycline and oxytetracycline by crude manganese peroxidase prepared from Phanerochaete chrysosporium. J. Hazard. Mater. 2010, 177, 924–928. [Google Scholar] [CrossRef]
- Inoue, S.; Igarashi, Y.; Yoneda, Y.; Kawai, S.; Okamura, H.; Nishida, T. Elimination and detoxification of fungicide miconazole and antidepressant sertraline by manganese peroxidase-dependent lipid peroxidation system. Int. Biodeterior. Biodegrad. 2015, 100, 79–84. [Google Scholar]
- García-Zamora, J.L.; León-Aguirre, K.; Quiroz-Morales, R.; Parra-Saldívar, R.; Gómez-Patiño, M.B.; Arrieta-Baez, D.; Rebollar-Pérez, G.; Torres, E. Chloroperoxidase-mediated halogenation of selected pharmaceutical micropollutants. Catalysts 2018, 8, 32. [Google Scholar] [CrossRef]
- Prieto, A.; Möder, M.; Rodil, R.; Adrian, L.; Marco-Urrea, E. Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour. Technol. 2011, 102, 10987–10995. [Google Scholar]
- Čvančarová, M.; Moeder, M.; Filipová, A.; Cajthaml, T. Biotransformation of fluoroquinolone antibiotics by ligninolytic fungi–metabolites, enzymes and residual antibacterial activity. Chemosphere 2015, 136, 311–320. [Google Scholar]
- Gao, N.; Liu, C.X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus. Chemosphere 2018, 195, 146–155. [Google Scholar] [CrossRef]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: A review. Sci. Total Environ. 2022, 806, 150500. [Google Scholar] [PubMed]
- Pylypchuk, I.V.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A. Removal of diclofenac, paracetamol, and carbamazepine from model aqueous solutions by magnetic sol–gel encapsulated horseradish peroxidase and lignin peroxidase composites. Nanomaterials 2020, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Al-Maqdi, K.A.; Hisaindee, S.; Rauf, M.A.; Ashraf, S.S. Detoxification and degradation of sulfamethoxazole by soybean peroxidase and UV+ H₂O₂ remediation approaches. Chem. Eng. J. 2018, 352, 450–458. [Google Scholar]
- Chen, W.; Lee, M.K.; Jefcoate, C.; Kim, S.C.; Chen, F.; Yu, J.H. Fungal cytochrome P450 monooxygenases: Their distribution, structure, functions, family expansion, and evolutionary origin. Genome Biol. Evol. 2014, 6, 1620–1634. [Google Scholar]
- Lin, S.; Wei, J.; Yang, B.; Zhang, M.; Zhuo, R. Bioremediation of organic pollutants by white rot fungal cytochrome P450: The role and mechanism of CYP450 in biodegradation. Chemosphere 2022, 301, 134776. [Google Scholar]
- Mori, T.; Ohno, H.; Ichinose, H.; Kawagishi, H.; Hirai, H. White-rot fungus Phanerochaete chrysosporium metabolizes chloropyridinyl-type neonicotinoid insecticides by an N-dealkylation reaction catalyzed by two cytochrome P450s. J. Hazard. Mater. 2021, 402, 123831. [Google Scholar]
- Khan, M.F.; Murphy, C.D. Cunninghamella spp. produce mammalian-equivalent metabolites from fluorinated pyrethroid pesticides. AMB Express 2021, 11, 101. [Google Scholar]
- Khan, M.F.; Murphy, C.D. Fluorotelomer alcohols are efficiently biotransformed by Cunninghamella elegans. Environ. Sci. Pollut. Res. 2023, 30, 23613–23623. [Google Scholar]
- Rodríguez-Rodríguez, C.E.; García-Galán, M.J.; Blánquez, P.; Díaz-Cruz, M.S.; Barceló, D.; Caminal, G.; Vicent, T. Continuous degradation of a mixture of sulfonamides by Trametes versicolor and identification of metabolites from sulfapyridine and sulfathiazole. J. Hazard. Mater. 2012, 213, 347–354. [Google Scholar]
- Marco-Urrea, E.; Pérez-Trujillo, M.; Vicent, T.; Caminal, G. Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 2009, 74, 765–772. [Google Scholar]
- Marco-Urrea, E.; Pérez-Trujillo, M.; Blánquez, P.; Vicent, T.; Caminal, G. Biodegradation of the analgesic naproxen by Trametes versicolor and identification of intermediates using HPLC-DAD-MS and NMR. Bioresour. Technol. 2010, 101, 2159–2166. [Google Scholar] [PubMed]
