Abstract
The current work investigates the intensification process of the biological oxidation (BO) of a pharmaceutical effluent using ultrasound (US)-based pretreatment methods. US, in combination with chemical oxidants, like hydrogen peroxide (H2O2), Fenton, potassium persulphate (KPS), and peroxone, was used as a pretreatment technique to enhance the efficacy of BO, as BO alone could only bring about 16.67% COD reduction. The application of US under the optimized conditions of a 70% duty cycle, 120W of power, pH 2, and at a 30 °C temperature resulted in 12.3% COD reduction after 60 min, whereas its combination with oxidants at optimized loadings resulted in a higher COD reduction of 20% for H2O2 (2000 ppm), 23.08% for Fenton (1:1 Fe:H2O2), and 30.77% for the US + peroxone approach (400 mg/h of ozone with 2000 ppm H2O2). The pretreated samples did not produce any toxic by-products, as confirmed by a toxicity analysis using the agar well diffusion method. A cow-dung-based sludge was acclimatised specifically for use in BO. The treatment time for BO was set to 8 h, and the US + peroxone-pretreated samples showed a maximum overall COD reduction of 60%, which is about three times that observed with only BO. This work clearly demonstrates the enhancement of the biodegradation of a complex recalcitrant pharmaceutical effluent using a US-based pretreatment.
1. Introduction
Rapid urbanisation, development, and population growth have increased the load of pollutants in the environment. Water pollution is one of the main drawbacks of urbanisation affecting human health and the environment. With only 2.5% of the earth’s water currently being potable, treatment methods must be devised to protect water resources. The rising use of pharmaceutical compounds for human and veterinary applications has led to their exposure in the environment, also severely affecting natural resources, specifically, water resources. The threat is significant, as the efficacy of the treatment of effluents from the pharmaceutical industry using conventional means is limited, and there is an immense need for research on novel technologies [1]. Wastewater streams from pharmaceutical industries usually contain hazardous substances, like complex and toxic aromatic compounds, nitrogen, phosphorous, sulphur-containing compounds, pharmaceutical drugs, and intermediates [2]. The compounds present in the wastewater can lead to genotoxic, mutagenic, carcinogenic, and ecotoxicological effects in plants, animals, and humans [3]. Regulatory agencies have introduced stringent environmental regulations and control measures on discharge limits, again necessitating the development of novel strategies to effectively regulate the release of emerging contaminants to permissible levels.
In the past, various techniques applied for wastewater treatment have included biological oxidation; carbon bed adsorption; coagulation and flocculation; and oxidation processes, such as chlorination and ozonation [4]. Most new pharmaceuticals are recalcitrant and are difficult to degrade using these commonly applied treatment methods, which necessitates developing newer and more efficient processes to degrade these compounds to the minimum concentration levels [5]. In recent years, advanced oxidation processes (AOPs) have been considered as a more effective way to remove organic pollutants from wastewater. AOPs, like photocatalytic oxidation, cavitation, and ozonation, as well as Fenton chemistry, rely on the generation of highly oxidizing hydroxyl radicals (•OH) to break down complex organic molecules. The short-lived •OH radicals react with various contaminants to form mineral end products, releasing inorganic ions and carbon dioxide [6]. Sulphate radicals are another highly effective species in AOPs, particularly when sulphates are employed as oxidizing agents. For instance, persulfate (PS) is effective mainly because of its direct oxidation properties or its ability to generate reactive species, such as the sulphate radical (SO4•−) [7].
Cavitation has proven to be a practical approach for oxidizing complex organic molecules. Cavitation involves the formation, growth, and collapse of cavities, releasing large amounts of energy in a short period coupled with the generation of oxidizing agents and local hotspots. Acoustic cavitation involves the chemical changes caused by ultrasound within the frequency range of 16 kHz and 2 MHz [8]; however, the degradation of pollutants using only ultrasound is difficult, and, hence, hybrid treatment methods that combine AOPs and cavitation are becoming promising. Hybrid methods drive synergism for effluent treatment, as they help to lower the required oxidant loading, treatment costs, and treatment time. Literature analysis indeed reveals studies with validated results demonstrating the effectiveness of combining AC with other chemical oxidants to enhance pollutant degradation in wastewater [9]. The main driving forces for the intensified oxidation of pollutants using cavitation are local hotspots, the generation of free radicals, and strong turbulence [10]. The present work focuses on the treatment of pharmaceutical wastewater by acoustic cavitation generated using ultrasound alone and in combination with several oxidants as a pre-treatment to biological oxidation.
