University of Oslo-66: A Versatile Zr-Based MOF for Water Purification Through Adsorption and Photocatalysis
Abstract
:1. Introduction
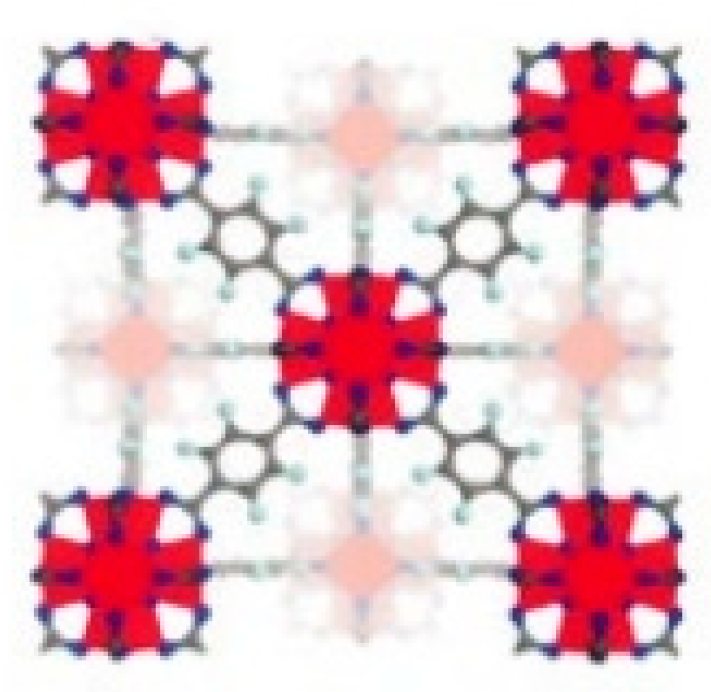
- MOFs can form a variety of topological structures, including cubic [31], tetrahedral [32], octahedral [33], as well as more complex three-dimensional network structures [34]. Figure 1 shows the structure of the regular tetrahedron and octahedron of UiO-66(Zr) [35]. By strategically choosing metal nodes and organic ligands, MOFs with specific topological structures can be tailored to meet targeted applications.
- 2.
- MOFs can have their structures and properties adjusted and optimized through various modification methods to enhance their performance across applications. For instance, doping metal ions [36] can alter the electronic structure and catalytic activity of MOFs. MOFs can also be combined with inorganic nanomaterials; compounding with carbon nanotubes [37] or graphene [38] can enhance their electron conduction performance, while integration with TiO2 [39], g-C3N4 [40], etc., can improve their photocatalytic performance. Similarly, MOFs can be paired with organic polymers; when combined with polyimide [41], they form a film material with enhanced mechanical properties and gas separation capabilities, suitable for gas separation membranes, and related technologies.
- 3.
- There are numerous synthesis methods for MOFs, including the solvothermal method [42], hydrothermal method [43], microwave-assisted synthesis method [44], ultrasound-assisted synthesis method [45], electrochemical synthesis method [46], and mechanochemical synthesis method [47]. These approaches allow precise control over synthesis conditions, producing MOFs with tailored properties suited to specific needs. In recent years, MOFs have gained increasing attention for their ability to treat water pollution. Among these MOFs, UiO-66 particularly stands out due to its exceptional stability and versatility in water treatment applications. The subsequent section delves into UiO-66’s structure and synthesis evolution, building on this foundation.
2. UiO-66 Structure and Synthesis Evolution
2.1. Structure
2.2. Synthesis Evolution
2.2.1. Solvothermal Method
2.2.2. Microwave-Assisted Method
2.2.3. Mechanochemical Method
2.2.4. Evaporation
2.2.5. Continuous Flow
2.2.6. Electrochemical Method
| Synthetic Method | Temp (°C) | Activation Solvent | Ref. |
|---|---|---|---|
| Solvothermal | 120 | DMF | [56] |
| Solvothermal | 120 | Methanol | [68] |
| Solvothermal | 120 | Ethanol | [69] |
| Solvothermal | 120 | Acetone | [70] |
| Solvothermal | 120 | Chloroform | [71] |
| Solvothermal | 50 | DMF | [72] |
| Solvothermal | 70 | DMF | [72] |
| Solvothermal | 90 | DMF | [72] |
| Solvothermal | 110 | DMF | [72] |
| Microwave-assisted | - | Ethanol | [59] |
| Mechanochemical | - | Methanol | [61] |
| Evaporation | 100 | DMF | [62] |
| Continuous flow | 130 | Methanol | [64] |
| Electrochemical | 80 | Ethanol | [67] |
3. Application of UiO-66 in Water Purification
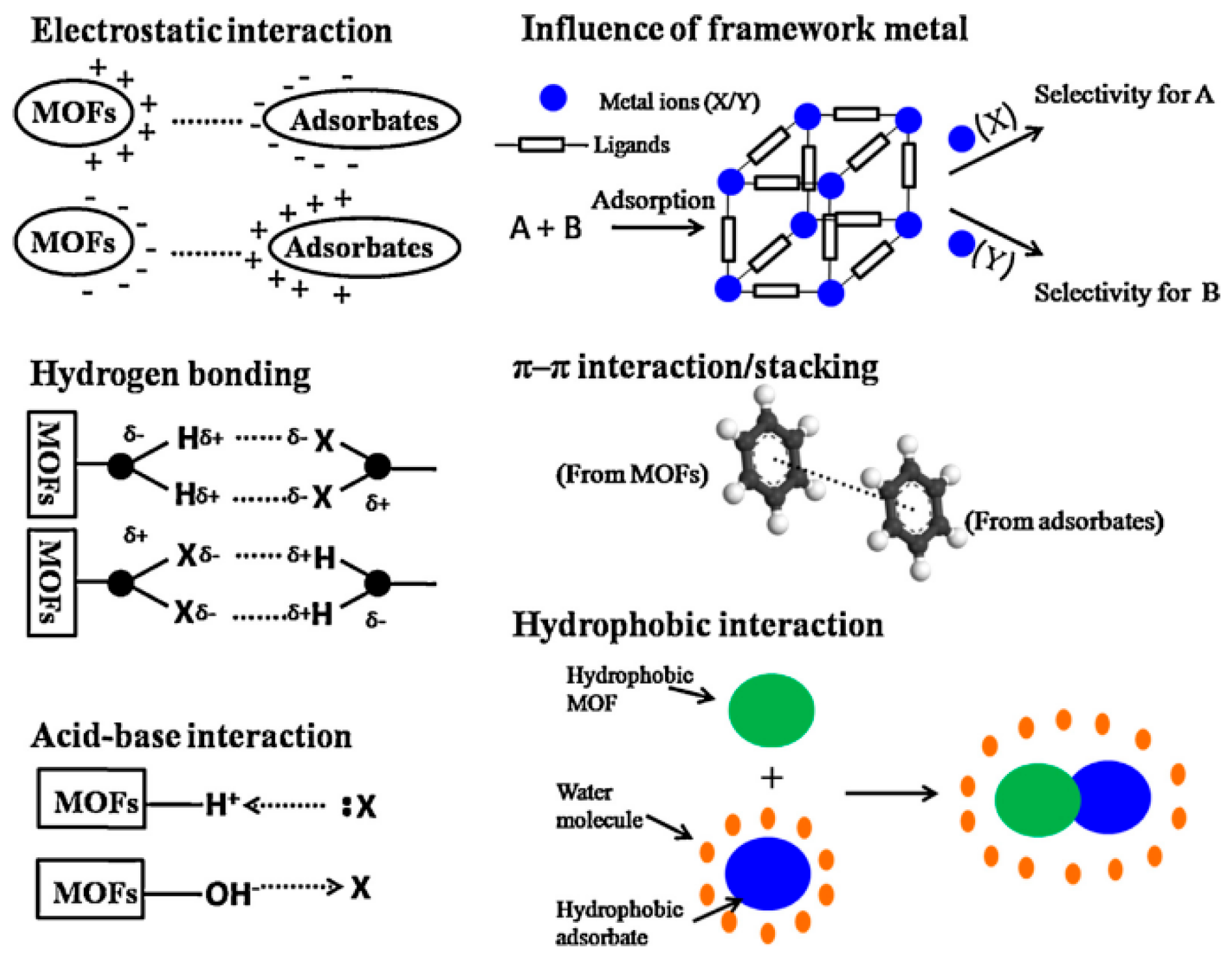
3.1. Removal of Pollutants from Water by Adsorption Method
3.1.1. Organic Dyes
3.1.2. Antibiotics
3.1.3. Heavy Metal Ions
3.1.4. Fluoride
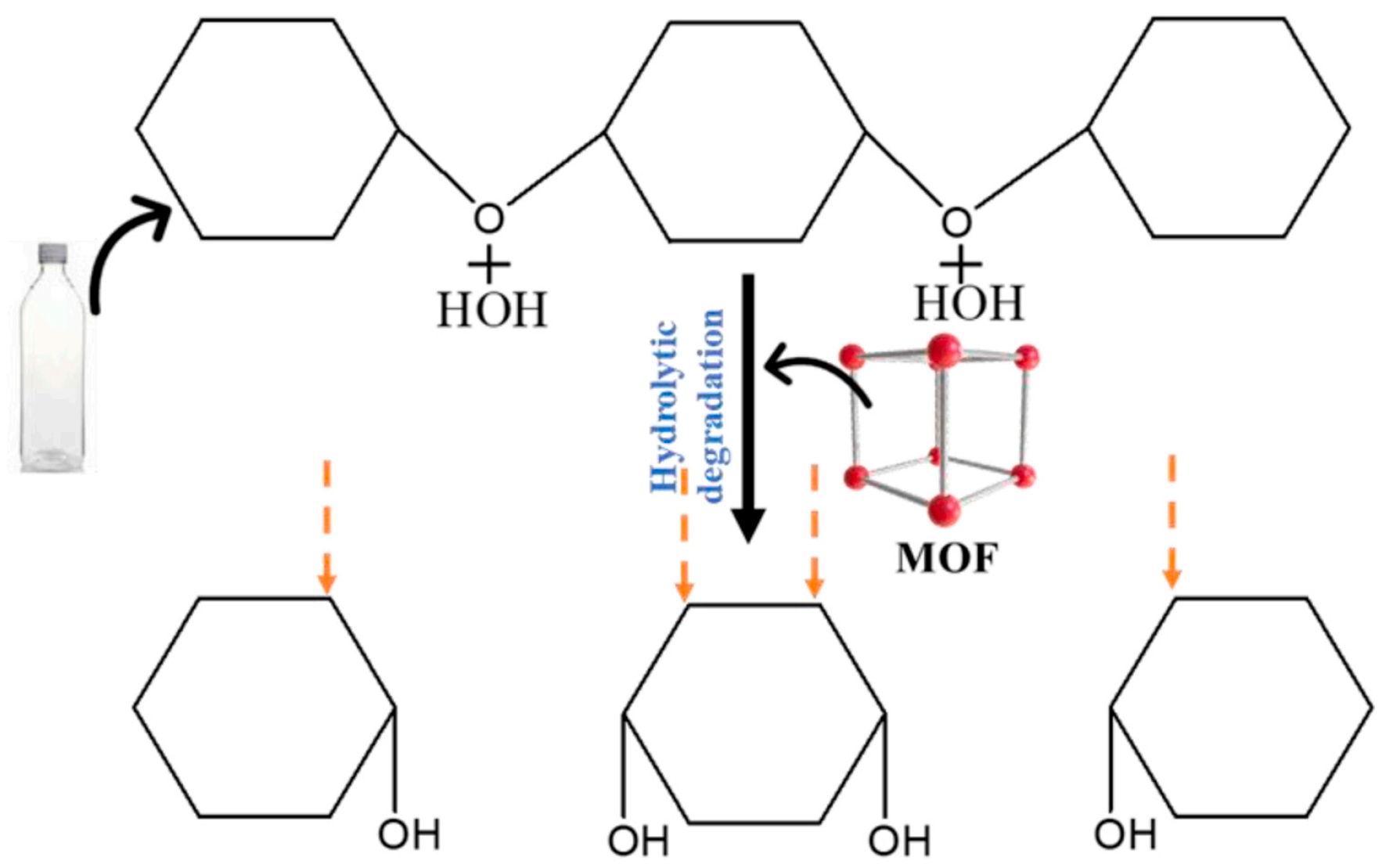
3.1.5. Microplastics
| Pollutant | Qe (mg/g) | Adsorptive Mechanism | Ref. |
| Methylene blue (MB) | 91 | Electrostatic interaction | [82] |
| Rhodamine B (Rh B) | 75.85 | Electrostatic interaction | [83] |
| Methyl red (MR) | 384 | Electrostatic interaction | [84] |
| Malachite green (MG) | 133 | π–π interactions, electrostatic interaction, and hydrogen bonding | [84] |
| Methylene blue (MB) | 370 | π–π interactions, electrostatic interaction, and hydrogen bonding | [84] |
| Alizarin red S (ARS) | 400 | Electrostatic interaction | [85] |
| Methyl orange (MO) | 188.6 | Electrostatic interaction | [86] |
| Congo red (CR) | 147.1 | Electrostatic interaction | [86] |
| Methylene blue (MB) | 107.5 | Electrostatic interaction,π–π interactions | [86] |
| Methylene blue (MB) | 79.78 | Electrostatic interaction | [104] |
| Methyl orange (MO) | 70.79 | Electrostatic interaction | [104] |
| Rhodamine B (Rh B) | 25.94 | Electrostatic interaction | [104] |
| Acid red 52 (AR52) | 5.42 | Electrostatic interaction | [104] |
| Doxycycline | 156.25 | Electrostatic interaction | [88] |
| Sulfachlorpyrazine (SCP) | 417 | π–π interactions, electrostatic interaction | [93] |
| Oxytetracycline (OTC) | 21.22 | Electrostatic interaction | [105] |
| Norfloxacin | 134.5 | π–π interactions, electrostatic interaction, and hydrogen bonding | [106] |
| Tetracycline (TC) | 208.68 | π–π interactions and hydrogen bonding | [107] |
| Sulfamethoxazole (SMX) | 25 | π–π interactions, electrostatic interaction, and hydrogen bonding | [108] |
| As (V) | 303 | Complexation (coordination) | [96] |
| As (III) | 143.95 | Electrostatic interaction | [107] |
| Pb (II) | 19.40 | Complexation (coordination) | [109] |