- Golan-Rozen, N.; Chefetz, B.; Ben-Ari, J.; Geva, J.; Hadar, Y. Transformation of the recalcitrant pharmaceutical compound carbamazepine by Pleurotus ostreatus: Role of cytochrome P450 monooxygenase and manganese peroxidase. Environ. Sci. Technol. 2011, 45, 6800–6805. [Google Scholar]
- Amadio, J.; Gordon, K.; Murphy, C.D. Biotransformation of flurbiprofen by Cunninghamella species. Appl. Environ. Microbiol. 2010, 76, 6299–6303. [Google Scholar]
- Tamborini, L.; Romano, D.; Pinto, A.; Contente, M.; Iannuzzi, M.C.; Conti, P.; Molinari, F. Biotransformation with whole microbial systems in a continuous flow reactor: Resolution of (RS)-flurbiprofen using Aspergillus oryzae by direct esterification with ethanol in organic solvent. Tetrahedron Lett. 2013, 54, 6090–6093. [Google Scholar]
- Williams, A.J.; Deck, J.; Freeman, J.P.; Chiarelli, M.P.; Adjei, M.D.; Heinze, T.M.; Sutherland, J.B. Biotransformation of flumequine by the fungus Cunninghamella elegans. Chemosphere 2007, 67, 240–243. [Google Scholar]
- Amadio, J.; Murphy, C.D. Production of human metabolites of the anti-cancer drug flutamide via biotransformation in Cunninghamella species. Biotechnol. Lett. 2011, 33, 321–326. [Google Scholar] [PubMed]
- Herath, W.; Khan, I.A. Microbial metabolism. Part 11. Metabolites of flutamide. Chem. Pharm. Bull. 2010, 58, 562–564. [Google Scholar]
- Sá, H.; Michelin, M.; Tavares, T.; Silva, B. Current challenges for biological treatment of pharmaceutical-based contaminants with oxidoreductase enzymes: Immobilization processes, real aqueous matrices and hybrid techniques. Biomolecules 2022, 12, 1489. [Google Scholar] [CrossRef]
- Kózka, B.; Nałęcz-Jawecki, G.; Turło, J.; Giebułtowicz, J. Application of Pleurotus ostreatus to efficient removal of selected antidepressants and immunosuppressants. J. Environ. Manag. 2020, 273, 111131. [Google Scholar]
- Kasonga, T.K.; Coetzee, M.A.; Kamika, I.; Momba, M.N. Assessing a co-culture fungal granule ability to remove pharmaceuticals in a sequencing batch reactor. Environ. Technol. 2022, 43, 1684–1699. [Google Scholar]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Biotransformation of carbamazepine by laccase-mediator system: Kinetics, by-products and toxicity assessment. Process Biochem. 2018, 67, 147–154. [Google Scholar]
- Lonappan, L.; Rouissi, T.; Laadila, M.A.; Brar, S.K.; Hernandez Galan, L.; Verma, M.; Surampalli, R.Y. Agro-industrial-produced laccase for degradation of diclofenac and identification of transformation products. ACS Sustain. Chem. Eng. 2017, 5, 5772–5781. [Google Scholar]
- Wang, X.; Meng, F.; Zhang, B.; Xia, Y. Elimination of tetracyclines in seawater by laccase-mediator system. Chemosphere 2023, 333, 138916. [Google Scholar] [PubMed]
- Tian, Q.; Dou, X.; Huang, L.; Wang, L.; Meng, D.; Zhai, L.; Shen, Y.; You, C.; Guan, Z.; Liao, X. Characterization of a robust cold-adapted and thermostable laccase from Pycnoporus sp. SYBC-L10 with a strong ability for the degradation of tetracycline and oxytetracycline by laccase-mediated oxidation. J. Hazard. Mater. 2020, 382, 121084. [Google Scholar]
- Yousefi-Ahmadipour, A.; Bozorgi-Koshalshahi, M.; Mogharabi, M.; Amini, M.; Ghazi-Khansari, M.; Faramarzi, M.A. Laccase-catalyzed treatment of ketoconazole, identification of biotransformed metabolites, determination of kinetic parameters, and evaluation of micro-toxicity. J. Mol. Catal. B Enzym. 2016, 133, 77–84. [Google Scholar]
- Navada, K.K.; Kulal, A. Enzymatic degradation of chloramphenicol by laccase from Trametes hirsuta and comparison among mediators. Int. Biodeterior. Biodegrad. 2019, 138, 63–69. [Google Scholar]
- Feng, Y.; Shen, M.; Wang, Z.; Liu, G. Transformation of atenolol by a laccase-mediator system: Efficiencies, effect of water constituents, and transformation pathways. Ecotoxicol. Environ. Saf. 2019, 183, 109555. [Google Scholar]