Industrial effluents with high concentrations of pharmaceutical compounds should ideally be treated at their source, as any combination with other wastewater streams containing biodegradable substrates would mean that the biological treatment only metabolizes the digestible compounds, leaving the toxic pharmaceuticals untreated. Past research on pharmaceutical wastewater treatment has mainly utilized synthetic effluents obtained by dissolving the pure contaminants in the water. For example, Lakshmi et al. [11] investigated the treatment of wastewater containing ciprofloxacin (CIP) using ultrasound combined with AOPs, such as AC, AC + O3, AC + KPS, and AC + H2O2. Almost complete (99.98%) degradation of CIP was observed for the combined operation of AC + O3 (flow rate = 400 mg/h) [11]. Another study reported the treatment of ibuprofen (IBP) using hybrid approaches, like sonophotofenton, sonophotocatalysis, and TiO2/Fe2+/sonolysis. The highest degradation (95%) was observed in the case of sonophoto-Fenton coupled with 60% mineralization [12]. Al Momani et al. [13] explored the degradation of 2,4-dichlorophenol using diverse AOPs, including UV, UV/H2O2, Fenton, and photo-Fenton combinations. While photolysis was not efficient in the complete degradation of DCP, photo-Fenton with initial loadings of oxidants of 75 mg l−1 of H2O2 and 10 mg l−1 of Fe(II) resulted in the complete degradation of the compound within 60 min of treatment [13]. There have been some studies dealing with real industrial effluents as well. For example, Saxena et al. studied the treatment of effluent from two tannery industry treatment units using hydrodynamic cavitation (HC) and AC. The COD reduction, applying AC, was 50.45% and 52.38% compared to 35.6% and 16.5% using HC in two units [14]. Similarly, Chandak et al. [15] studied the application of cavitation-based hybrid treatment techniques for real pharmaceutical industrial effluent. The authors used AC in combination with ozone, peroxide, Fenton, and a CuO catalyst. A maximum COD reduction of 92% was observed using AC/O3/ CuO and 72% using the AC/O3/H2O2 system [15]. Other studies of treating real industrial wastewater using hybrid cavitation-based technologies include effluents from coke oven plants, the textile industry, polymer processing, and specialty chemicals [10,16,17,18,19]. It was also observed that the share of the literature for the treatment of pharmaceutical wastewater using cavitation-based AOPs is only 3.3%, suggesting the limited amount of cavitation-based studies on pharmaceutical industry effluent (PIE) [20]. Based on this analysis, the novelty of the current work, dealing with the treatment of real pharmaceutical wastewater using hybrid technologies, such as AC and AOPs, like H2O2, Fenton, potassium persulfate, and ozone, is justified. In this work, we studied, optimized, and compared the various hybrid treatment processes using COD as the parameter for characteristics of real PIE. By systematically investigating the PIE treatment with ultrasound alone, the optimal conditions in terms of pH and temperature were identified and subsequently applied in the hybrid cavitation-based technique. The effect of varying oxidant loadings was evaluated, incorporating H2O2, KPS, ozone, and Fenton. The pretreated effluent was subsequently subjected to biological oxidation using acclimatised cow dung-based sludge, and the effect of pretreatment on the efficacy of biological oxidation was validated. This study also analysed the process by looking into energy and cost inputs to understand the feasibility of the process. The outcomes of this work are extremely important to commercial installations, as current effluent treatment plants are struggling quite a bit with treatments using the conventional approach of only biological oxidation.
2. Materials and Methods
2.1. Materials
The effluent was obtained from a pharmaceutical company in Mumbai. The chemicals used in this study, such as hydrogen peroxide (30% w/v) (H2O2), ferrous sulphate (FeSO4), potassium persulfate (KPS), mercuric sulphate (HgSO4), silver sulphate (AgSO4), sulphuric acid (H2SO4), potassium dichromate (K2Cr2O7), ammonium ferrous sulphate (FAS) and a Ferroin indicator, were analytical-grade reagents and were procured from Loba Chemie, Mumbai, India. A nutrient agar and a gram staining kit were procured from Himedia, Mumbai, India. Deionised water was procured freshly from the purification unit (model: Millipore Milli-Q Gradient A10, Millipore, Mumbai, India). pH was adjusted using 0.1N H2SO4 and 0.1N NaOH, as required. Ozonation studies were conducted using a portable ozone generator supplied by Eltech Ozone Pvt. Ltd. (Mumbai, India). A silicone pipe attached to a sparger introduced ozone into the treatment system.
2.2. Experimental Setup
2.2.1. Ultrasonic Horn
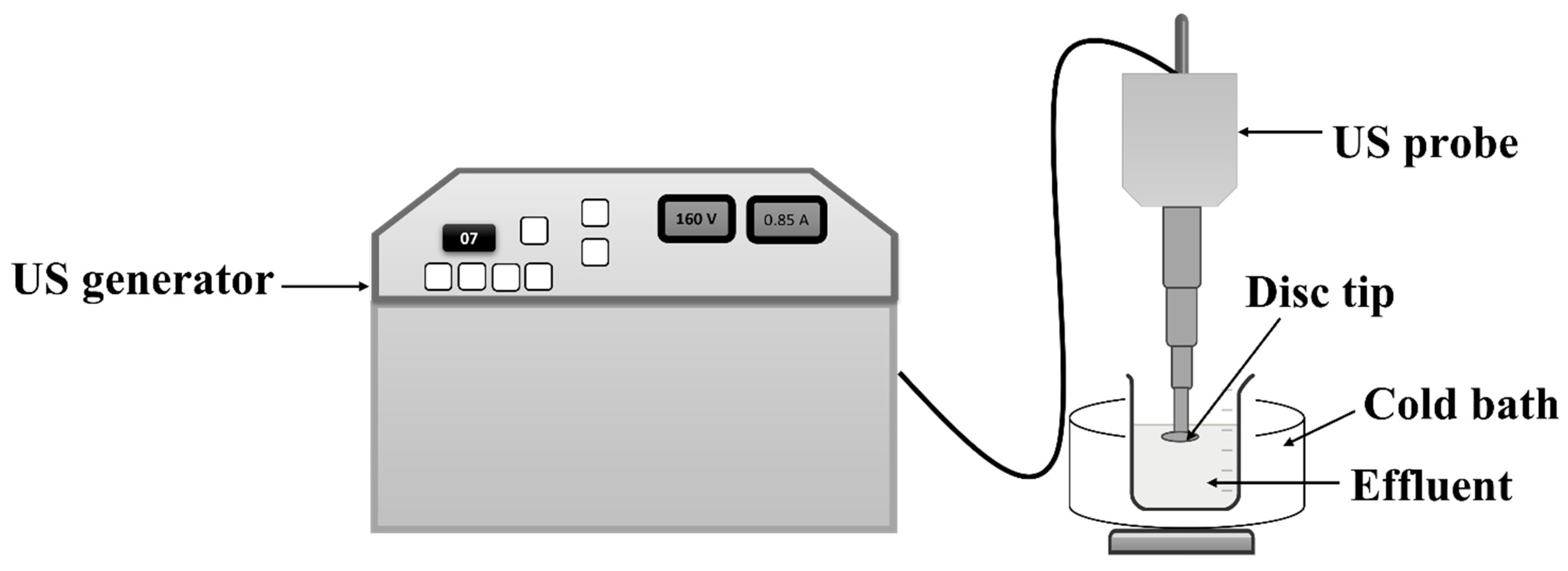
All the ultrasonic treatments were performed using a US horn procured from M/s Dakshin, Mumbai, India. The horn has a disc-shaped tip and operates at a fixed frequency of 22 kHz, with a maximum operating power of 150 W. A schematic representation of the horn is shown in Figure 1. In this study, the operating power and duty cycle were kept constant at 120 W and 70%, respectively, based on the optimization performed in previous studies [11]. All reactions were performed in the fume hood to control the environment and prevent any emissions that could impact the people working in the area.
