High Cu-Cu Bonding Strength Achievement Using Micron Copper Particles Under Formic Acid Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
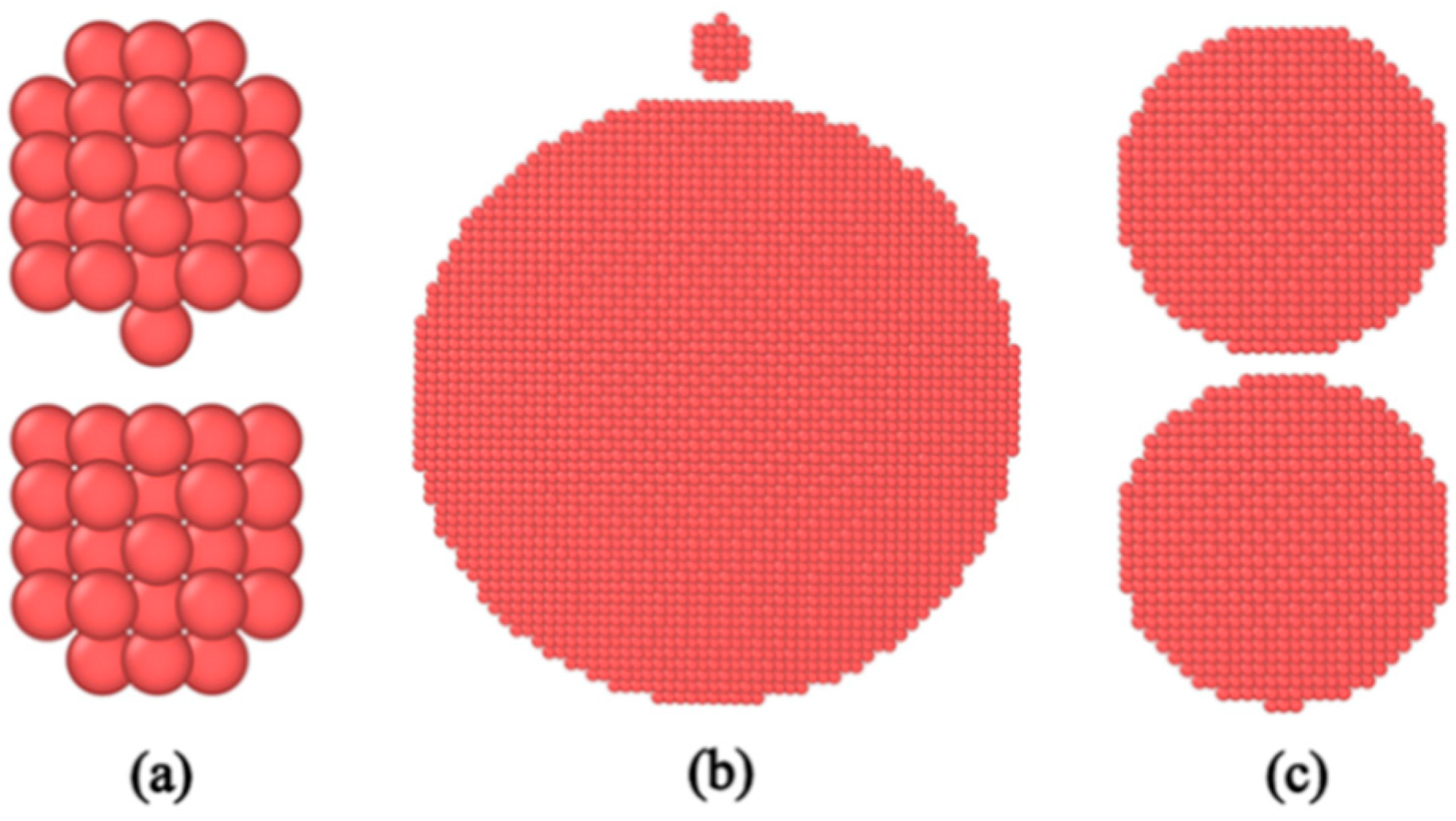
2.1. Simulation Methodology
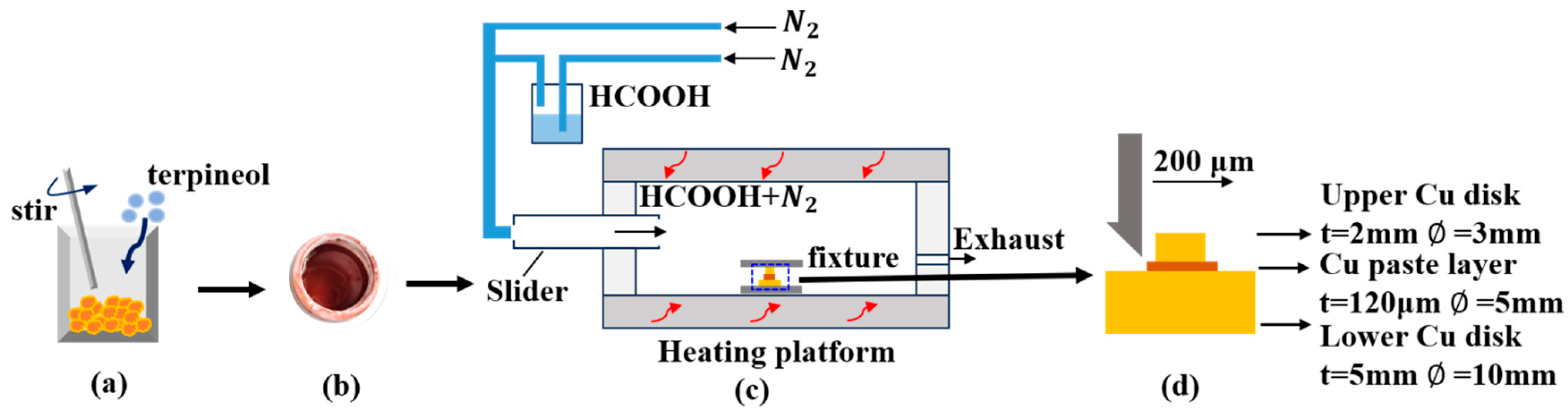
2.2. Experimental Procedure
2.3. Sintering Process
3. Results and Discussion
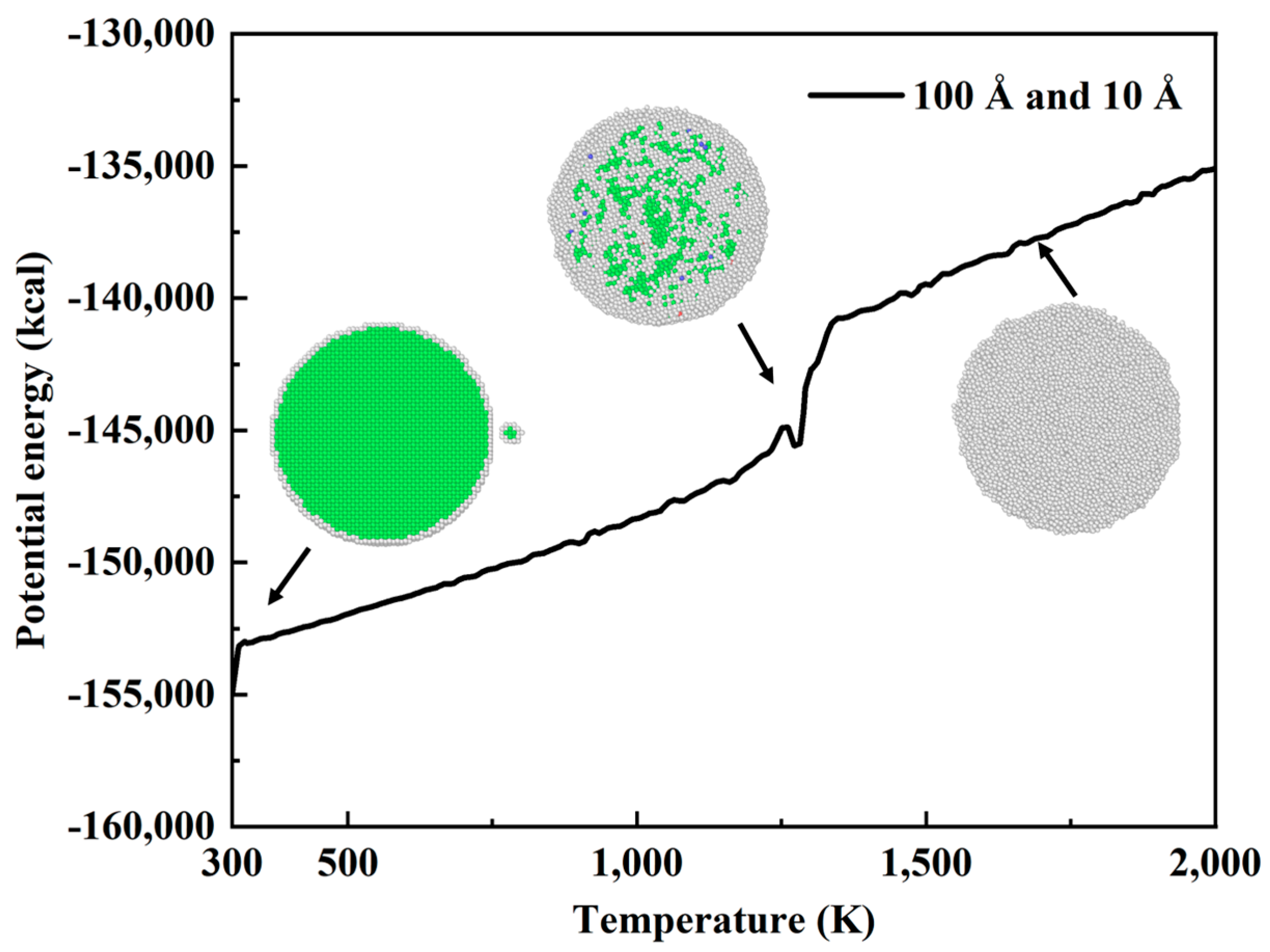