- Khan, M.F.; Kundu, D.; Gogoi, M.; Shrestha, A.K.; Karanth, N.G.; Patra, S. Enzyme-Responsive and Enzyme Immobilized Nanoplatforms for Therapeutic Delivery: An Overview of Research Innovations and Biomedical Applications. In Nanopharmaceuticals: Principles and Applications, 3rd ed.; Yata, V., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; Volume 48, pp. 165–200. [Google Scholar]
- Kundu, D.; Khan, M.F.; Gogoi, M.; Patra, S. Environmental Impact and Econanotoxicity of Engineered Nanomaterials. In Nanotoxicology and Nanoecotoxicology, 1st ed.; Springer: New York, NY, USA, 2021; Volume 1, pp. 287–312. [Google Scholar]
- Khan, M.F.; Murphy, C.D. Environmental remediation by novel nanomaterials and fungi with high-degradation capacity of hazardous contaminants. In Bio and Nanoremediation of Hazardous Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2023; pp. 283–310. [Google Scholar]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C 2019, 103, 109789. [Google Scholar]
- Masjoudi, M.; Golgoli, M.; Nejad, Z.G.; Sadeghzadeh, S.; Borghei, S.M. Pharmaceuticals removal by immobilized laccase on poly-vinylidene fluoride nanocomposite with multi-walled carbon nanotubes. Chemosphere 2021, 263, 128043. [Google Scholar]
- García-Morales, R.; García-García, A.; Orona-Navar, C.; Osma, J.F.; Nigam, K.D.; Ornelas-Soto, N. Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J. Environ. Chem. Eng. 2018, 6, 710–717. [Google Scholar]
- Lonappan, L.; Liu, Y.; Rouissi, T.; Pourcel, F.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem. Eng. J. 2018, 351, 985–994. [Google Scholar]
- Yang, J.; Lin, Y.; Yang, X.; Ng, T.B.; Ye, X.; Lin, J. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazard. Mater. 2017, 322, 525–531. [Google Scholar] [PubMed]
- Primožič, M.; Kravanja, G.; Knez, Ž.; Crnjac, A.; Leitgeb, M. Immobilized laccase in the form of (magnetic) cross-linked enzyme aggregates for sustainable diclofenac (bio) degradation. J. Clean. Prod. 2020, 275, 124121. [Google Scholar]
- Huber, D.; Bleymaier, K.; Pellis, A.; Vielnascher, R.; Daxbacher, A.; Greimel, K.J.; Guebitz, G.M. Laccase catalyzed elimination of morphine from aqueous systems. New Biotechnol. 2018, 42, 19–25. [Google Scholar]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-Pour, A.; Verma, M.; Surampalli, R.Y. Immobilized laccase on oxygen functionalized nanobiochars through mineral acids treatment for removal of carbamazepine. Sci. Total Environ. 2017, 584, 393–401. [Google Scholar]
- Zdarta, J.; Feliczak-Guzik, A.; Siwińska-Ciesielczyk, K.; Nowak, I.; Jesionowski, T. Mesostructured cellular foam silica materials for laccase immobilization and tetracycline removal: A comprehensive study. Microporous Mesoporous Mater. 2020, 291, 109688. [Google Scholar]
- Wen, X.; Zeng, Z.; Du, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Xu, P.; Zhang, C.; Wan, J.; et al. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 2019, 222, 865–871. [Google Scholar]
- Dong, S.; Jing, X.; Cao, Y.; Xia, E.; Gao, S.; Mao, L. Non-covalent assembled laccase-graphene composite: Property, stability and performance in beta-blocker removal. Environ. Pollut. 2019, 252, 907–916. [Google Scholar]
- Sharifi-Bonab, M.; Rad, F.A.; Mehrabad, J.T. Preparation of laccase-graphene oxide nanosheet/alginate composite: Application for the removal of cetirizine from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 3013–3020. [Google Scholar] [CrossRef]
- Maryšková, M.; Schaabová, M.; Tomankova, H.; Novotný, V.; Rysová, M. Wastewater treatment by novel polyamide/polyethylenimine nanofibers with immobilized laccase. Water 2020, 12, 588. [Google Scholar] [CrossRef]