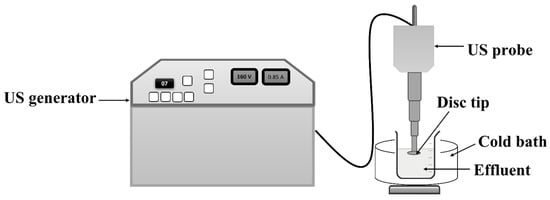
Figure 1.
Schematic representation of US horn-based treatment.
2.2.2. Biological Oxidation Setup
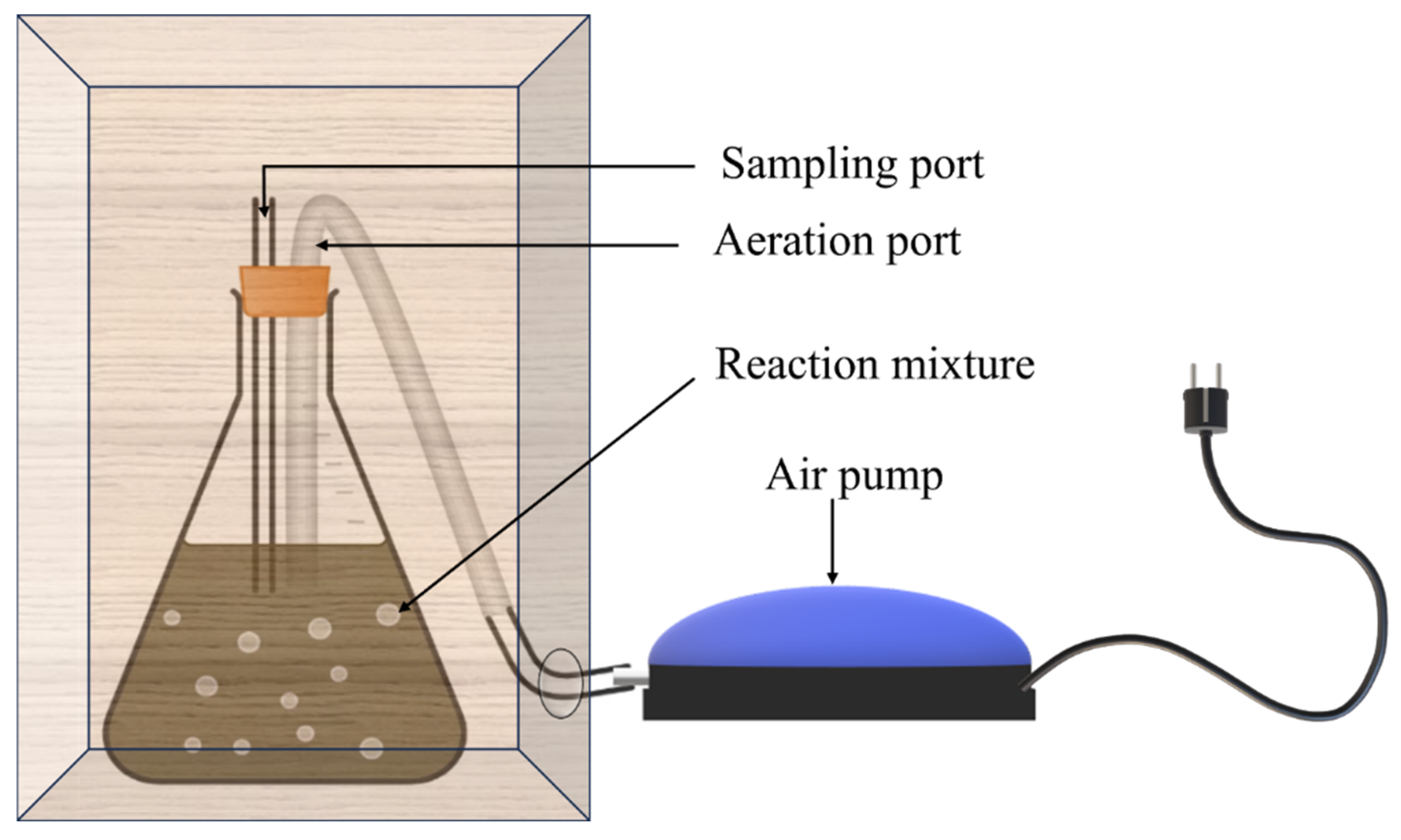
For BO, a 500 mL conical flask was used as the reaction vessel. A rubber cork with 2 outlets (each for sampling and aeration) was used to seal the flask. Aeration was facilitated using an aquarium pump supplied with silica tubing. The entire setup was encased in a box. Samples were regularly withdrawn from the sampling port. A pictorial representation of the setup is depicted in Figure 2.
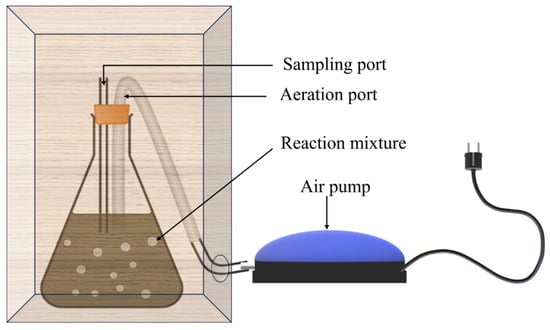
Figure 2.
Schematic representation of the setup of biological oxidation.