| Sb (V) | 127.5 | Electrostatic interaction | [110] |
| Pb (II) | 48.7 | Complexation (coordination) | [111] |
| Hg (II) | 59 | Complexation (coordination) | [112] |
| Cr (VI) | 36.4 | Electrostatic interaction | [113] |
| Au (III) | 53.6 | Electrostatic interaction | [114] |
| Fluoride | 41.36 | Complexation (coordination) | [98] |
| Polyethylene terephthalate (PET) | 226.8 | Hydrogen bonding | [102] |
| Melamine foam (MF) | 955 | Electrostatic attraction, hydrogen bonding, and van der Waals force | [103] |
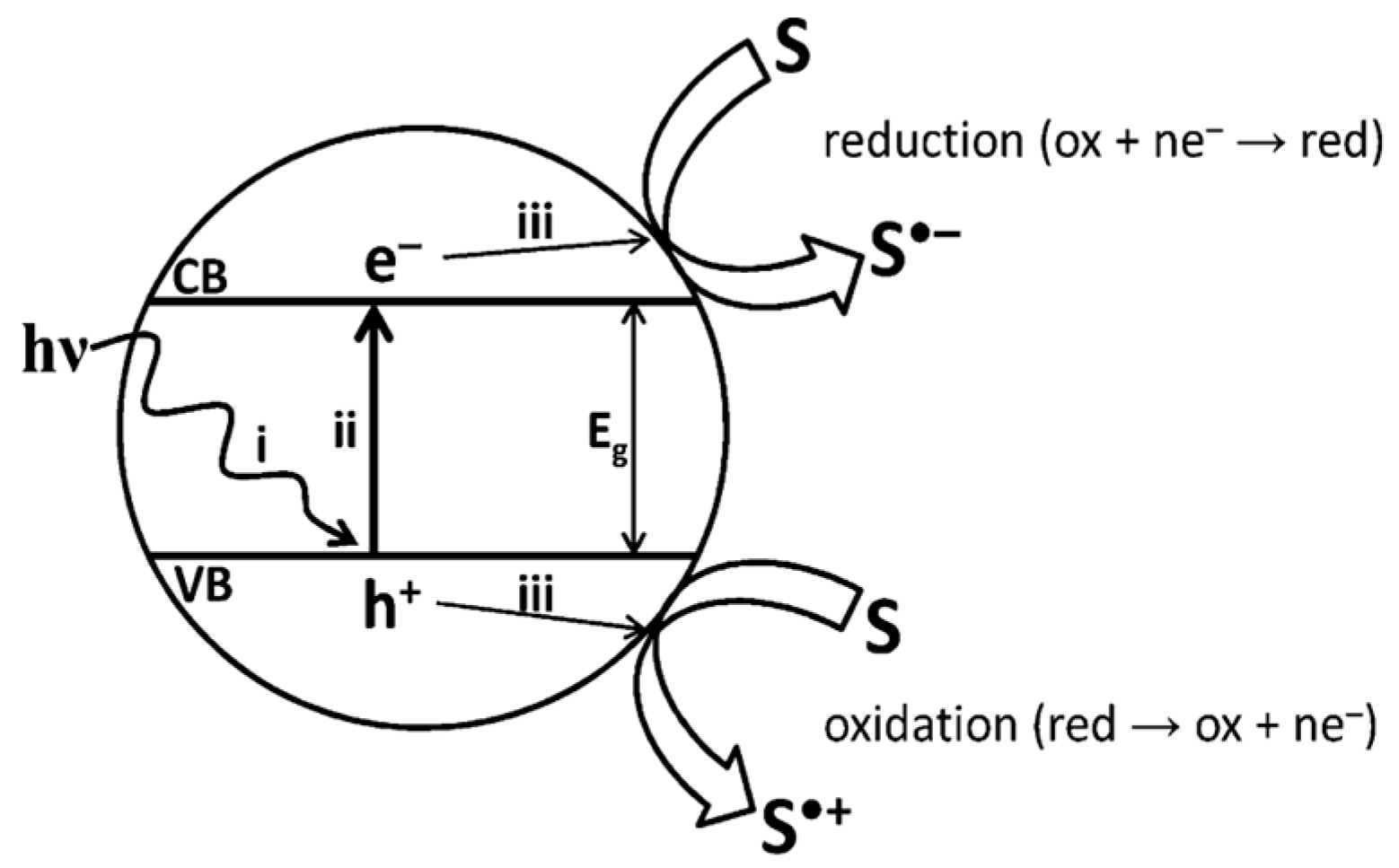
3.2. Removal of Pollutants from Water by Photocatalysis
3.2.1. Organic Dyes
3.2.2. Antibiotics
3.2.3. Hexavalent Chromium (Cr (VI))
| Pollutant | Photocatalysts | Efficiency (%) | Time (min) | Ref. |
|---|---|---|---|---|
| Rhodamine B (Rh B) | g-C3N4/UiO-66 | 96.08 | 360 | [120] |
| Methyl orange (MO) | TiO2@UiO-66 | 97.59 | 150 | [121] |
| Rhodamine B (Rh B) | UiO-66 | 69 | 360 | [120] |
| Rhodamine B (Rh B) | UiO-66 | 14.55 | 12 | [128] |
| Methylene blue (MB) | NH2-UiO-66/ZnO | 96.7 | 60 | [129] |
| Malachite green (MG) | NH2-UiO-66/ZnO | 98 | 60 | [129] |
| Ciprofloxacin (CIP) | Cu/UiO-66 | 93 | 120 | [122] |
| Tetracycline (TC) | Co/UiO-66 | 94 | 60 | [123] |
| Ciprofloxacin (CIP) | Bi2MoO6/UiO-66-NH2 | 96 | 90 | [130] |
| Oxytetracycline (OTC) | MnO2/UiO-66 | 49.9 | 60 | [131] |
| Oxytetracycline (OTC) | UiO-66 | 40.2 | 60 | [131] |
| Sulfameter | Fe@UiO-66 | 89.9 | 300 | [132] |
| Tetracycline (TC) | ZnO@NH2-UiO-66 | 61.9 | 30 | [133] |
| Cr (VI) | UiO-66 | 99 | 360 | [127] |
| Cr (VI) | g-C3N4/UiO-66 | 99 | 40 | [134] |
| Cr (VI) | UiO-66-NH2 | 99 | 180 | [135] |
| Cr (VI) | UiO-66 | 8 | 80 | [136] |
| Cr (VI) | UiO-66-NH2 | 20 | 60 | [137] |
4. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmadijokani, F.; Molavi, H.; Rezakazemi, M.; Tajahmadi, S.; Bahi, A.; Ko, F.; Aminabhavi, T.M.; Li, J.-R.; Arjmand, M. UiO-66 metal–organic frameworks in water treatment: A critical review. Prog. Mater. Sci. 2022, 125, 100904. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Abu Bakar, H.H.; Garba, Z.N.; Isiyaka, H.A.; Saad, B. Selective adsorption of dyes and pharmaceuticals from water by UiO metal–organic frameworks: A comprehensive review. Polyhedron 2021, 210, 115515. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, C.; Fang, Z.; Qin, M.; Zhu, Z.; Qu, J.; Zheng, H. Copper-supported MOF-derived carbon materials for highly efficient antibiotics removal. J. Environ. Chem. Eng. 2024, 12, 113756. [Google Scholar] [CrossRef]
- Mondol, M.M.H.; Jhung, S.H. Adsorptive removal of pesticides from water with metal–organic framework-based materials. Chem. Eng. J. 2021, 421, 129688. [Google Scholar] [CrossRef]
- Mohan, B.; Kamboj, A.; Virender; Singh, K.; Priyanka; Singh, G.; Pombeiro, A.J.L.; Ren, P. Metal-organic frameworks (MOFs) materials for pesticides. heavy metals, and drugs removal: Environmental safety. Sep. Purif. Technol. 2023, 310, 123175. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, G.; Li, Y.; Yao, Y.; Huang, J.; Zhang, P.; Ren, S.; Shen, J.; Zhang, Z. Removal of pharmaceutical and personal care products (PPCPs) by MOF-derived carbons: A review. Sci. Total Environ. 2023, 857, 159279. [Google Scholar] [CrossRef]
- Song, J.; Yu, Y.; Han, X.; Yang, W.; Pan, W.; Jian, S.; Duan, G.; Jiang, S.; Hu, J. Novel MOF (Zr)-on-MOF (Ce) adsorbent for elimination of excess fluoride from aqueous solution. J. Hazard. Mater. 2024, 463, 132843. [Google Scholar] [CrossRef]
- Nikhar, S.; Kumar, P.; Chakraborty, M. A review on microplastics degradation with MOF: Mechanism and action. Next Nanotechnol. 2024, 5, 100060. [Google Scholar] [CrossRef]
- Al-Muttair, A.K.; Al Easawi, N.A.; Mustafa, S.A. Using Adsorption as Means to Treat Water Pollution. J. Botechnol. Res. Cent. 2022, 16, 37–47. [Google Scholar] [CrossRef]
- Martini, S. Membrane technology for water pollution control: A review of recent hybrid mechanism. J. Rekayasa Kim. Lingkung. 2022, 17, 83–96. [Google Scholar] [CrossRef]
- Liu, H.; Tang, S.; Wang, Z.; Zhang, Q.; Yuan, D. Organic cocatalysts improved Fenton and Fenton-like processes for water pollution control: A review. Chemosphere 2024, 353, 141581. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Dai, Q.; Gao, G.; Tang, J.; Jiang, R.; Sun, S.; Ye, Y.; Li, S.; Xie, R.; Zhang, J. The MIL-125 (Ti)/Co3O4 towards efficiently removing tetracycline by synergistic adsorption-photocatalysis roles. Mater. Des. 2025, 113608. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. A critical review on latest innovations and future challenges of electrochemical technology for the abatement of organics in water. Appl. Catal. B Environ. 2023, 328, 122430. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, H.; Chen, Y. Recent advances in biological removal of nitroaromatics from wastewater. Environ. Pollut. 2022, 307, 119570. [Google Scholar] [CrossRef]
- Rojas, S.; Rodríguez-Diéguez, A.; Horcajada, P. Metal–organic frameworks in agriculture. ACS Appl. Mater. Interfaces 2022, 14, 16983–17007. [Google Scholar] [CrossRef]