- Apriceno, A.; Astolfi, M.L.; Girelli, A.M.; Scuto, F.R. A new laccase-mediator system facing the biodegradation challenge: Insight into the NSAIDs removal. Chemosphere 2019, 215, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Covalent immobilization of laccase onto nanofibrous membrane for degradation of pharmaceutical residues in water. ACS Sustain. Chem. Eng. 2017, 5, 10430–10438. [Google Scholar] [CrossRef]
- Simón-Herrero, C.; Naghdi, M.; Taheran, M.; Brar, S.K.; Romero, A.; Valverde, J.L.; Ramirez, A.A.; Sánchez-Silva, L. Immobilized laccase on polyimide aerogels for removal of carbamazepine. J. Hazard. Mater. 2019, 376, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Guardado, A.L.; Druon-Bocquet, S.; Belleville, M.P.; Sanchez-Marcano, J. A novel process for the covalent immobilization of laccases on silica gel and its application for the elimination of pharmaceutical micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 25579–25593. [Google Scholar] [CrossRef]
- Asif, M.B.; Hai, F.I.; Dhar, B.R.; Ngo, H.H.; Guo, W.; Jegatheesan, V.; Price, W.E.; Nghiem, L.D.; Yamamoto, K. Impact of simultaneous retention of micropollutants and laccase on micropollutant degradation in enzymatic membrane bioreactor. Bioresour. Technol. 2018, 267, 473–480. [Google Scholar] [CrossRef] [PubMed]
- De Cazes, M.; Belleville, M.P.; Petit, E.; Llorca, M.; Rodríguez-Mozaz, S.; De Gunzburg, J.; Barceló, D.; Sanchez-Marcano, J. Design and optimization of an enzymatic membrane reactor for tetracycline degradation. Catal. Today 2014, 236, 146–152. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Vu, M.T.; Johir, M.A.; Pathak, N.; Zdarta, J.; Jesionowski, T.; Semblante, G.U.; Hai, F.I.; Khanh, D.N.; Nghiem, L.D. A novel approach in crude enzyme laccase production and application in emerging contaminant bioremediation. Processes 2020, 8, 648. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Kang, J.; Leusch, F.D.; Roddick, F.; van de Merwe, J.P.; Magram, S.F.; Nghiem, L.D. Degradation of a broad spectrum of trace organic contaminants by an enzymatic membrane reactor: Complementary role of membrane retention and enzymatic degradation. Int. Biodeterior. Biodegrad. 2015, 99, 115–122. [Google Scholar] [CrossRef]
- Abejón, R.; De Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J. Large-scale enzymatic membrane reactors for tetracycline degradation in WWTP effluents. Water Res. 2015, 73, 118–131. [Google Scholar] [CrossRef]
- Ba, S.; Jones, J.P.; Cabana, H. Hybrid bioreactor (HBR) of hollow fiber microfilter membrane and cross-linked laccase aggregates eliminate aromatic pharmaceuticals in wastewaters. J. Hazard. Mater. 2014, 280, 662–670. [Google Scholar] [CrossRef]
- Ba, S.; Haroune, L.; Soumano, L.; Bellenger, J.P.; Jones, J.P.; Cabana, H. A Hybrid Bioreactor Based on Insolubilized Tyrosinase and Laccase Catalysis and Microfiltration Membrane Removal of Pharmaceuticals from Wastewater. Chemosphere 2018, 201, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Paul Guin, J.; Thampi, R.K.; Sullivan, J.A.; Murphy, C.D. Enhanced Removal of Perfluorooctanoic Acid with Sequential Photocatalysis and Fungal Treatment. Environ. Sci. Pollut. Res. 2023, 30, 91478–91486. [Google Scholar]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current Insights into Fungal Species Diversity and Perspective on Naming the Environmental DNA Sequences of Fungi. Mycology 2019, 10, 127–140. [Google Scholar] [PubMed]
- Li, G.; Jian, T.; Liu, X.; Lv, Q.; Zhang, G.; Ling, J. Application of Metabolomics in Fungal Research. Molecules 2022, 27, 7365. [Google Scholar] [CrossRef]
- Damasio, A.; Goldman, G.H.; Silva, R.N.; Segato, F. Advances in the Regulation and Production of Fungal Enzymes by Transcriptomics, Proteomics, and Recombinant Strain Design. Front. Bioeng. Biotechnol. 2019, 7, 157. [Google Scholar]
- Sanches, P.H.; de Melo, N.C.; Porcari, A.M.; de Carvalho, L.M. Integrating Molecular Perspectives: Strategies for Comprehensive Multi-Omics Integrative Data Analysis and Machine Learning Applications in Transcriptomics, Proteomics, and Metabolomics. Biology 2024, 13, 848. [Google Scholar] [CrossRef]