2.3. Methodology
2.3.1. Pretreatment Studies
All the pretreatment studies were conducted with 200 mL of the effluent in a 250 mL-capacity beaker. The reaction time was fixed at 120 min, with samples being withdrawn at regular intervals. The withdrawn samples were filtered before being used for analysis. Initially, the operating parameters (pH and temperature) were optimised using US treatment applied as a standalone treatment. The effect of US in combination with other chemical AOPs, like H2O2, Fenton, KPS and peroxone, was studied under optimized conditions of only ultrasound. The effect of oxidant loading was also studied, and the synergy between US and AOPs was analysed by treating the effluent without US at the optimum loading of the oxidant. Peroxide and Fenton reactions were carried out at acidic pH, and the samples were neutralised with NaOH to stop the action of any residual quantum of H2O2. KPS and the peroxone-based treatment were performed at basic pH.
2.3.2. Aerobic Oxidation
For BO, cow-dung-based sludge was used as the microbial agent. The cow dung, obtained from a local farm, was diluted and filtered to remove large particles and applied in the form of a slurry. The cow dung was incubated at 37 °C, throughout. Initially, raw sludge was used for treatment without any modifications. Subsequently, the sludge was acclimatised by introducing the effluent periodically in the sludge and exposing the microbes to it. The detailed methodology for the sludge acclimatisation was described by Iyer et al. [21].
2.4. Analysis
COD analysis was performed using the closed reflux method (ISO 15705:2002 [22]) to analyse the extent of effluent treatment. The analysis was performed using a digester obtained from Hanna Equipment Pvt. Ltd. (Mumbai, India), in which the prepared samples were subjected to digestion at 150 °C for 2 h. The digested samples were titrated against FAS with a Ferroin indicator.
A toxicity analysis was performed to reveal the efficacy of pretreatment to reduce effluent toxicity and negate the absence of any toxic intermediates. Agar well diffusion was performed to understand microbial toxicity against 2 test organisms: Staphylococcus aureus (gram-positive) and Escherichia coli (gram-negative). Doxycycline was the positive control, and sterile deionised water was the negative control. The detailed method for the toxicity analysis protocol is given in earlier work by Iyer et al. [21].
The organisms in the sludge were studied using agar plating. The sludge solution was straked on a sterile nutrient agar plate. Prominent colonies were isolated and plated in individual agar plates. The growth of the colonies on the agar surface was studied and tabulated to understand the colony characteristics. Additionally, gram staining was performed according to the standard procedure.
3. Results and Discussion
3.1. Pretreatment Using US Reactor
The primary goal of the detailed study of a pretreatment is the optimisation of conditions like pH and temperature using a US reactor. The characteristics of the effluent used in this study are given in Table 1. The obtained effluent is the wash water stream released from a plant, consisting of ammonia and water as the main components, along with chloroxime and other cyclic compounds.

Table 1.
Characteristics of effluent.
3.1.1. Effect of Initial pH on COD Reduction Using Only US
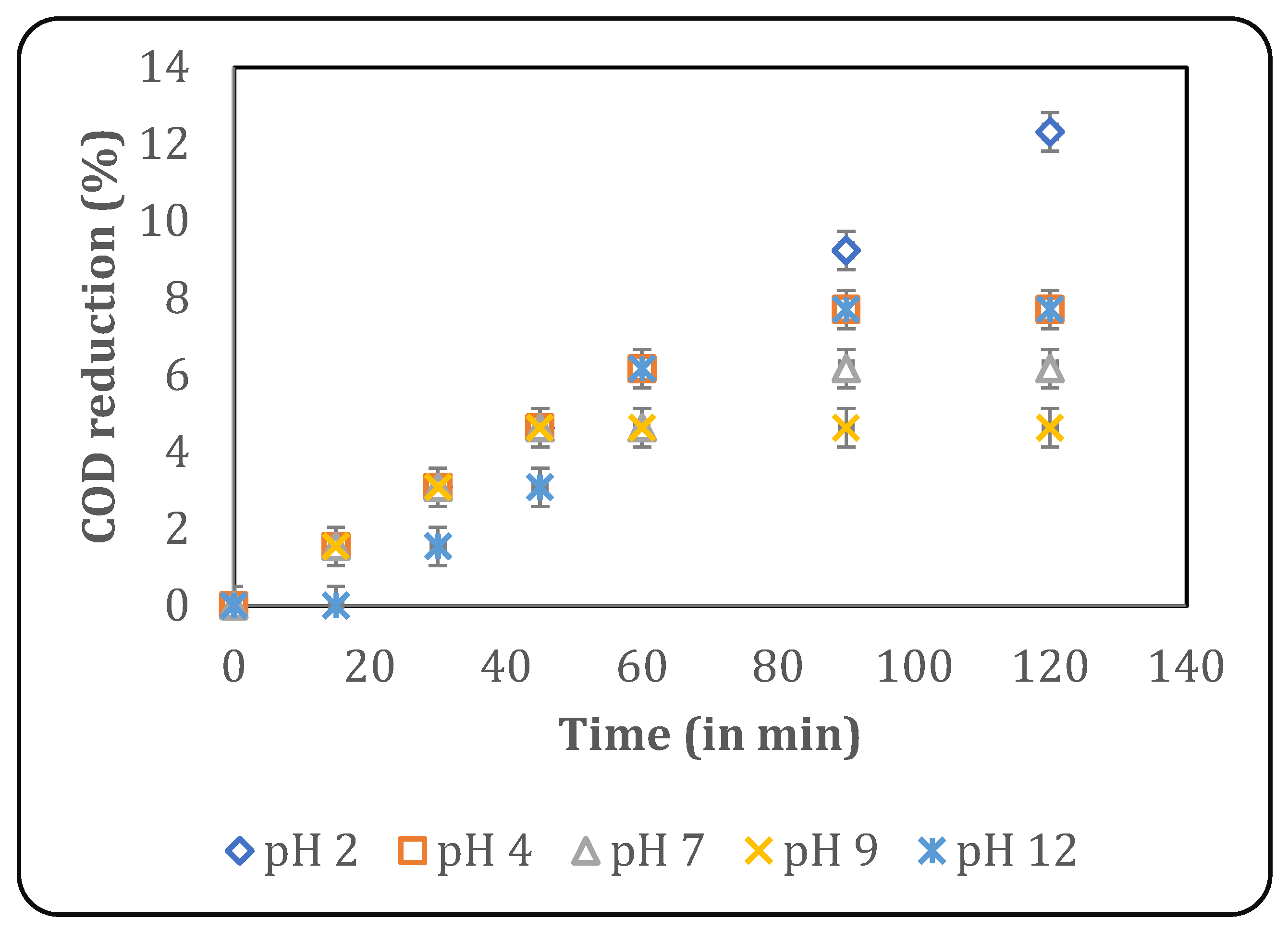
A study on the effect of pH is essential, as it affects radical formation, the physicochemical properties of the pollutants, and degradation kinetics [23]. The study of pH is simpler with a synthetic effluent, as the components are minimal and known, whose pKa can be used to determine the effect of pH. Conversely, the presence of a matrix in real industrial effluents results in an inconsistent influence of pH on degradation kinetics, making any study more important. In the present work, the effect of initial pH was studied at a constant temperature of 30 °C using various pH values, such as 2, 4, 7, 9 and 12. The results are depicted in Figure 3, which shows that the maximum COD reduction of 12.3% was obtained after 120 min of treatment at a pH of 2.