- Salama, R.S.; El-Sayed, E.-S.M.; El-Bahy, S.M.; Awad, F.S. Silver nanoparticles supported on UiO-66 (Zr): As an efficient and recyclable heterogeneous catalyst and efficient adsorbent for removal of indigo carmine. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127089. [Google Scholar] [CrossRef]
- Qu, N.; Sun, H.; Sun, Y.; He, M.; Xing, R.; Gu, J.; Kong, J. 2D/2D coupled MOF/Fe composite metamaterials enable robust ultra–broadband microwave absorption. Nat. Commun. 2024, 15, 5642. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wu, H.; Shi, Z.; Duan, X.; Ma, S.; Chen, J.; Kong, Z.; Chen, A.; Sun, Y.; Liu, X. Mussel-inspired durable superhydrophobic/superoleophilic MOF-PU sponge with high chemical stability, efficient oil/water separation and excellent anti-icing properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129142. [Google Scholar] [CrossRef]
- Song, K.; Liang, S.; Zhong, X.; Wang, M.; Mo, X.; Lei, X.; Lin, Z. Tailoring the crystal forms of the Ni-MOF catalysts for enhanced photocatalytic CO2-to-CO performance. Appl. Catal. B Environ. 2022, 309, 121232. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Lin, X.; Wang, B.; Zhang, Z.; Xu, Y.; Suo, Y. Facile synthesis of MOF-5-derived porous carbon with adjustable pore size for CO2 capture. J. Solid State Chem. 2023, 322, 123984. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, Q.; Li, Z.; Dong, J.; Liu, J.; Zhang, L.; Xia, T.; He, Y.; Zhao, D. Water-stable methyl-modified MOF and mixed matrix membrane for efficient adsorption and separation of cationic dyes. Sep. Purif. Technol. 2024, 330, 125268. [Google Scholar] [CrossRef]
- Song, J.; Liu, C.K.; Piradi, V.; Chen, C.; Zhu, Y.; Zhu, X.; Li, L.; Wong, W.Y.; Yan, F. Large-Area Fabrication of Hexaazatrinaphthylene-Based 2D Metal-Organic Framework Films for Flexible Photodetectors and Optoelectronic Synapses. Adv. Sci. 2024, 11, 2305551. [Google Scholar] [CrossRef]
- Sağlam, S.; Türk, F.N.; Arslanoğlu, H. Use and applications of metal-organic frameworks (MOF) in dye adsorption: Review. J. Environ. Chem. Eng. 2023, 11, 110568. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H. Metal-organic framework (MOF) composites as promising materials for energy storage applications. Adv. Colloid Interface Sci. 2022, 307, 102732. [Google Scholar] [CrossRef]
- Kazemi, A.; Pordsari, M.A.; Tamtaji, M.; Afshari, M.H.; Keshavarz, S.; Zeinali, F.; Baesmat, H.; Zahiri, S.; Manteghi, F.; Ghaemi, A. Unveiling the power of defect engineering in MOF-808 to enhance efficient carbon dioxide adsorption and separation by harnessing the potential of DFT analysis. Chem. Eng. J. 2024, 494, 153049. [Google Scholar] [CrossRef]
- Li, C.; Qi, A.; Ling, Y.; Tao, Y.; Zhang, Y.-B.; Li, T. Establishing gas transport highways in MOF-based mixed matrix membranes. Sci. Adv. 2023, 9, eadf5087. [Google Scholar] [CrossRef]
- Hammad, S.F.; Abdallah, I.A.; Bedair, A.; Abdelhameed, R.M.; Locatelli, M.; Mansour, F.R. Metal organic framework-derived carbon nanomaterials and MOF hybrids for chemical sensing. TrAC Trends Anal. Chem. 2024, 170, 117425. [Google Scholar] [CrossRef]
- Mohanty, B.; Kumari, S.; Yadav, P.; Kanoo, P.; Chakraborty, A. Metal-organic frameworks (MOFs) and MOF composites based biosensors. Coord. Chem. Rev. 2024, 519, 216102. [Google Scholar] [CrossRef]
- Khan, M.S.; Li, Y.; Li, D.-S.; Qiu, J.; Xu, X.; Yang, H.Y. A review of metal-organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 2023, 5, 6318–6348. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Huang, Z.; Tu, W.; Liu, R.; Chen, M. Cubic MOF coated stainless steel mesh with underwater superoleophobicity for highly efficient oil/water separation. Mater. Chem. Phys. 2023, 297, 127346. [Google Scholar] [CrossRef]
- Zhan, Y.; Cao, J.; Wang, Y.; Li, X.; Li, Y.; Zeng, H.; Huang, W.; Cheng, H.; Gao, S.; Li, L. Au/Ag@ ZIF-8 nanocomposite as solid phase extraction adsorbent and SERS substrate for tacrolimus label-free therapeutic drug monitoring in human serum. Talanta 2025, 281, 126813. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Li, Q.; Wei, L.; Shi, R.; Liu, X.; Zhang, Q.; Liu, K.; Li, Z.; Xiao, Z.; Wang, L. Occupying in Metal−Organic Frameworks’ Pores: Vein-Like PANI Cross-Coupled Hierarchical Porous UiO-66 Flexible Electrode for Supercapacitor Application. Adv. Funct. Mater. 2025, 35, 2413546. [Google Scholar] [CrossRef]
- Rong, H.; Song, P.; Gao, G.; Jiang, Q.; Chen, X.; Su, L.; Liu, W.-L.; Liu, Q. A three-dimensional Mn-based MOF as a high-performance supercapacitor electrode. Dalton Trans. 2023, 52, 1962–1969. [Google Scholar] [CrossRef]
- Ramsahye, N.; Gao, J.; Jobic, H.; Llewellyn, P.; Yang, Q.; Wiersum, A.; Koza, M.; Guillerm, V.; Serre, C.; Zhong, C. Adsorption and diffusion of light hydrocarbons in UiO-66 (Zr): A combination of experimental and modeling tools. J. Phys. Chem. C 2014, 118, 27470–27482. [Google Scholar] [CrossRef]