- Alaidaroos, B.A. Advancing Eco-Sustainable Bioremediation for Hydrocarbon Contaminants: Challenges and Solutions. Processes 2023, 11, 3036. [Google Scholar] [CrossRef]
- Marques, H.M. Electron Transfer in Biological Systems. JBIC J. Biol. Inorg. Chem. 2024, 1, 641–683. [Google Scholar]
- Corbu, V.M.; Gheorghe-Barbu, I.; Dumbravă, A.Ș.; Vrâncianu, C.O.; Șesan, T.E. Current Insights in Fungal Importance—A Comprehensive Review. Microorganisms 2023, 11, 1384. [Google Scholar] [CrossRef]
- Kelbert, M.; Pereira, C.S.; Daronch, N.A.; Cesca, K.; Michels, C.; de Oliveira, D.; Soares, H.M. Laccase as an Efficacious Approach to Remove Anticancer Drugs: A Study of Doxorubicin Degradation, Kinetic Parameters, and Toxicity Assessment. J. Hazard. Mater. 2021, 409, 124520. [Google Scholar]
- Frasconi, M.; Favero, G.; Boer, H.; Koivula, A.; Mazzei, F. Kinetic and Biochemical Properties of High and Low Redox Potential Laccases from Fungal and Plant Origin. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 899–908. [Google Scholar]
- Singh, A.K.; Abellanas-Perez, P.; de Andrades, D.; Cornet, I.; Fernandez-Lafuente, R.; Bilal, M. Laccase-Based Biocatalytic Systems Application in Sustainable Degradation of Pharmaceutically Active Contaminants. J. Hazard. Mater. 2024, 1, 136803. [Google Scholar]
- Tinoco-Valencia, R.; Gómez-Cruz, C.; Galindo, E.; Serrano-Carreon, L. Toward an Understanding of the Effects of Agitation and Aeration on Growth and Laccases Production by Pleurotus ostreatus. J. Biotechnol. 2014, 177, 67–73. [Google Scholar] [PubMed]
- Sang, B.I.; Kim, Y.H.; Yoo, Y.J. Shear Effects on Production of Lignin Peroxidase by Phanerochaete chrysosporium. Biotechnol. Bioprocess Eng. 1996, 1, 26–31. [Google Scholar]
- Li, J.; Liu, Z.; Zhao, J.; Wang, G.; Xie, T. Molecular Insights into Substrate Promiscuity of CotA Laccase Catalyzing Lignin-Phenol Derivatives. Int. J. Biol. Macromol. 2024, 256, 128487. [Google Scholar]
- Guengerich, F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018, 8, 10964–10976. [Google Scholar]
- Lorenzo, M.; Moldes, D.; Couto, S.R.; Sanromán, M.A. Inhibition of Laccase Activity from Trametes versicolor by Heavy Metals and Organic Compounds. Chemosphere 2005, 60, 1124–1128. [Google Scholar]
- Okal, E.J.; Heng, G.; Magige, E.A.; Khan, S.; Wu, S.; Ge, Z.; Zhang, T.; Mortimer, P.E.; Xu, J. Insights into the Mechanisms Involved in the Fungal Degradation of Plastics. Ecotoxicol. Environ. Saf. 2023, 262, 115202. [Google Scholar]
- Mitra, S.; Murthy, G.S. Bioreactor Control Systems in the Biopharmaceutical Industry: A Critical Perspective. Syst. Microbiol. Biomanuf. 2022, 1, 91–112. [Google Scholar]
- Mendes, S.; Robalo, M.P.; Martins, L.O. Bacterial Enzymes and Multi-Enzymatic Systems for Cleaning-Up Dyes from the Environment. Microb. Degrad. Synth. Dyes Wastewaters 2015, 1, 27–55. [Google Scholar]
- Sharma, K.; Kaushik, G.; Thotakura, N.; Raza, K.; Sharma, N.; Nimesh, S. Enhancement Effects of Process Optimization Technique While Elucidating the Degradation Pathways of Drugs Present in Pharmaceutical Industry Wastewater Using Micrococcus yunnanensis. Chemosphere 2020, 238, 124689. [Google Scholar] [CrossRef] [PubMed]
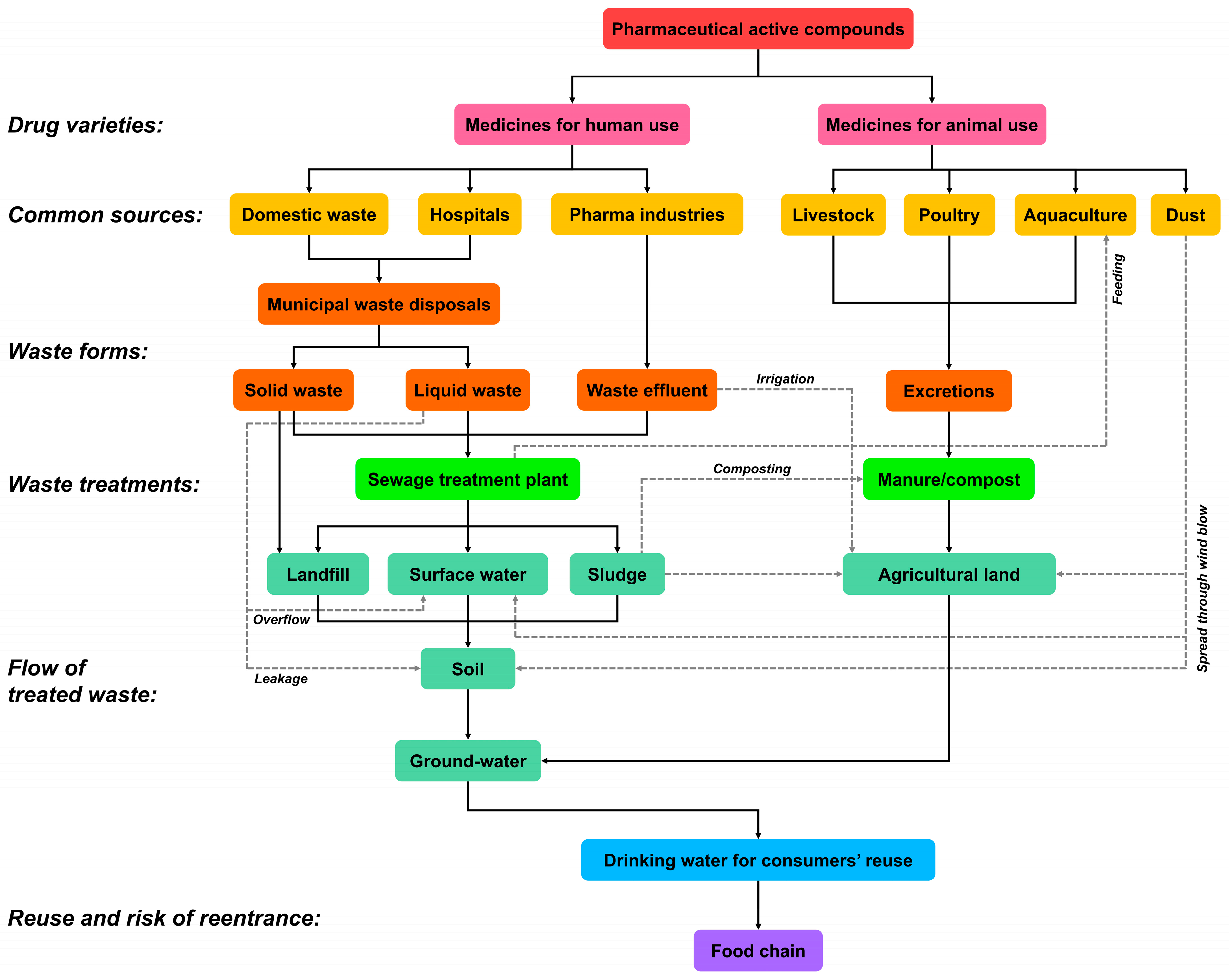

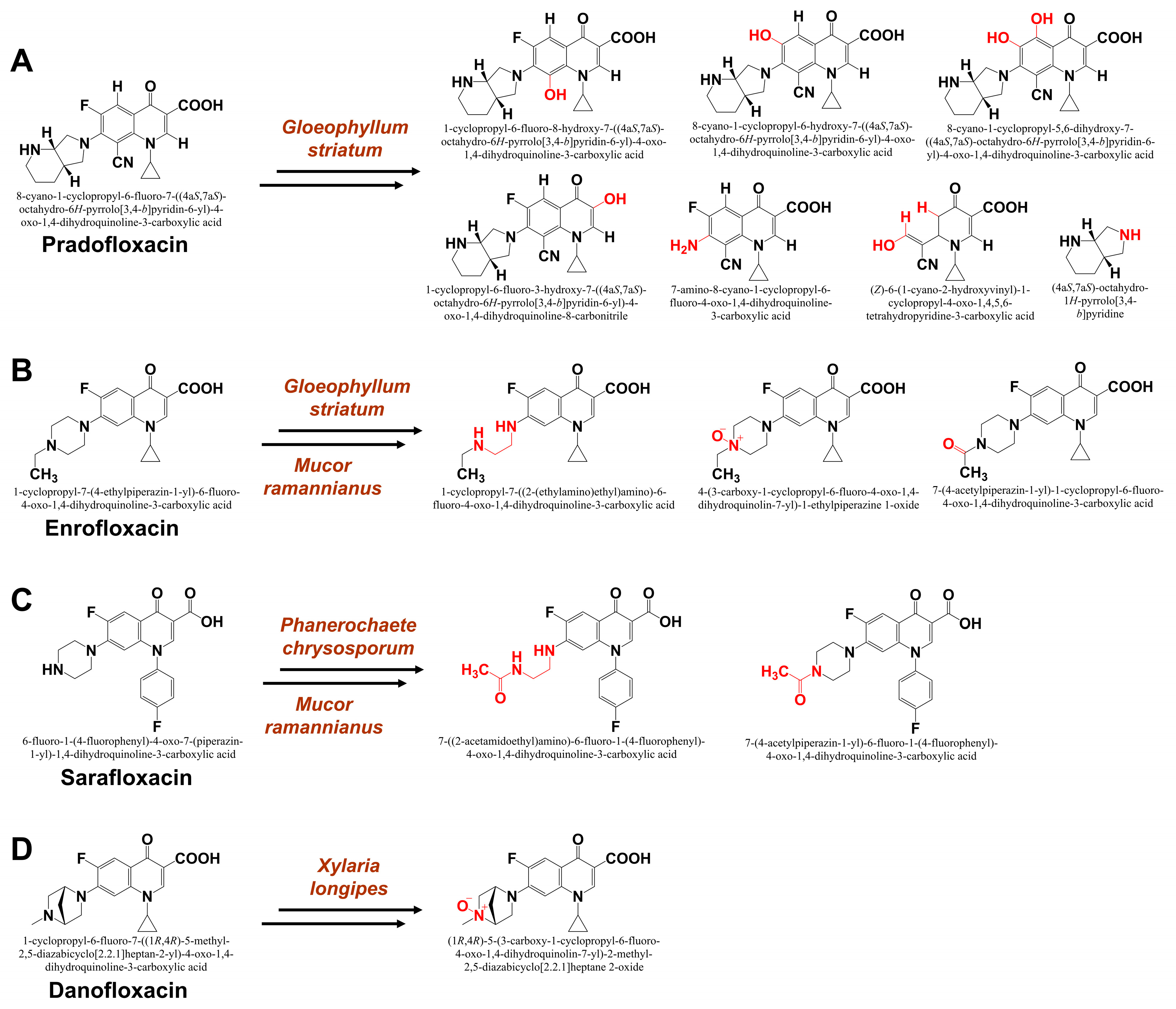


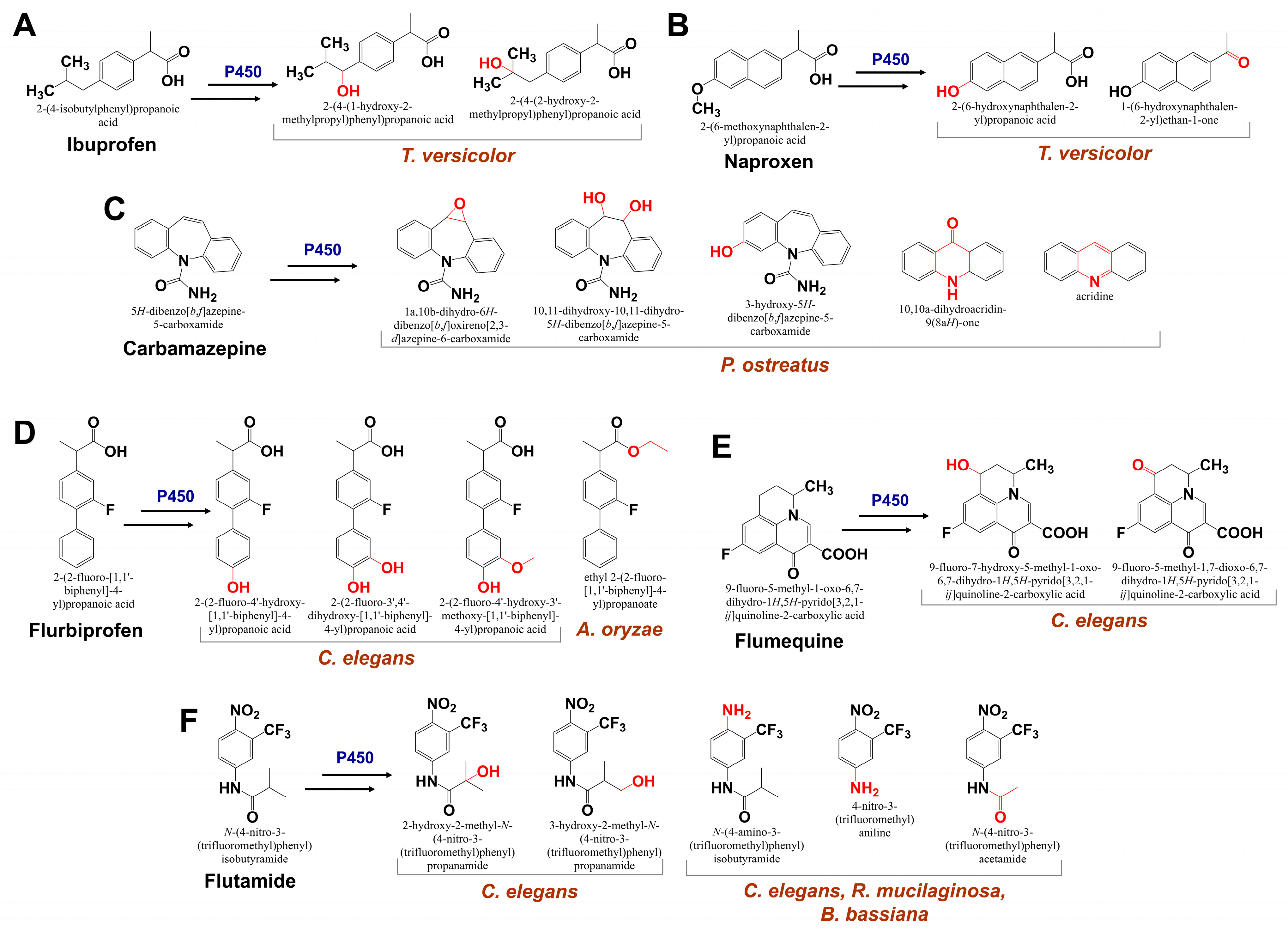

| Drug Name | Environmental Source | Concentration (ng L−1 or ng g−1) * | Contributing Country | Harmful Side Effects on Human Health | References |
|---|---|---|---|---|---|
| Antibiotics | |||||
| Sulfamethoxazole | Industrial effluent | 1,340,000 | China/Taiwan | Gastrointestinal disturbances and skin irritations | [31] |
| Penicilloic acids | Industrial effluent | 44,000,000 | China | Hypersensitivity, angioedema, and anaphylaxis | [32] |