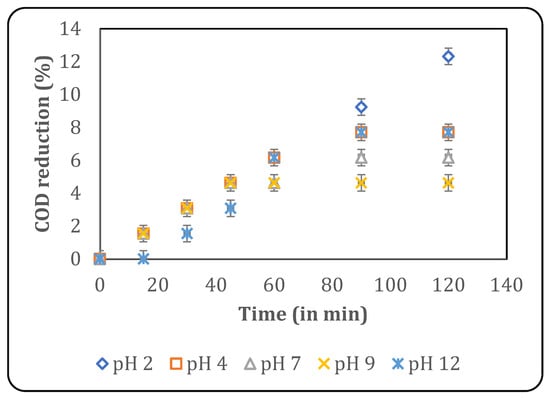
Figure 3.
Effect of pH on US-induced treatment (temperature: 30 °C).
The pH of the solution determines the solubility of the system’s components and, thus, their availability to hydroxyl radicals. pH also determines the oxidation capacity and reactivity of hydroxyl radicals. An optimum pH favours the reaction of the pollutant with the radicals at a faster rate, also preventing the recombination of radicals. Additionally, pH decides molecular interactions among the matrix, which contributes to the effectiveness of oxidation [23]. In the present work, the best treatment was observed at acidic conditions, attributed to the higher oxidation capacity and interaction of the oxidizing species, with the pollutants present in the effluent in favourable forms.
Naddeo et al. [24] studied the effect of sonolysis in treating urban wastewater containing pharmaceuticals. Pharmaceuticals were spiked in the effluent collected from an urban wastewater treatment plant, and the effect of pH on sonolysis showed that a faster reaction was achieved at acidic pH conditions, attributed to the molecular state of pharmaceuticals at a lower pH, which makes them hydrophobic and transports them to the bubble–water interface, where the maximum concentration of the oxidizing species is expected. A comparison of the data at three pH levels (3, 7.5, and 11) revealed that the reaction was fastest at pH 3 (~0.02 min−1), while at pH 11, the rate constant was minimum at 0.007 min−1. Alkaline pH also resulted in a higher production of H2O2, which reduced the availability of radicals [24]. Similarly, Chandak et al. [15] studied the efficacy of ultrasound-based pharmaceutical effluent treatment. The native pH of the effluent was 6.5, but at an adjusted pH of 2.5, maximum degradation efficiency using US treatment was reported. Saharan et al. [25] studied the effect of pH on the treatment of textile effluents using hydrodynamic cavitation. The authors found ~60% decolourisation at acidic pH, while at basic pH, the molecules became hydrophilic and dispersed into the bulk liquid, which caused lesser degradation of the compound. The authors clearly elucidated that the pH of the solution decided the state of the molecule and thus the efficiency of degradation [25]. The studies clearly highlight the effect of pH on the reaction rate while also iterating the importance of optimisation for any specific system, as the quantitative trends are quite different for each system.
3.1.2. Effect of Temperature on COD Reduction Using Only US
Temperature determines the energy supplied to the reaction system and thus regulates the rate of reaction. Different effects of temperature have been reported in the literature, often elucidating the existence of an optimum temperature which gives the highest treatment efficacy with the minimum energy input. The degradation of organics can be favoured by increasing the temperature, as the molecules migrate to the gas–liquid interface with the supply of energy, leading to higher rates of degradation. An increase in the temperature can be detrimental to the cavitation, especially when vaporous solvents are present, as their presence reduces the collapse energy release. The optimum temperature is mostly affected by characteristics of the pollutant and the kinetics of the interaction between the pollutants and the radicals as well as effect on cavitational intensity.