- Abdel-Mageed, A.M.; Rungtaweevoranit, B.; Parlinska-Wojtan, M.; Pei, X.; Yaghi, O.M.; Behm, R.J.R. Highly active and stable single-atom Cu catalysts supported by a metal–organic framework. J. Am. Chem. Soc. 2019, 141, 5201–5210. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, J.; Li, J.; Li, Y.; Yang, P.; Zhao, P.; Fei, J.; Xie, Y. A novel dopamine electrochemical sensor based on 3D flake nickel oxide/cobalt oxide@ porous carbon nanosheets/carbon nanotubes/electrochemical reduced of graphene oxide composites modified glassy carbon electrode. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131284. [Google Scholar] [CrossRef]
- Nazir, M.A.; Javed, M.S.; Islam, M.; Assiri, M.A.; Hassan, A.M.; Jamshaid, M.; Najam, T.; Shah, S.S.A.; ur Rehman, A. MOF@ graphene nanocomposites for energy and environment applications. Compos. Commun. 2024, 45, 101783. [Google Scholar] [CrossRef]
- Bathla, A.; Lee, J.; Younis, S.A.; Kim, K.-H. Recent advances in photocatalytic reduction of CO2 by TiO2–and MOF–based nanocomposites impregnated with metal nanoparticles. Mater. Today Chem. 2022, 24, 100870. [Google Scholar] [CrossRef]
- Durmus, Z.; Maijenburg, A.W. A review on graphitic carbon nitride (g-C3N4)–metal organic framework (MOF) heterostructured photocatalyst materials for photo (electro) chemical hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 36784–36813. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, H.; Wang, Q.; Ma, W.; Yang, G.; Xu, S.; Li, S.; Su, G.; Qu, Y.; Zhang, M. Optimization of a MOF blended with modified polyimide membrane for high-performance gas separation. Membranes 2021, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, Y.; Wang, Y.; Yin, L. One-pot solvothermal synthesis of Cu–Fe-MOF for efficiently activating peroxymonosulfate to degrade organic pollutants in water: Effect of electron shuttle. Chemosphere 2024, 352, 141333. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U.; Aftab, S.; Wabaidur, S.M.; Siddique, S.; Iqbal, M.J. A hydrothermally prepared lithium and copper MOF composite as anode material for hybrid supercapacitor applications. ChemistrySelect 2023, 8, e202204554. [Google Scholar] [CrossRef]
- Fernández-Andrade, K.J.; Fernández-Andrade, A.A.; Zambrano-Intriago, L.Á.; Arteaga-Perez, L.E.; Alejandro-Martin, S.; Baquerizo-Crespo, R.J.; Luque, R.; Rodríguez-Díaz, J.M. Microwave-assisted MOF@ biomass layered nanomaterials: Characterization and applications in wastewater treatment. Chemosphere 2023, 314, 137664. [Google Scholar] [CrossRef]
- Yi, J.; Lee, G.; Park, S.S. Solvent-Induced Structural Rearrangement in Ultrasound-Assisted Synthesis of Metal–Organic Frameworks. Small Methods 2024, 8, 2400363. [Google Scholar] [CrossRef]
- Ren, H.; Wei, T. Electrochemical synthesis methods of metal-organic frameworks and their environmental analysis applications: A review. ChemElectroChem 2022, 9, e202200196. [Google Scholar] [CrossRef]
- Khosroshahi, N.; Bakhtian, M.; Safarifard, V. Mechanochemical synthesis of ferrite/MOF nanocomposite: Efficient photocatalyst for the removal of meropenem and hexavalent chromium from water. J. Photochem. Photobiol. A Chem. 2022, 431, 114033. [Google Scholar] [CrossRef]
- Kadhom, M.; Al-Furaiji, M.; Salih, S.; Al-Obaidi, M.A.; Abdullah, G.H.; Albayati, N. A review on UiO-66 applications in membrane-based water treatment processes. J. Water Process Eng. 2023, 51, 103402. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Eshaghi, M.M.; Ostovar, S.; Shamsabadipour, A.; Safakhah, S.; Mousavi, M.S.; Rahdar, A.; Pandey, S. UiO-66 metal-organic framework nanoparticles as gifted MOFs to the biomedical application: A comprehensive review. J. Drug Deliv. Sci. Technol. 2022, 76, 103758. [Google Scholar] [CrossRef]
- Kandiah, M.; Usseglio, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P.; Tilset, M. Post-synthetic modification of the metal–organic framework compound UiO-66. J. Mater. Chem. 2010, 20, 9848–9851. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A decade of UiO-66 research: A historic review of dynamic structure. synthesis mechanisms, and characterization techniques of an archetypal metal–organic framework. Cryst. Growth Des. 2019, 20, 1347–1362. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated synthesis of Zr-based metal–organic frameworks: From nano to single crystals. Chem.–A Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Ethiraj, J.; Vitillo, J.G.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. Tuned to perfection: Ironing out the defects in metal–organic framework UiO-66. Chem. Mater. 2014, 26, 4068–4071. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metal–organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef]
- Melillo, A.; Cabrero-Antonino, M.; Navalón, S.; Álvaro, M.; Ferrer, B.; García, H. Enhancing visible-light photocatalytic activity for overall water splitting in UiO-66 by controlling metal node composition. Appl. Catal. B Environ. 2020, 278, 119345. [Google Scholar] [CrossRef]