| Oxytetracycline | Industrial effluent | 19,500,000 | China | Skin irritations and gastrointestinal disturbances | [32] |
| Sulfaguanidine | Industrial effluent | >1,100,000 | Croatia | Kidney damage and destroys red blood cells | [33] |
| Ciprofloxacin | Industrial effluent | 14,000,000 | India | Tendon problems, nerve damage, and low blood sugar | [34] |
| Lincomycin | Industrial effluent | 43,900,000 | Korea | Nausea, vomiting, swollen tongue, and vaginal itching | [35] |
| Cetirizine | Surface water | 28,000 | India | Drowsiness, fatigue, dry mouth, nausea, and vomiting | [34] |
| Oxytetracycline | Surface water | 712,000 | China | Skin irritations and gastrointestinal disturbances | [32] |
| Penicilloic acids | Surface water | 11,600,000 | China | Hypersensitivity, angioedema, and anaphylaxis | [32] |
| Sulfamethoxazole | Surface water | 49,000 | Pakistan | Gastrointestinal disturbances and skin irritations | [36] |
| Ciprofloxacin | Groundwater | 770 | India | Tendon problems, nerve damage, and low blood sugar | [37] |
| Clarithromycin | Seawater (coastal) | 17.6 | USA | Hearing loss, mood swings, myopathy, and vision issues | [38] |
| Erythromycin | Seawater (coastal) | 5–70 | China | Liver disease, stomach cramps, and diarrhea | [39] |
| Sulfadiazine | Seawater (coastal) | 0.6–71.8 | China | Hallucinations, seizure, liver problems, and dark urine | [39] |
| Norfloxacin | Seawater (coastal) | 3.0–6800 | China | Headache, dark urine, muscle weakness, and diarrhea | [40] |
| Ofloxacin | Seawater (coastal) | 3.5–5100 | China | Nausea, headache, insomnia, and vaginitis | [40] |
| Roxithromycin | Seawater (coastal) | 6–630 | China | Skin irritations and gastrointestinal disturbances | [40] |
| Sulfadimidine | Seawater (coastal) | 1.3–219 | China | Allergies, gastric issues, anemia, and crystalluria | [41] |
| Sulfamethoxazole | Seawater (coastal) | 4.2–765 | China | Gastrointestinal disturbances and skin irritations | [42] |
| Trimethoprim | Seawater (coastal) | 60–870 | Ireland | Itching and rash, stomach upset, and headache | [43] |
| Ciprofloxacin | Soil | 1900 | India | Tendon problems, nerve damage, and low blood sugar | [37] |
| Norfloxacin | Soil | 61.9 | China | Headache, dark urine, muscle weakness, and diarrhea | [44] |
| Sulfamerazine | Soil | 16 | China | Nausea, diarrhea, and hypersensitivity reactions | [44] |
| Triclosan | Soil | 0.4–35.5 | Mexico | Interferes with thyroid hormone metabolism | [45] |
| Analgesic | |||||
| Ibuprofen | Industrial effluent | 1,500,000 | China | Nausea, dyspepsia, and hypertension | [31] |
| Acetaminophen | Seawater (coastal) | 1.9–1952 | Costa Rica | Nausea, vomiting, liver damage, and polyuria | [46] |
| Sea sediments | 96–100 | Spain | [47] | ||
| Ibuprofen | Sea sediments | 98–100 | Spain | Nausea, dyspepsia, and hypertension | [47] |
| Anti-convulsant | |||||
| Carbamazepine | Seawater (coastal) | 50–1400 | Ireland | Ataxia, dizziness, drowsiness, nausea, and vomiting | [43] |
| Venlafaxine | Industrial effluent | 11,700,000 | Israel | Dyspepsia, tachycardia, insomnia, and sweating | [48] |
| Industrial effluent | 2600 | Spain | [49] | ||
| NSAID | |||||
| Diclofenac | Surface water | 27,000 | China/Taiwan | Indigestion, headache, dizziness, and drowsiness | [50] |
| Seawater (coastal) | 283–843 | China | [42] | ||
| Seawater (coastal) | 60–550 | Ireland | [43] | ||
| Ketoprofen | Seawater (coastal) | 185–805 | Costa Rica | Abdominal pain, diarrhea, edema, and headaches | [46] |
| Indomethacin | Sea sediments | 12–164 | China | Heart attack, stroke, skin changes, weight gain, etc. | [42] |
| Beta-blockers | |||||
| Atenolol | Seawater (coastal) | 80–293 | Belgium | Constipation, indigestion, depression, and insomnia | [51] |
| Propranolol | Seawater (coastal) | 0.3–142 | China | Constipation, decreased sex drive, and insomnia | [42] |