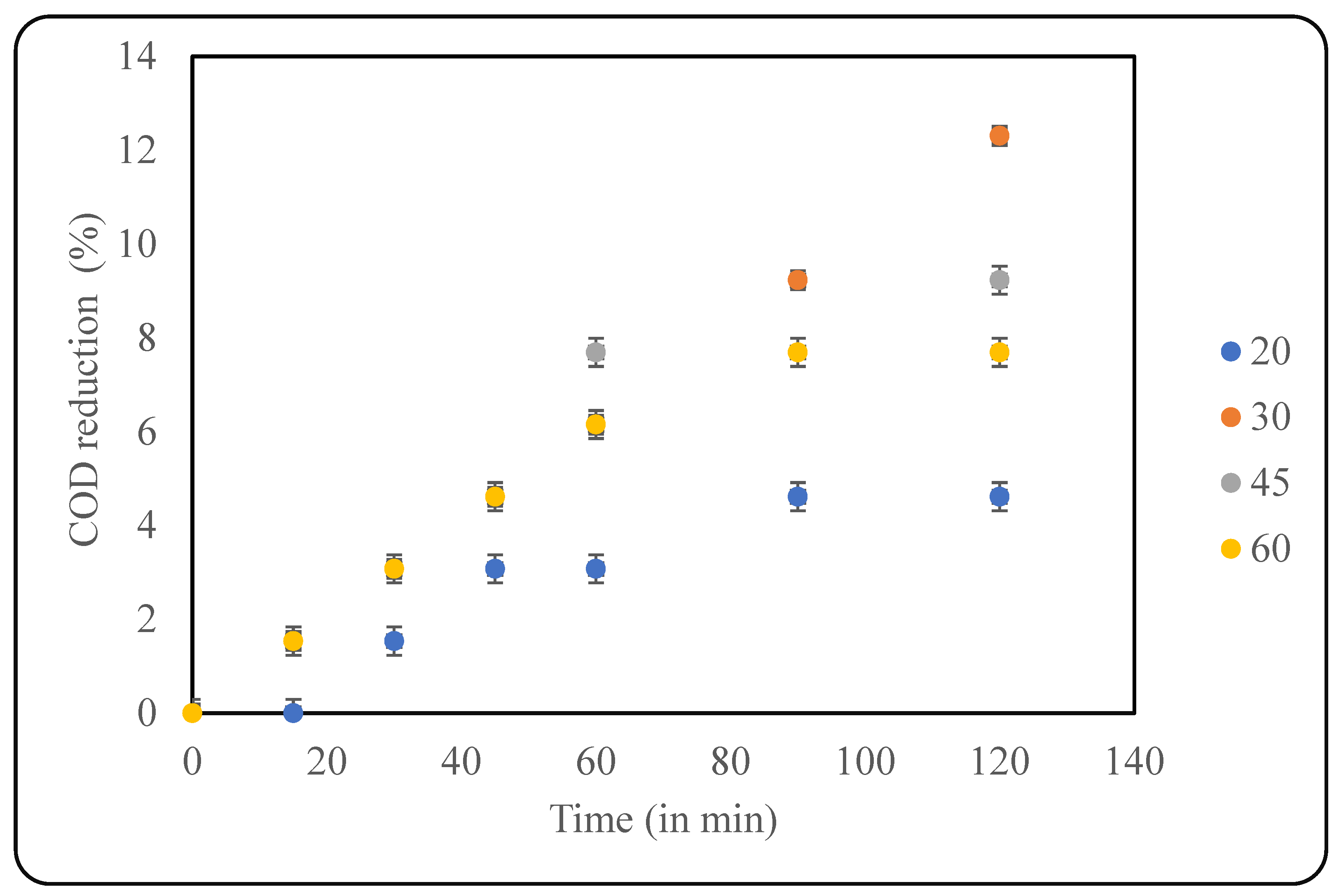
In this study, four different temperatures were applied for optimisation, including 20 °C, 30 °C, 45 °C, and 60 °C. As seen in Figure 4, a maximum COD reduction of 12.3% was observed at 30 °C after 120 min of treatment. At lower temperatures (20 °C), there was not enough energy for the treatment to proceed, and, hence, the COD reduction was only 4.6%. Similarly, at higher temperatures, the bubbles were huge and burst quicker, which was not beneficial to the system due to lower intensity of cavitation, resulting in less COD reduction. It has been reported that gaseous cavities are efficient than vapour-filled cavities in terms of energy dissipation, which is achieved at a lower temperature [26]. The counteracting effects lead to the existence of the optimum temperature for best results, which, in the current study, was established as 30 °C. Similar qualitative trends can be seen in the literature; however, the specific value of the optimum temperature varies, clearly elucidating the importance of the current study.
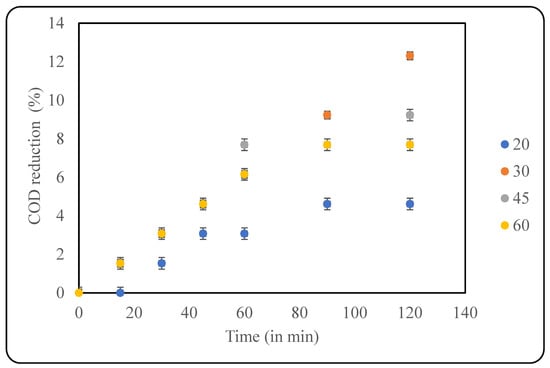
Figure 4.
Effect of temperature on US-induced treatment (pH: 2).
In a similar study on the use of US for the treatment of various pharmaceuticals, the authors recommended that ambient temperature favours the successful degradation of endocrine disruptors. Under these conditions, the compounds are broken down into simpler aliphatic acids that are easily biodegradable [27]. Conversely, a study on Olive Mill wastewater revealed that increasing the temperature from 25 °C to 45 °C increased the efficiency of the degradation of components using a US-assisted AOP. The authors attributed the results to increasing vapour pressure at higher temperatures, subsequently increasing bubble formation and also the presence of contaminants at the site of collapse [28]. Im et al. [29] studied the effects of temperature and US frequency on the degradation of Acetaminophen and Naproxen. The temperatures varied from 15 °C to 55 °C, and the frequencies tested were 28 kHz and 1000 kHz. At 28 kHz, the degradation increased as the temperature increased from 15 °C to 25 °C, after which the degradation rate decreased. The use of a higher frequency resulted in an optimum degradation temperature of 35 °C. Although the effect of temperature is dependent on the frequency of US, it is evident that extreme temperatures do not favour the progress of the reaction, and the optimum temperature revolves around ambient conditions [29]. A comparative analysis of available studies helped in understanding the effect of temperature for different effluents, also implying that the effect is system-specific.