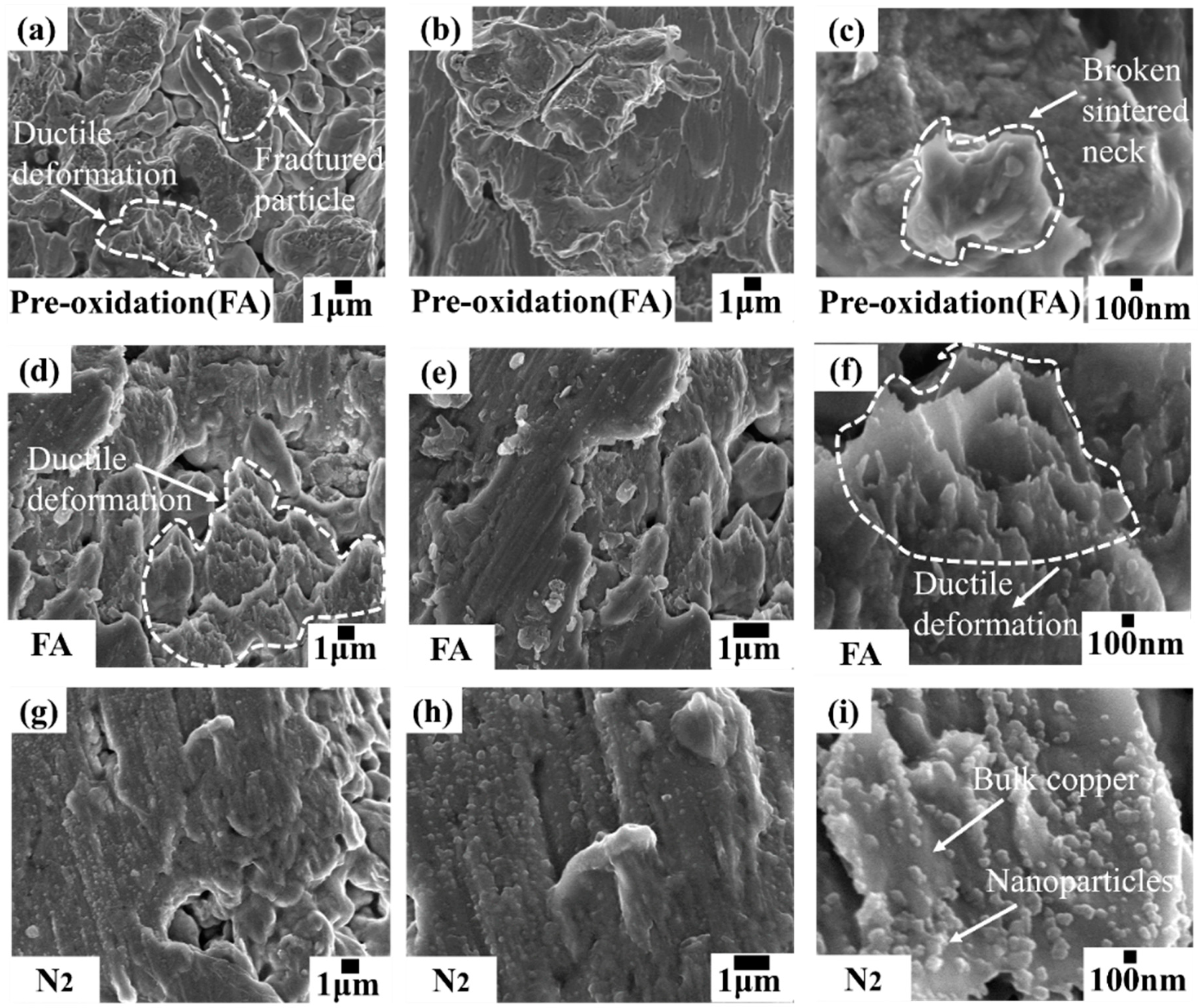
3.1. Effect of Oxidatively Grown Small Copper Particles on the Sintering Process
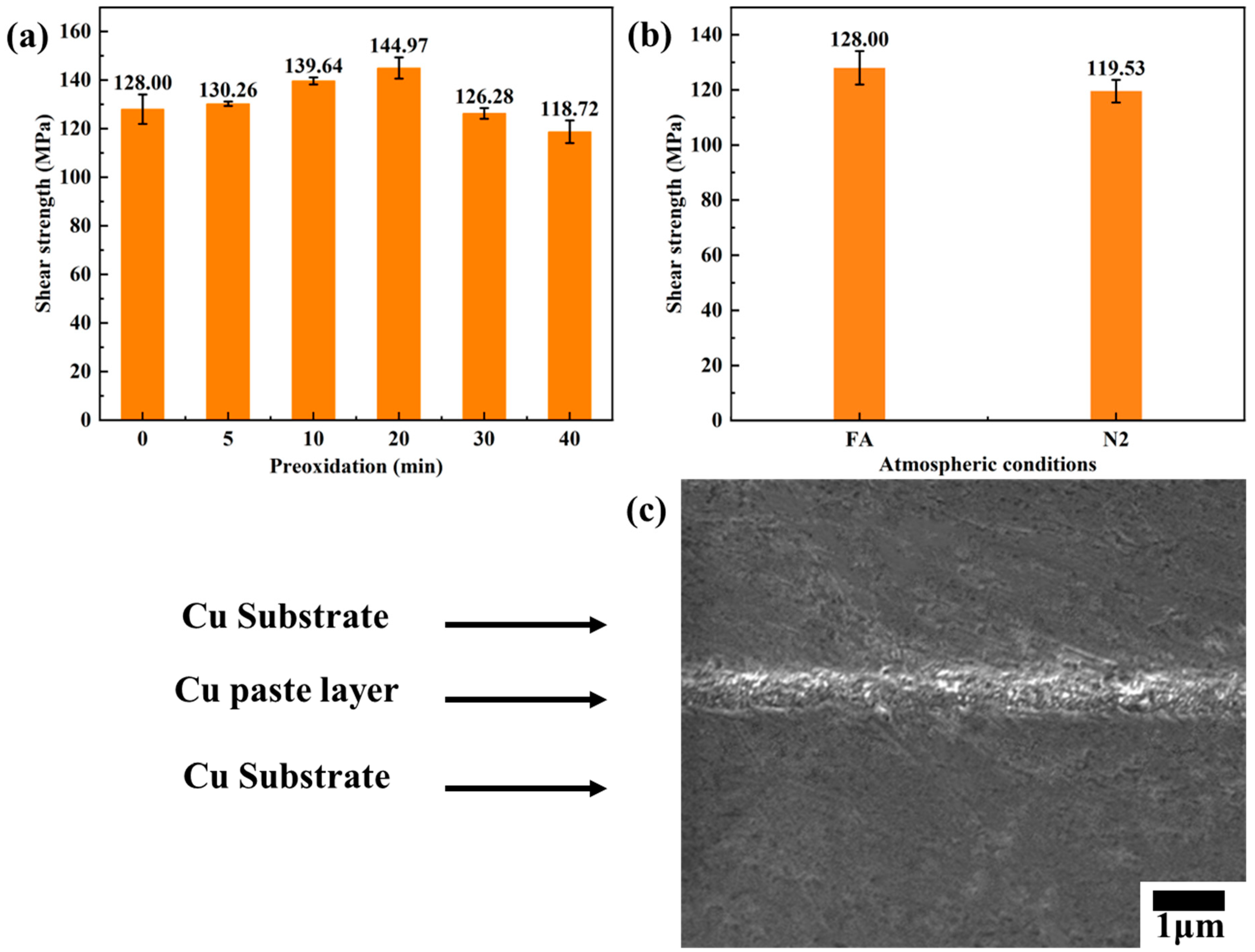
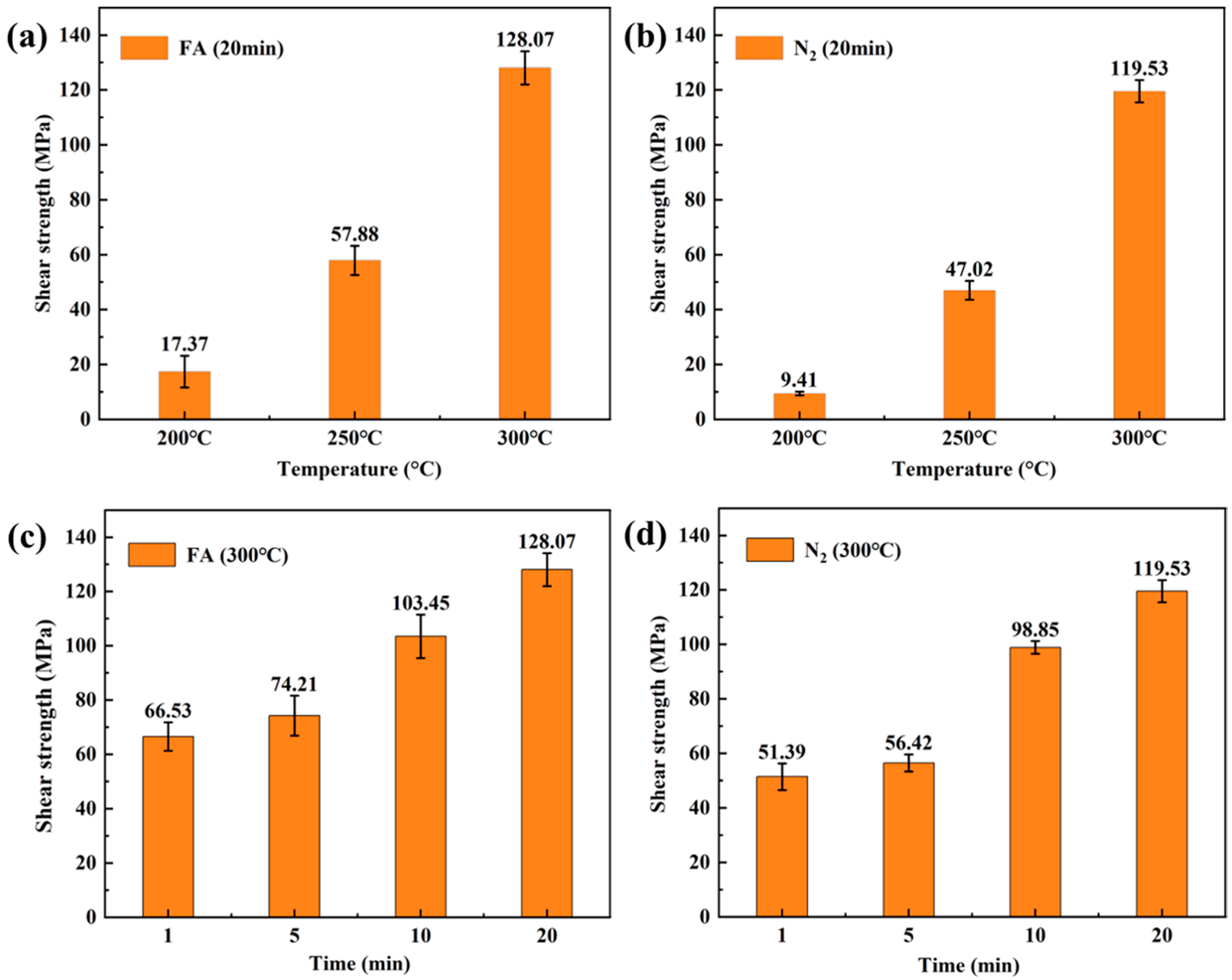
3.2. Effect of Preoxidation Time on TCB
3.3. Effect of Different Sintering Environment on TCB
4. Conclusions
- (1)
- As the sintering temperature increases, the motion mode changes from atomic vibration to diffusion. The smaller the copper particle size, the easier the sintering, and the smaller copper particle size facilitates the sintering process of the larger copper particle size. The introduction of small copper particle sintering can improve the local structure orderliness on a short-range scale and increase the local structure disorderliness on a large scale. Small copper particles can increase the phase change point, increase the total potential energy, and promote the sintering of copper particles. The oxidized small particles on the surface of micron copper particles can promote the sintering process of micron copper particles.
- (2)
- Preoxidation has minimal effect on the improvement of the mechanical properties of TCB. TCB provides extremely thin and excellent mechanical properties. The nail acid atmosphere helps to reduce micro-oxide particles on the particle surface and promote sintering of oxide particles. Oxides on the surface of copper atoms impede sintering, making the mechanical properties lower than those of the environment.