- Amani, V.; Norouzi, F.; Akrami, Z. A review of UiO-based MOF detection and removal strategies for antibiotics in water. New J. Chem. 2024, 48, 18600–18617. [Google Scholar] [CrossRef]
- Liang, W.; Babarao, R.; D’Alessandro, D.M. Microwave-Assisted Solvothermal Synthesis and Optical Properties of Tagged MIL-140A Metal–Organic Frameworks. Inorg. Chem. 2013, 52, 12878–12880. [Google Scholar] [CrossRef]
- Vakili, R.; Xu, S.; Al-Janabi, N.; Gorgojo, P.; Holmes, S.M.; Fan, X. Microwave-assisted synthesis of zirconium-based metal organic frameworks (MOFs): Optimization and gas adsorption. Microporous Mesoporous Mater. 2018, 260, 45–53. [Google Scholar] [CrossRef]
- Mahmoud, M.M. Microwave-assisted fast synthesis of MOF-801. Next Mater. 2025, 6, 100316. [Google Scholar] [CrossRef]
- Germann, L.S.; Katsenis, A.D.; Huskić, I.; Julien, P.A.; Uzarevic, K.; Etter, M.; Farha, O.K.; Friscic, T.; Dinnebier, R.E. Real-time in situ monitoring of particle and structure evolution in the mechanochemical synthesis of UiO-66 metal–organic frameworks. Cryst. Growth Des. 2019, 20, 49–54. [Google Scholar] [CrossRef]
- Shearer, G.C.; Forselv, S.; Chavan, S.; Bordiga, S.; Mathisen, K.; Bjørgen, M.; Svelle, S.; Lillerud, K.P. In situ infrared spectroscopic and gravimetric characterisation of the solvent removal and dehydroxylation of the metal organic frameworks UiO-66 and UiO-67. Top. Catal. 2013, 56, 770–782. [Google Scholar] [CrossRef]
- Batten, M.P.; Rubio-Martinez, M.; Hadley, T.; Carey, K.-C.; Lim, K.-S.; Polyzos, A.; Hill, M.R. Continuous flow production of metal-organic frameworks. Curr. Opin. Chem. Eng. 2015, 8, 55–59. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Batten, M.P.; Polyzos, A.; Carey, K.-C.; Mardel, J.I.; Lim, K.-S.; Hill, M.R. Versatile, high quality and scalable continuous flow production of metal-organic frameworks. Sci. Rep. 2014, 4, 5443. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Van Assche, T.; Campagnol, N.; Fransaer, J.; Denayer, J.; Tan, J.-C.; Falcaro, P.; De Vos, D.; Ameloot, R. Electrochemical Film Deposition of the Zirconium Metal–Organic Framework UiO-66 and Application in a Miniaturized Sorbent Trap. Chem. Mater. 2015, 27, 1801–1807. [Google Scholar] [CrossRef]
- Liu, X. Metal-organic framework UiO-66 membranes. Front. Chem. Sci. Eng. 2020, 14, 216–232. [Google Scholar] [CrossRef]
- Hod, I.; Bury, W.; Karlin, D.M.; Deria, P.; Kung, C.W.; Katz, M.J.; So, M.; Klahr, B.; Jin, D.; Chung, Y.W. Directed growth of electroactive metal-organic framework thin films using electrophoretic deposition. Adv. Mater. 2014, 26, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Zhao, Y.; Bedia, J.; Rodriguez, J.; Belver, C. UiO-66-based metal organic frameworks for the photodegradation of acetaminophen under simulated solar irradiation. J. Environ. Chem. Eng. 2021, 9, 106087. [Google Scholar] [CrossRef]
- Mirzaei, K.; Jafarpour, E.; Shojaei, A.; Khasraghi, S.S.; Jafarpour, P. An investigation on the influence of highly acidic media on the microstructural stability and dye adsorption performance of UiO-66. Appl. Surf. Sci. 2023, 618, 156531. [Google Scholar] [CrossRef]
- Bůžek, D.; Adamec, S.; Lang, K.; Demel, J. Metal–organic frameworks vs. buffers: Case study of UiO-66 stability. Inorg. Chem. Front. 2021, 8, 720–734. [Google Scholar] [CrossRef]
- Molavi, H.; Zamani, M.; Aghajanzadeh, M.; Kheiri Manjili, H.; Danafar, H.; Shojaei, A. Evaluation of UiO-66 metal organic framework as an effective sorbent for Curcumin’s overdose. Appl. Organomet. Chem. 2018, 32, e4221. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, W.; Gao, Y.; Wu, J.; Tang, B. Facile synthesis and supercapacitive properties of Zr-metal organic frameworks (UiO-66). RSC Adv. 2015, 5, 17601–17605. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Ahmadipouya, S.; Haris, M.H.; Rezakazemi, M.; Bokhari, A.; Molavi, H.; Ahmadipour, M.; Pung, S.-Y.; Klemeš, J.I.J.R.; Aminabhavi, T.M. Magnetic nitrogen-rich UiO-66 metal–organic framework: An efficient adsorbent for water treatment. ACS Appl. Mater. Interfaces 2023, 15, 30106–30116. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, B.; Liu, M.; Xie, Z.; Wang, D.; Feng, G. The adsorption properties of defect controlled metal-organic frameworks of UiO-66. Sep. Purif. Technol. 2021, 270, 118842. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.; Lan, J.; Huang, Z.; Li, T.; Wang, B.; Wu, L.; Liang, L.; Duan, X.; Kong, Z. Selectivity adsorption mechanism of different phenolic organic pollutants on UiO-66 by molecular dynamics simulation. J. Mol. Liq. 2024, 398, 124228. [Google Scholar] [CrossRef]
- Driscoll, D.M.; Troya, D.; Usov, P.M.; Maynes, A.J.; Morris, A.J.; Morris, J.R. Geometry and energetics of CO adsorption on hydroxylated UiO-66. Phys. Chem. Chem. Phys. 2019, 21, 5078–5085. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Zhao, D.; Cai, C. Cerium-based UiO-66 metal-organic framework for synergistic dye adsorption and photodegradation: A discussion of the mechanism. Dye. Pigment. 2021, 185, 108957. [Google Scholar] [CrossRef]