3.2. US + AOPs
3.2.1. US + KPS
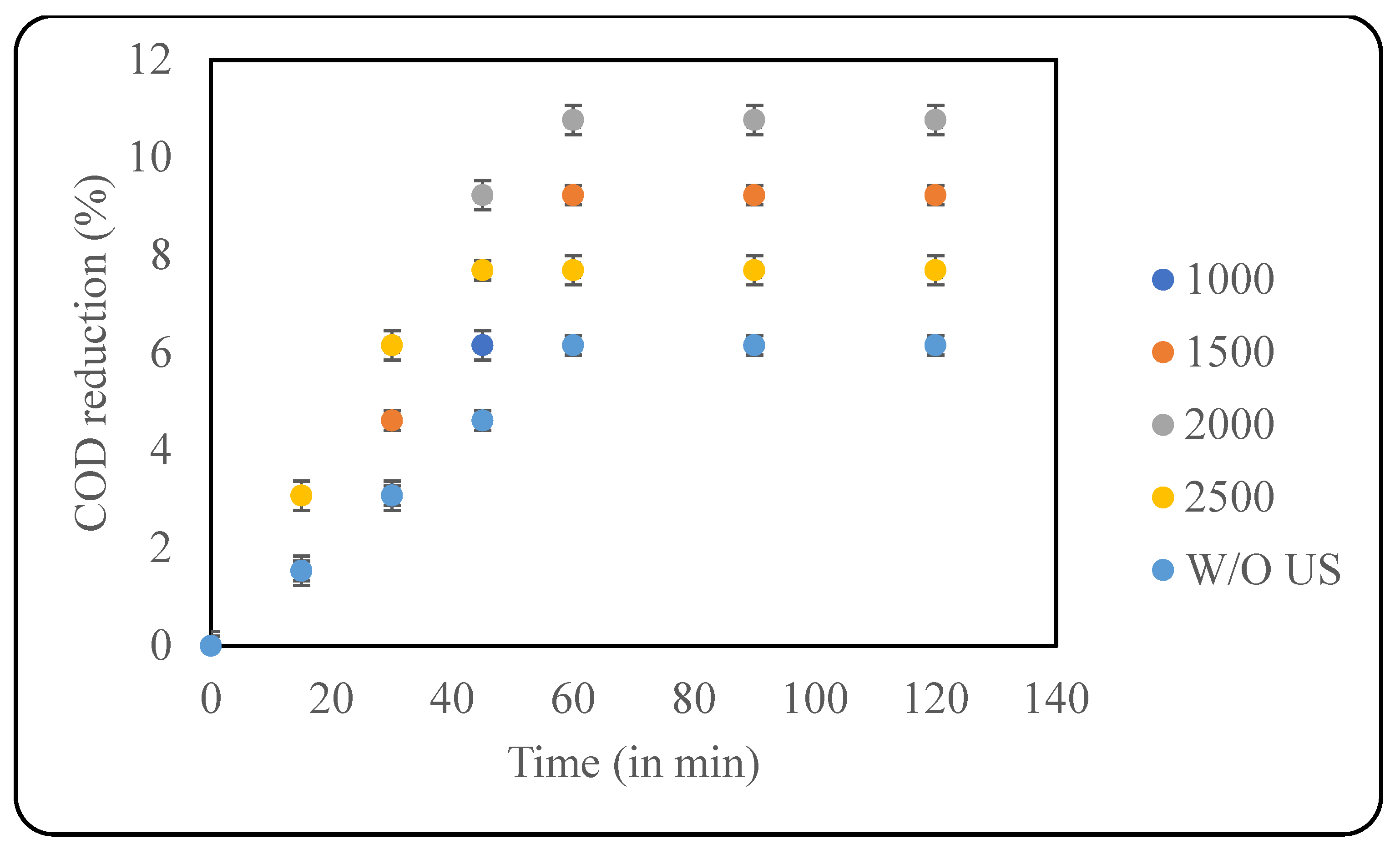
Persulphate and Peroxymonosulphate are strong oxidising agents and are useful in effluent treatment. Along with hydroxyl radicals, persulphates also generate sulphate radicals that are extremely efficient in effluent treatment. Another advantage of persulphate ions is that they can be activated through multiple ways, like photolysis, sonolysis, heat, and using catalysts [30]. In the current study, KPS was used alone and in combination with US for the pretreatment. The oxidant loading varied from 1000 ppm to 2500 ppm, and the effect of US in combination with KPS was analysed. The US parameters were kept constant based on the optimisation study; however, the pH used was 9, as KPS is observed to work better at basic pH. It was observed that an increase in the oxidant loading resulted in increased COD reduction. A maximum reduction of 10.77% was observed with 2000 ppm KPS after 60 min of treatment. Increasing the oxidant loading any further resulted in a decrease in COD reduction. Studies revealed that excess KPS can lead to scavenging effects where the persulphate ions combine with sulphate radicals, making them unavailable for the desired reaction of oxidation of contaminants. Scavenging effects are system-dependent and vary greatly with the type of pollutant under study [31]. In this study, the effect of KPS alone was also tested using the optimised KPS load (2000 ppm), and it was observed that only KPS resulted in 6.15% COD reduction after 60 min of treatment. The results of this study reveal that there is no significant synergy between US and KPS, and the results of combined processes match the additional effects of single processes. A graphical representation of the results is depicted in Figure 5.
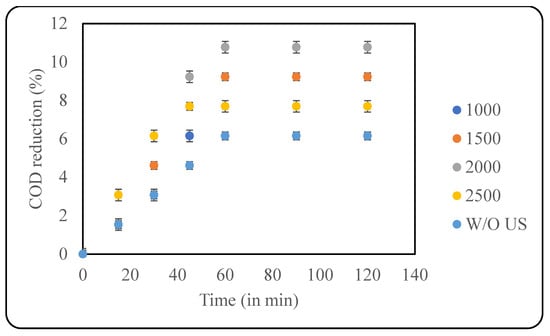
Figure 5.
Effect of KPS loading (in ppm) on COD reduction (pH: 9; temp.: 30 °C).