- (3)
- Different redox processes have a limited effect on the mechanical properties of low-temperature and high-pressure sintering joints, and the sintering joints are ductile fractures. TCB can realize short-term sintering and obtain sintering joints with excellent mechanical properties, thereby providing theoretical support for the rapid realization of Cu-Cu bond exploration in the future.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TCB | Thermocompression bonding |
| FA | Formic acid |
| MSD | Mean square displacement |
| ORB | Oxidation reduction bonding |
| RDF | Radial distribution function |
| SEM | Scanning electronic microscope |
References
- Loulijat, H.; Zerradi, H.; Mizani, S.; Achhal, E.M.; Dezairi, A.; Ouaskit, S. The behavior of the thermal conductivity near the melting temperature of copper nanoparticle. J. Mol. Liq. 2015, 211, 695–704. [Google Scholar] [CrossRef]
- Feng, D.; Feng, Y.; Yuan, S.; Zhang, X.; Wang, G. Melting behavior of Ag nanoparticles and their clusters. Appl. Therm. Eng. 2017, 111, 1457–1463. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, X.; Chen, M. Improved thermal conductivity and reliability through graphene reinforced nanopaste for power devices in new energy vehicles. IEEE Trans. Compon. Packag. Manuf. Technol. 2024, 14, 52–60. [Google Scholar] [CrossRef]
- Qu, G.; Guo, W.; Zhang, C.; Xue, J.; Peng, Z.; Yin, C.; He, S.; Zou, G.; Jia, Q.; Zhang, H. Improving thermal stability and reliability of power chips by sintering foam structure layer. Appl. Mater. Today 2024, 40, 102397. [Google Scholar]
- Yamagiwa, D.; Matsuda, T.; Furusawa, H.; Sato, K.; Tatsumi, H.; Sano, T.; Kashiba, Y.; Hirose, A. Pressureless sinter joining of bare Cu substrates under forming gas atmosphere by surface-oxidized submicron Cu particles. J. Mater. Sci. Mater. Electron. 2021, 32, 19031–19041. [Google Scholar]
- Zuo, Y.; Carter-Searjeant, S.; Green, M.; Mills, L.; Mannan, S.H. High bond strength Cu joints fabricated by rapid and pressureless in situ reduction-sintering of Cu nanoparticles. Mater. Lett. 2020, 276, 128260. [Google Scholar]
- Mou, Y.; Peng, Y.; Zhang, Y.; Cheng, H.; Chen, M. Cu-Cu bonding enhancement at low temperature by using carboxylic acid surface-modified Cu nanoparticles. Mater. Lett. 2018, 227, 179–183. [Google Scholar] [CrossRef]
- Yan, J.; Zou, G.; Hu, A.; Zhou, Y.N. Preparation of PVP coated Cu NPs and the application for low-temperature bonding. J. Mater. Chem. 2011, 21, 15981–15986. [Google Scholar]
- Jeong, S.; Woo, K.; Kim, D.; Lim, S.; Kim, J.S.; Shin, H.; Xia, Y.; Moon, J. Controlling the thickness of the surface oxide layer on Cu nanoparticles for the fabrication of conductive structures by inkjet printing. Adv. Funct. Mater. 2008, 18, 679–686. [Google Scholar] [CrossRef]
- Nishikawa, H.; Hirano, T.; Takemoto, T.; Terada, N. Effects of joining conditions on joint strength of Cu/Cu joint using Cu nanoparticle paste. Open Surf. Sci. J. 2011, 3, 60–64. [Google Scholar]
- Cheon, J.; Lee, J.; Kim, J. Inkjet printing using copper nanoparticles synthesized by electrolysis. Thin Solid Film. 2012, 520, 2639–2643. [Google Scholar] [CrossRef]
- Ishizaki, T.; Watanabe, R. A new one-pot method for the synthesis of Cu nanoparticles for low temperature bonding. J. Mater. Chem. 2012, 22, 25198–25206. [Google Scholar] [CrossRef]
- Zuo, Y.; Shen, J.; Xie, J.; Xiang, L. Influence of Cu micro/nano-particles mixture and surface roughness on the shear strength of Cu-Cu joints. J. Mater. Process. Technol. 2018, 257, 250–256. [Google Scholar] [CrossRef]
- Zuo, Y.; Shen, J.; Hu, Y.; Gao, R. Improvement of oxidation resistance and bonding strength of Cu nanoparticles solder joints of Cu–Cu bonding by phosphating the nanoparticle. J. Mater. Process. Technol. 2018, 253, 27–33. [Google Scholar] [CrossRef]
- Gao, R.; He, S.; Li, J.; Shen, Y.-A.; Nishikawa, H. Interfacial transformation of preoxidized Cu microparticles in a formic-acid atmosphere for pressureless Cu–Cu bonding. J. Mater. Sci. Mater. Electron. 2020, 31, 14635–14644. [Google Scholar]