- Beydaghdari, M.; Hooriabad Saboor, F.; Babapoor, A.; Karve, V.V.; Asgari, M. Recent advances in MOF-based adsorbents for dye removal from the aquatic environment. Energies 2022, 15, 2023. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Sweleh, A.O. Optimizing textile dye removal by activated carbon prepared from olive stones. Environ. Technol. Innov. 2019, 16, 100488. [Google Scholar] [CrossRef]
- Mohammadi, A.; Alinejad, A.; Kamarehie, B.; Javan, S.; Ghaderpoury, A.; Ahmadpour, M.; Ghaderpoori, M. Metal-organic framework Uio-66 for adsorption of methylene blue dye from aqueous solutions. Int. J. Environ. Sci. Technol. 2017, 14, 1959–1968. [Google Scholar] [CrossRef]
- He, Q.; Chen, Q.; Lü, M.; Liu, X. Adsorption behavior of rhodamine B on UiO-66. Chin. J. Chem. Eng. 2014, 22, 1285–1290. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Mohammadkhani, R.; Ahmadipouya, S.; Shokrgozar, A.; Rezakazemi, M.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Superior chemical stability of UiO-66 metal-organic frameworks (MOFs) for selective dye adsorption. Chem. Eng. J. 2020, 399, 125346. [Google Scholar] [CrossRef]
- Embaby, M.S.; Elwany, S.D.; Setyaningsih, W.; Saber, M.R. The adsorptive properties of UiO-66 towards organic dyes: A record adsorption capacity for the anionic dye Alizarin Red S. Chin. J. Chem. Eng. 2018, 26, 731–739. [Google Scholar] [CrossRef]
- Hidayat, A.R.P.; Zulfa, L.L.; Widyanto, A.R.; Abdullah, R.; Kusumawati, Y.; Ediati, R. Selective adsorption of anionic and cationic dyes on mesoporous UiO-66 synthesized using a template-free sonochemistry method: Kinetic, isotherm and thermodynamic studies. RSC Adv. 2023, 13, 12320–12343. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, D.V.; Ahmadipouya, S.; Shokrgozar, A.; Molavi, H.; Rezakazemi, M.; Ahmadijokani, F.; Arjmand, M. Adsorption performance of UiO-66 towards organic dyes: Effect of activation conditions. J. Mol. Liq. 2021, 321, 114487. [Google Scholar] [CrossRef]
- Alsaedi, M.; Alothman, G.; Alnajrani, M.; Alsager, O.; Alshmimri, S.; Alharbi, M.; Alawad, M.; Alhadlaq, S.; Alharbi, S. Antibiotic adsorption by metal-organic framework (UiO-66): A comprehensive kinetic. thermodynamic, and mechanistic study. Antibiotics 2020, 9, 722. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Hu, X.; Tan, W.; Li, J.; Su, D.; Wang, H.; Yang, M. A study on the performance of a novel adsorbent UiO-66 modified by a nickel on removing tetracycline in wastewater. Chemosphere 2023, 338, 139399. [Google Scholar] [CrossRef]
- Huang, L.; Shen, R.; Shuai, Q. Adsorptive removal of pharmaceuticals from water using metal-organic frameworks: A review. J. Environ. Manag. 2021, 277, 111389. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Ullah, H.; Iqbal, M.; Khan, M.S.; Ullah, R.S.; Gul, Z.; Rehman, R.; Altaf, A.A.; Ullah, S. MOF UiO-66 and its composites: Design strategies and applications in drug and antibiotic removal. Environ. Sci. Pollut. Res. 2025, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.R.; Abid, H.R.; Periasamy, V.; Sun, H.; Tade, M.O.; Wang, S. Adsorptive removal of antibiotic sulfonamide by UiO-66 and ZIF-67 for wastewater treatment. J. Colloid Interface Sci. 2017, 500, 88–95. [Google Scholar] [CrossRef]
- Zhai, L.; Zheng, X.; Liu, M.; Wang, X.; Li, W.; Zhu, X.; Yuan, A.; Xu, Y.; Song, P. Tuning surface functionalizations of UiO-66 towards high adsorption capacity and selectivity eliminations for heavy metal ions. Inorg. Chem. Commun. 2023, 154, 110937. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, J.; Quan, W.; Chen, Q.; Long, X.; Wang, A. Advances in the adsorption of heavy metal ions in water by UiO-66 composites. Front. Chem. 2023, 11, 1211989. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Chen, J.P.; Li, K. Superior removal of arsenic from water with zirconium metal-organic framework UiO-66. Sci. Rep. 2015, 5, 16613. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Malloum, A.; Igwegbe, C.A.; Ighalo, J.O.; Ahmadi, S.; Dehghani, M.H.; Othmani, A.; Gökkuş, Ö.; Mubarak, N.M. New generation adsorbents for the removal of fluoride from water and wastewater: A review. J. Mol. Liq. 2022, 346, 118257. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D.; Huang, H.; Zhang, W.; Yang, Q.; Zhong, C. The stability and defluoridation performance of MOFs in fluoride solutions. Microporous Mesoporous Mater. 2014, 185, 72–78. [Google Scholar] [CrossRef]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The potential toxicity of microplastics on human health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef]
- Rafa, N.; Ahmed, B.; Zohora, F.; Bakya, J.; Ahmed, S.; Ahmed, S.F.; Mofijur, M.; Chowdhury, A.A.; Almomani, F. Microplastics as carriers of toxic pollutants: Source. transport, and toxicological effects. Environ. Pollut. 2024, 343, 123190. [Google Scholar] [CrossRef]