| CNS stimulant | |||||
| Methamphetamine | River sediments | 2.6–32.4 | China | Distractibility, memory loss, and mood disturbances | [52] |
| Ephedrine | River sediments | 2.6–32.4 | China | Anxiety, dizziness, headache, and insomnia | [52] |
| Anti-spasmodic | |||||
| Mebeverine | Sea sediments | 18–415 | China | Heartburn, malaise, insomnia, and bradycardia | [42] |
| Antiviral | |||||
| Oseltamivir | Surface water | 160 | Switzerland | Nausea, vomiting, insomnia, and headache | [53] |
| Fibric acid agent | |||||
| Gemfibrozil | Seawater (coastal) | 77–758 | Costa Rica | Indigestion, drowsiness, joint pain, and impotence | [47] |
| Muscle relaxant | |||||
| Metaxalone | Industrial effluent | 3,800,000 | USA | Gastrointestinal issues, nervousness, and drowsiness | [54] |
| SERM | |||||
| Tamoxifen | Sea sediments | 212–431 | China | Increased tumor or bone pain, hot flashes, and nausea | [42] |
| Fungi | Biodegrades Pharmaceutical Compound(s) | Pharmaceutical Category | References |
|---|---|---|---|
| Phanerochaete chrysosporium | Sulfamethoxazole | Antibiotic | [72] |
| Diazepam | Psychiatric drug | ||
| Ibuprofen, naproxen, and diclofenac | Anti-inflammatory | ||
| Citalopram and fluoxetine | Antidepressant | ||
| Carbamazepine | Anti-epileptic | ||
| Ibuprofen, diclofenac, and naproxen | Anti-inflammatory | ||
| Carbamazepine | Anti-epileptic | ||
| Pleurotus ostreatus | Diclofenac and ketoprofen | Anti-inflammatory | [76] |
| Atenolol | Antihypertensive | ||
| Carbamazepine | Anti-epileptic | [73] | |
| Clarithromycin | Antibiotic | ||
| Trametes versicolor | Azithromycin, ciprofloxacin, tetracycline, and cephalexin | Antibiotic | [77] |
| Metoprolol and carazolol | β-blockers | ||
| Diazepam | Psychiatric drug | ||
| Ciprofloxacin and ofloxacin | Antibiotic | [78] | |
| Acetaminophen, ibuprofen, and ketoprofen | Anti-inflammatory | ||
| Carbamazepine | Psychiatric drug | [78,79] | |
| Erythromycin | Antibiotic | [80] | |
| Salicylic acid | Keratolytic agent | ||
| Codeine and acetaminophen | Analgesic | ||
| Ibuprofen and ketoprofen | Anti-inflammatory | ||
| Sulfamethoxazole | Antibiotic | [79] | |
| Antipyrine | Analgesic | ||
| Clofibric acid | Antilipidemic | ||
| Atenolol | Antihypertensive | ||
| Hydrochlorothiazide | Diuretic | ||
| Ranitidine | Histamine 2 blocker | ||
| Diclofenac | Anti-inflammatory | [81] | |
| Propyphenazone | Analgesic | [82] | |
| Fenoprofen, naproxen, ketoprofen, and indomethacin | Anti-inflammatory | ||
| Clofibric acid | Antilipidemic | ||
| Gemfibrozil | Lipid regulation | ||
| Bisphenol A, nonylphenol, parabens, and phthalates | Endocrine-disrupting chemicals | [83] | |
| 17α-ethinyl-estradiol, 17β-estradiol, estriol, and estrone | Hormones | [84] | |
| Trichoderma harzianum | Carbamazepine | Anti-epileptic | [73] |
| Clarithromycin | Antibiotic | ||
| Trichoderma pubescens | Amoxicillin | Antibiotic | [74] |
| Aspergillus niger | Sulfamethoxazole | Antibiotic | [71] |
| Metoprolol | β-blockers | ||
| Acetaminophen | Analgesic | ||
| Diclofenac and naproxen | Anti-inflammatory | ||
| Ranitidine | Histamine 2 blocker | ||
| Carbamazepine | Anti-epileptic | ||
| Bjerkandera adusta | Diclofenac | Anti-inflammatory | [63] |
| Sulfamethoxazole | Antibiotic | [73] | |
| Diazepam | Psychiatric drug | ||
| Ibuprofen, naproxen, and diclofenac | Anti-inflammatory | ||
| Citalopram and fluoxetine | Antidepressant | ||
| Carbamazepine | Anti-epileptic | ||
| Fomitopsis meliae | Diclofenac | Anti-inflammatory | [63] |
| Myceliophthora thermophila | 17α-ethinyl-estradiol, 17β-estradiol, estriol, and estrone | Hormones | [84] |
| Factor | Effect on Enzyme Activity | Example | Reference |
|---|---|---|---|