- Zuo, Y.; Shen, J.; Xu, H.; Gao, R. Effect of different sizes of Cu nanoparticles on the shear strength of Cu-Cu joints. Mater. Lett. 2017, 199, 13–16. [Google Scholar] [CrossRef]
- Yamakawa, T.; Takemoto, T.; Shimoda, M.; Nishikawa, H.; Shiokawa, K.; Terada, N. Influence of joining conditions on bonding strength of joints: Efficacy of low-temperature bonding using Cu nanoparticle paste. J. Electron. Mater. 2013, 42, 1260–1267. [Google Scholar]
- Zuo, Y.; Zhao, C.; Robador, A.; Wickham, M.; Mannan, S.H. Quasi-in-situ observation of the grain growth and grain boundary movement in sintered Cu nanoparticle interconnects. Acta Mater. 2022, 236, 118135. [Google Scholar]
- Gao, Y.; Zhang, H.; Li, W.; Jiu, J.; Nagao, S.; Sugahara, T.; Suganuma, K. Die bonding performance using bimodal Cu particle paste under different sintering atmospheres. J. Electron. Mater. 2017, 46, 4575–4581. [Google Scholar] [CrossRef]
- Liang, P.; Pan, Z.; Tang, L.; Zhang, G.; Yang, D.; He, S.; Yan, H. Molecular dynamics simulation of sintering densification of multi-scale silver layer. Materials 2022, 15, 2232. [Google Scholar] [CrossRef]
- Liu, X.; Nishikawa, H. Low-pressure Cu-Cu bonding using in-situ surface-modified microscale Cu particles for power device packaging. Scr. Mater. 2016, 120, 80–84. [Google Scholar] [CrossRef]
- Namgoong, D.; Siow, K.S.; Lee, J.H. Improvement of bondability by addition of carboxylic acid to the sinter-bonding paste containing bimodal-sized Cu particles and rapid bonding in air. Met. Mater. Int. 2023, 29, 457–466. [Google Scholar] [CrossRef]
- Kim, D.; Chen, C.; Lee, S.-J.; Nagao, S.; Suganuma, K. Strengthening of DBA substrate with Ni/Ti/Ag metallization for thermal fatigue-resistant Ag sinter joining in GaN power modules. J. Mater. Sci. Mater. Electron. 2020, 31, 3715–3726. [Google Scholar]
- Jangam, S.C.; Bajwa, A.A.; Mogera, U. Fine-Pitch (≤10 m) Direct Cu-Cu Interconnects Using In-Situ Formic Acid Vapor Treatment. In Proceedings of the 2019 IEEE 69th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 28–31 May 2019; pp. 620–627. [Google Scholar]
- Song, Z.; Luo, W.; Fan, X.; Zhu, Y. Atomic fast dynamic motion on the Cu Nanoparticle’s surface before melting: A molecular dynamics study. Appl. Surf. Sci. 2022, 606, 154901. [Google Scholar]
- Bingqing, C.; Alfonso, H.; Ngan, W. The crystal structures of sintered copper nanoparticles: A molecular dynamics study. Int. J. Plast. 2013, 47, 65–79. [Google Scholar]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The open visualization tool. Model. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar]
- Mishin, Y.; Mehl, M.J.; Papaconstantopoulos, D.A.; Voter, A.F.; Kress, J.D. Structural stability and lattice defects in copper: Ab initio, tight-binding, and embedded-atom calculations. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 63, 224106. [Google Scholar]
- Li, Q.; Liu, C.; Chen, X. Molecular characteristics of dissociated water with memory effect from methane hydrates. Int. J. Mod. Phys. B 2014, 28, 1450062. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, P.; Sun, W. Monitoring micro-structural evolution during aluminum sintering and understanding the sintering mechanism of aluminum nanoparticles: A molecular dynamics study. J. Mater. Sci. Technol. 2020, 57, 92–100. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Gan, Y.; Chen, Z.; Peng, H. Molecular dynamics study of neck growth in laser sintering of hollow silver nanoparticles with different heating rates. J. Phys. D Appl. Phys. 2013, 46, 335302. [Google Scholar]
- Marquardt, R. Mean square displacement of a free quantum particle in a thermal state. Mol. Phys. 2021, 119, 17–18. [Google Scholar]
- Malti, A.; Kardani, A.; Montazeri, A. An insight into the temperature-dependent sintering mechanisms of metal nanoparticles through MD-based microstructural analysis. Powder Technol. 2021, 386, 30–39. [Google Scholar]