- Meng, Y.; Kelly, F.J.; Wright, S.L. Advances and challenges of microplastic pollution in freshwater ecosystems: A UK perspective. Environ. Pollut. 2020, 256, 113445. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, X.; Kirlikovali, K.O.; Gong, X.; Atilgan, A.; Ma, K.; Schweitzer, N.M.; Gianneschi, N.C.; Li, Z.; Zhang, X. Catalytic degradation of polyethylene terephthalate using a phase-transitional zirconium-based metal–organic framework. Angew. Chem. Int. Ed. 2022, 61, e202117528. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chen, Y.; Miao, C.; Wang, Y.-R.; Gao, G.-K.; Yang, R.-X.; Zhu, H.-J.; Wang, J.-H.; Li, S.-L.; Lan, Y.-Q. Metal–organic framework-based foams for efficient microplastics removal. J. Mater. Chem. A 2020, 8, 14644–14652. [Google Scholar] [CrossRef]
- Konno, H.; Tsukada, A. Size-and ion-selective adsorption of organic dyes from aqueous solutions using functionalized UiO-66 frameworks. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129749. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Han, R.; Li, X.; Cui, S.; Yang, J. Hierarchical porous UiO-66 composites modified by dual competitive strategy for adsorption of oxytetracycline. J. Environ. Chem. Eng. 2024, 12, 111662. [Google Scholar] [CrossRef]
- Wei, F.; Liu, H.; Ren, Q.; Yang, L.; Qin, L.; Chen, H.; Ma, Y.; Liang, Z.; Wang, S. Preparation of Zr-MOF for the removal of Norfloxacin from an aqueous Solution. Inorg. Chem. Commun. 2023, 153, 110819. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Q.; Wang, Y.; Zhu, Z.; Guo, Z.; Li, J.; Lv, Y.; Chow, Y.T.; Wang, X.; Zhu, L. Water-stable metal–organic framework (UiO-66) supported on zirconia nanofibers membrane for the dynamic removal of tetracycline and arsenic from water. Appl. Surf. Sci. 2022, 596, 153559. [Google Scholar] [CrossRef]
- Ouyang, J.; Chen, J.; Ma, S.; Xing, X.; Zhou, L.; Liu, Z.; Zhang, C. Adsorption removal of sulfamethoxazole from water using UiO-66 and UiO-66-BC composites. Particuology 2022, 62, 71–78. [Google Scholar] [CrossRef]
- Ali, S.; Zuhra, Z.; Abbas, Y.; Shu, Y.; Ahmad, M.; Wang, Z. Tailoring defect density in UiO-66 frameworks for enhanced Pb (II) adsorption. Langmuir 2021, 37, 13602–13609. [Google Scholar] [CrossRef]
- Peng, M.; You, D.; Shi, H.; Shao, P.; Ren, W.; Yang, L.; Sheng, X.; Shao, J.; Ding, X.; Ding, L.; et al. Disclosing the role of defective UiO-66 over Sb(V) removal: A joint experimental and theoretical study. Chem. Eng. J. 2022, 448, 137612. [Google Scholar] [CrossRef]
- Morcos, G.S.; Ibrahim, A.A.; El-Sayed, M.M.; El-Shall, M.S. High performance functionalized UiO metal organic frameworks for the efficient and selective adsorption of Pb (II) ions in concentrated multi-ion systems. J. Environ. Chem. Eng. 2021, 9, 105191. [Google Scholar] [CrossRef]
- Lam, I.T.Y.; Yuan, Y.; Bang, K.-T.; Choi, S.-J.; Shin, D.-M.; Lu, D.; Kim, Y. Towards the fastest kinetics and highest uptake of post-functionalized UiO-66 for Hg 2+ removal from water. Nanoscale 2023, 15, 10558–10566. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Chen, D.; Ma, D.; Liu, G.; Zou, X.; Chen, Y.; Shu, R.; Song, Q.; Lv, W. Facile synthesis of acid-modified UiO-66 to enhance the removal of Cr (VI) from aqueous solutions. Sci. Total Environ. 2019, 682, 118–127. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, X.; Wang, Z.; Yang, J.; Li, Y.; Gu, J. Specific recovery and in situ reduction of precious metals from waste to create MOF composites with immobilized nanoclusters. Ind. Eng. Chem. Res. 2017, 56, 13975–13982. [Google Scholar] [CrossRef]
- Mahata, P.; Madras, G.; Natarajan, S. Novel photocatalysts for the decomposition of organic dyes based on metal-organic framework compounds. J. Phys. Chem. B 2006, 110, 13759–13768. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Philo, D.; Li, Y.; Shi, L.; Chang, K.; Ye, J. Recent advances of low-dimensional phosphorus-based nanomaterials for solar-driven photocatalytic reactions. Coord. Chem. Rev. 2020, 424, 213516. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal–organic framework (MOF) compounds: Photocatalysts for redox reactions and solar fuel production. Angew. Chem. Int. Ed. 2016, 55, 5414–5445. [Google Scholar] [CrossRef] [PubMed]
- Gomes Silva, C.; Luz, I.; Llabres i Xamena, F.X.; Corma, A.; García, H. Water stable Zr–benzenedicarboxylate metal–organic frameworks as photocatalysts for hydrogen generation. Chem.–A Eur. J. 2010, 16, 11133–11138. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic Self-Assembly of Nanosized Carbon Nitride Nanosheet onto a Zirconium Metal–Organic Framework for Enhanced Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Huang, W.; Yang, Y.; Wang, Y.; He, C.; Liu, N.; Wu, M.; Tang, L. g-C3N4/UiO-66 nanohybrids with enhanced photocatalytic activities for the oxidation of dye under visible light irradiation. Mater. Res. Bull. 2018, 99, 349–358. [Google Scholar] [CrossRef]