| Fungal strain type | -Different fungal species produce different enzyme types -Some fungi have high oxidative enzyme activity, while others lack key metabolic pathways | -T. versicolor produces laccases that degrade doxorubicin -P. chrysosporium secretes ligninolytic enzymes that degrade diclofenac -Cunninghamella elegans CYPs degrade flutamide | [11,104,174] |
| pH | -Affects enzyme structure and substrate binding -High pH disrupts internal electron transfer in laccases and peroxidases | -T. versicolor laccases degrade doxorubicin at pH 4 but not at pH 3 -Cytochrome P450 enzymes require a neutral pH for optimal drug metabolism -P. chrysosporium lignin peroxidase degrades melanin at pH 4 | [12,101,174] |
| Temperature | -Low temperatures slow reaction rates -High temperatures can denature enzymes, reducing activity | -Laccase-ABTS system achieves complete ketoprofen and aspirin removal at 35 °C -Cytochrome P450 enzymes function best at 30 °C for flurbiprofen, diclofenac, and ibuprofen biodegradation but lose stability at higher temperatures -P. chrysosporium lignin peroxidase LiPH8 isozyme performs optimally at 25 °C in ABTS oxidation | [56,88,102] |
| Oxygen levels | -Oxygen is required for oxidation reactions -Low oxygen levels limit enzymatic activity, particularly for laccases and cytochrome P450 enzymes | -Low oxygen reduces laccase oxidation capacity -Cytochrome P450 enzymes require molecular oxygen for pharmaceutical breakdown | [8,175] |
| Nutrient availability | -Limited nutrients can reduce enzyme production -Carbon and nitrogen sources affect fungal metabolism | -White-rot fungi produce more laccase in nutrient-limited environments, enhancing pharmaceutical degradation | [176] |
| Agitation | -Increases oxygen transfer and substrate availability -Excessive agitation can reduce fungal growth or deactivate enzymes | -Higher agitation increases P. ostreatus and T. versicolor laccase activity but may lower fungal biomass -Shear sensitivity impacts lignin peroxidase overproduction in P. chrysosporium | [172,173] |
| Electron-donating and -withdrawing groups | -EDGs enhance enzymatic oxidation -EWGs reduce enzyme affinity for substrates | -DHQ (hydroxyl group as EDG) shows high biotransformation despite iodine (EWG) -Laccases and cytochrome P450 oxidize EDG-containing substrates efficiently | [174,175] |
| Influence of Ions | -Some ions inhibit enzyme activity by blocking active sites -Certain metal ions enhance electron transfer and enzymatic oxidation -Inorganic anions interfere with radical formation and alter pH | -5 mM NaCl inhibits T. versicolor laccase by 20% -Cu2+ enhances triclosan degradation, while Mn2+ inhibits tetracycline removal -HCO3− competes with substrates, reducing degradation efficiency | [176] |
| Humic substances | -Humic acids compete with pharmaceuticals for enzyme binding | -HA inhibits TEMPO-mediated Lac reactions | [138] |
| Drug properties | -Solubility, polarity, and redox potential affect bioavailability to enzymes | -Highly hydrophobic drugs resist enzymatic breakdown | [21,171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.F. Fungi for Sustainable Pharmaceutical Remediation: Enzymatic Innovations, Challenges, and Applications—A Review. Processes 2025, 13, 1034. https://doi.org/10.3390/pr13041034
Khan MF. Fungi for Sustainable Pharmaceutical Remediation: Enzymatic Innovations, Challenges, and Applications—A Review. Processes. 2025; 13(4):1034. https://doi.org/10.3390/pr13041034
Chicago/Turabian StyleKhan, Mohd Faheem. 2025. "Fungi for Sustainable Pharmaceutical Remediation: Enzymatic Innovations, Challenges, and Applications—A Review" Processes 13, no. 4: 1034. https://doi.org/10.3390/pr13041034
APA StyleKhan, M. F. (2025). Fungi for Sustainable Pharmaceutical Remediation: Enzymatic Innovations, Challenges, and Applications—A Review. Processes, 13(4), 1034. https://doi.org/10.3390/pr13041034