- Choi, E.B.; Lee, J.H. Sub-1 min sinter-bonding technique in air using modified Cu dendritic particles for formation of a high-temperature sustainable bondline. Met. Mater. Int. 2021, 27, 5278–5284. [Google Scholar]
- Li, J.; Xu, Y.; Zhao, X.; Meng, Y.; Yin, Z.; Wang, Y.; Suga, T. Enhancement and mechanism of copper nanoparticle sintering in activated formic acid atmosphere at low temperature. ECS J. Solid State Sci. Technol. 2021, 10, 054004. [Google Scholar]
- Li, J.; Yu, X.; Shi, T.; Cheng, C.; Fan, J.; Cheng, S.; Liao, G.; Tang, Z. Low-temperature and low-pressure Cu–Cu bonding by highly sinterable Cu nanoparticle paste. Nanoscale Res. Lett. 2017, 12, 255. [Google Scholar] [PubMed]
- Gao, Y.; Jiu, J.; Chen, C.; Suganuma, K.; Sun, R.; Liu, Z.-Q. Oxidation-enhanced bonding strength of Cu sinter joints during thermal storage test. J. Mater. Sci. Technol. 2022, 115, 251–255. [Google Scholar]
- Aliouat, A.; Antou, G.; Rat, V.; Pradeilles, N.; Geffroy, P.-M.; Maître, A. Investigation of electrical transitions in the first steps of spark plasma sintering: Effects of pre-oxidation and mechanical loading within copper granular media. Materials 2022, 15, 4096. [Google Scholar] [CrossRef]
- Yang, W.; Wan, J.; Wu, C.; Xie, C.; Huang, Z. Sintering of mixed Cu Ag nanoparticles pretreated by formic acid vapor for Cu-Cu low temperature bonding. Microelectron. Reliab. 2023, 141, 114890. [Google Scholar] [CrossRef]
- Liu, J.; Mou, Y.; Liu, J.; Peng, Y.; Chen, M. Low-temperature Cu-Cu bonding by using Cu2O nanoparticle coated hierarchical structure. IEEE Trans. Compon. Packag. Manuf. Technol. 2022, 12, 878–882. [Google Scholar]
- Yuan, Y.; Wu, H.; Li, J.; Zhu, P.; Sun, R. Cu-Cu joint formation by low-temperature sintering of self-reducible Cu nanoparticle paste under ambient condition. Appl. Surf. Sci. 2021, 570, 151220. [Google Scholar]
- Xie, J.; Shen, J.; Deng, J.; Chen, X. Influence of aging atmosphere on the thermal stability of low-temperature rapidly sintered Cu nanoparticle paste joint. J. Electron. Mater. 2020, 49, 2669–2676. [Google Scholar]
- Dai, D.; Li, J.; Qian, J.; Wang, Z.; Zheng, K.; Yu, J.; Chen, X. The formation of Cu-Cu joints by low temperature sintering Cu NPs with copper formate layer and its oxidation enhancement. Mater. Lett. 2023, 339, 134087. [Google Scholar] [CrossRef]



| Experiment 1 | Oxidation Bonding | Reduction Bonding | Atmosphere | ||
|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | Temperature (°C) | Time (min) | ||
| 1 | 250 | 40 | 300 | 20 | FA |
| 2 | 30 | 20 | FA | ||
| 3 | 20 | 20 | FA | ||
| 4 | 10 | 20 | FA | ||
| 5 | 5 | 20 | FA | ||
| 6 | 0 | 20 | FA | ||
| 7 | 0 | 20 | N2 | ||
| Experiment 2 | Temperature (°C) | Time (min) | Atmosphere |
|---|---|---|---|
| 1 | 300 | 1 | FA/N2 |
| 2 | 5 | ||
| 3 | 10 | ||
| 4 | 20 | ||
| 1 | 250 | 1 | |
| 2 | 5 | ||
| 3 | 10 | ||
| 4 | 20 | ||
| 1 | 200 | 1 | |
| 2 | 5 | ||
| 3 | 10 | ||
| 4 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Luo, Y.; Li, D.; Li, D.; Yang, B.; Gong, B.; Han, S.; He, S.; Cai, M. High Cu-Cu Bonding Strength Achievement Using Micron Copper Particles Under Formic Acid Atmosphere. Processes 2025, 13, 1042. https://doi.org/10.3390/pr13041042
Li B, Luo Y, Li D, Li D, Yang B, Gong B, Han S, He S, Cai M. High Cu-Cu Bonding Strength Achievement Using Micron Copper Particles Under Formic Acid Atmosphere. Processes. 2025; 13(4):1042. https://doi.org/10.3390/pr13041042
Chicago/Turabian StyleLi, Bofu, Yinyin Luo, Dejian Li, Dameng Li, Baobin Yang, Baoliang Gong, Shunfeng Han, Siliang He, and Miao Cai. 2025. "High Cu-Cu Bonding Strength Achievement Using Micron Copper Particles Under Formic Acid Atmosphere" Processes 13, no. 4: 1042. https://doi.org/10.3390/pr13041042
APA StyleLi, B., Luo, Y., Li, D., Li, D., Yang, B., Gong, B., Han, S., He, S., & Cai, M. (2025). High Cu-Cu Bonding Strength Achievement Using Micron Copper Particles Under Formic Acid Atmosphere. Processes, 13(4), 1042. https://doi.org/10.3390/pr13041042