- Yang, J.; Chang, X.; Wei, F.; Lv, Z.; Liu, H.; Li, Z.; Wu, W.; Qian, L. High performance photocatalyst TiO2@ UiO-66 applied to degradation of methyl orange. Discov. Nano 2023, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, D.; Li, X.; He, Y.; Liu, X.; Xu, Y.; Chen, H. One-pot synthesis of oxygen-vacancy-rich Cu-doped UiO-66 for collaborative adsorption and photocatalytic degradation of ciprofloxacin. Sci. Total Environ. 2022, 815, 151962. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, Z.-H.; Xiong, W.-P.; Zhou, Y.-Y.; Peng, Y.-R.; Li, X.; Zhou, C.-Y.; Xu, R.; Zhang, Y.-R. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, C.-C.; Yi, X.-H.; Chu, H.-Y. UiO-66 (Zr)-based functional materials for water purification: An updated review. Environ. Funct. Mater. 2023, 2, 93–132. [Google Scholar] [CrossRef]
- Shen, B.; Dong, C.; Ji, J.; Xing, M.; Zhang, J. Efficient Fe(III)/Fe(II) cycling triggered by MoO2 in Fenton reaction for the degradation of dye molecules and the reduction of Cr(VI). Chin. Chem. Lett. 2019, 30, 2205–2210. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, Y.; Tian, R.; Chen, J.; Wang, A.; Yao, J. Defect rich UiO-66 with enhanced adsorption and photosensitized reduction of Cr (VI) under visible light. Ind. Eng. Chem. Res. 2019, 58, 21562–21568. [Google Scholar] [CrossRef]
- He, J.; Zhou, H.; Peng, Q.; Wang, Y.; Chen, Y.; Yan, Z.; Wang, J. UiO-66 with confined dyes for adsorption and visible-light photocatalytic reduction of aqueous Cr(VI). Inorg. Chem. Commun. 2022, 140, 109441. [Google Scholar] [CrossRef]
- Tong, X.; Yang, Z.; Feng, J.; Li, Y.; Zhang, H. BiOCl/UiO-66 composite with enhanced performance for photo-assisted degradation of dye from water. Appl. Organomet. Chem. 2018, 32, e4049. [Google Scholar] [CrossRef]
- Teng, D.; Zhang, J.; Luo, X.; Jing, F.; Wang, H.; Chen, J.; Yang, C.; Zang, S.; Zhou, Y. Remarkably Enhanced Photodegradation of Organic Pollutants by NH2UiO-66/ZnO Composite under Visible-Light Irradiation. J. Renew. Mater. 2022, 10, 2378–2391. [Google Scholar] [CrossRef]
- Su, Q.; Li, J.; Wang, B.; Li, Y.; Hou, L.A. Direct Z-scheme Bi2MoO6/UiO-66-NH2 heterojunctions for enhanced photocatalytic degradation of ofloxacin and ciprofloxacin under visible light. Appl. Catal. B Environ. 2022, 318, 121820. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, S.; Duan, X.; Zheng, W.; Shao, C.; Wu, W.; Jiang, Z.; Lai, W. MnO2/UIO-66 improves the catalysed degradation of oxytetracycline under UV/H2O2/PMS system. J. Solid State Chem. 2021, 300, 122231. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Li, G. Promotion of sulfameter degradation by coupling persulfate and photocatalytic advanced oxidation processes with Fe-doped MOFs. Sep. Purif. Technol. 2022, 282, 119632. [Google Scholar] [CrossRef]
- Du, Q.; Wu, P.; Sun, Y.; Zhang, J.; He, H. Selective photodegradation of tetracycline by molecularly imprinted ZnO@NH2-UiO-66 composites. Chem. Eng. J. 2020, 390, 124614. [Google Scholar] [CrossRef]
- Yi, X.-H.; Ma, S.-Q.; Du, X.-D.; Zhao, C.; Fu, H.; Wang, P.; Wang, C.-C. The facile fabrication of 2D/3D Z-scheme g-C3N4/UiO-66 heterojunction with enhanced photocatalytic Cr (VI) reduction performance under white light. Chem. Eng. J. 2019, 375, 121944. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Cao, M.; Ling, N.; Yao, J. Defect-tailoring and titanium substitution in metal–organic framework UiO-66-NH2 for the photocatalytic degradation of Cr (VI) to Cr (III). ACS Appl. Nano Mater. 2019, 2, 5973–5980. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Xie, K.; Zhang, X.-F.; Feng, Y.; Jia, M.; Yao, J. Noble metal nanoparticle-functionalized Zr-metal organic frameworks with excellent photocatalytic performance. J. Colloid Interface Sci. 2019, 538, 569–577. [Google Scholar] [CrossRef]
- Zhou, Y.-C.; Xu, X.-Y.; Wang, P.; Fu, H.; Zhao, C.; Wang, C.-C. Facile fabrication and enhanced photocatalytic performance of visible light responsive UiO-66-NH2/Ag2CO3 composite. Chin. J. Catal. 2019, 40, 1912–1923. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Pan, W.; Li, K.; Chen, M.; Li, P.; Liu, Y.; Li, Z.; Lu, H. University of Oslo-66: A Versatile Zr-Based MOF for Water Purification Through Adsorption and Photocatalysis. Processes 2025, 13, 1133. https://doi.org/10.3390/pr13041133
Chen L, Pan W, Li K, Chen M, Li P, Liu Y, Li Z, Lu H. University of Oslo-66: A Versatile Zr-Based MOF for Water Purification Through Adsorption and Photocatalysis. Processes. 2025; 13(4):1133. https://doi.org/10.3390/pr13041133
Chicago/Turabian StyleChen, Lei, Wenbo Pan, Ke Li, Miaomiao Chen, Pan Li, Yu Liu, Zeyu Li, and Hai Lu. 2025. "University of Oslo-66: A Versatile Zr-Based MOF for Water Purification Through Adsorption and Photocatalysis" Processes 13, no. 4: 1133. https://doi.org/10.3390/pr13041133
APA StyleChen, L., Pan, W., Li, K., Chen, M., Li, P., Liu, Y., Li, Z., & Lu, H. (2025). University of Oslo-66: A Versatile Zr-Based MOF for Water Purification Through Adsorption and Photocatalysis. Processes, 13(4), 1133. https://doi.org/10.3390/pr13041133