Various studies on the use of KPS for effluent treatment were analysed. A study by Daware and Gogate [31] on the US-assisted degradation of 2-Picoline at a loading of 30 mg/L, at 30 °C, a pH of 6.7, at 70 W of US power, and at a treatment time of 90 min revealed that increasing the KPS concentration from 4 mg/L to 20 mg/L increased the degradation from 58.2~ to 81.4%. Any further increase in the oxidant loading decreased the degradation of the pollutant marginally due to the scavenging effect [31]. In another study by Daware et al. [32] on the degradation of 4-methylpyridine using the sonochemical method (a pH of 9, a temperature of 45 °C, a pollutant concentration of 50 mg/L, and a time of 120 min), the authors observed that by increasing the oxidant loading from 5 mg/L to 30 mg/L, the degradation increased from ~70% to ~80%. It was also observed that beyond 20 mg/L of oxidant loading, the pollutant degradation only marginally increased, confirming that 20 mg/L was optimum [32]. Hou et al. [33] studied the use of UV/persulphate for the degradation of haloacetonitriles. Their study reported that 100% degradation of dichloroacetonitrile (DCAN) using the UV/persulfate process was achieved. At an initial DCAN concentration of 2µM, when the persulfate varied from 20 µM to 200 µM, the degradation of DCAN increased with an increase in oxidant loading. The authors also observed that increasing the temperature from 10 °C to 30 °C increased the degradation efficiency [33]. A comparative analysis of the studies elucidated the varied nature of the action of KPS at different conditions and the importance of optimisation of oxidant loading.
3.2.2. US + H2O2
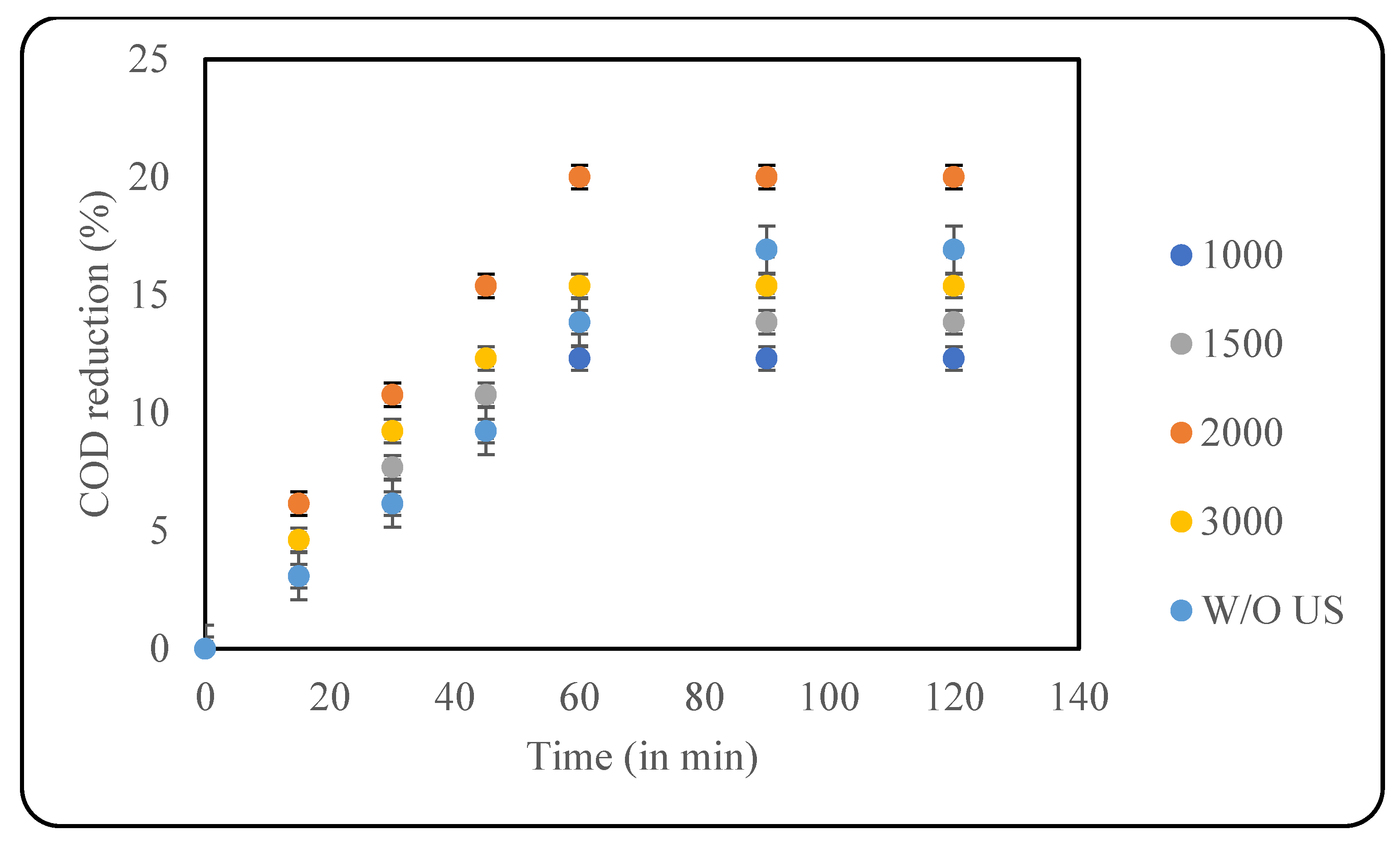
Hydrogen peroxide was the next oxidant applied in this study, as it is a convenient, easy-to-use, and cheap oxidant that has also proven to degrade pollutants in effluent efficiently [34]. It is also an environmentally friendly oxidant that does not produce any toxic residues [35]. In the presence of US, H2O2 dissociates into hydroxyl radicals that can efficiently degrade and mineralise the pollutants [11]. The current work checked four loadings of H2O2 for optimisation, including 1000, 1500, 2000 and 3000 ppm. As observed from Figure 6, a maximum COD reduction of 20% was achieved with 2000 ppm of oxidant loading after 60 min, and the use of further higher loadings of the oxidant lead to negative results. The effect of the oxidant in the absence of US was also studied, where the use of only 2000 ppm of H2O2 resulted in 13.5% COD reduction.
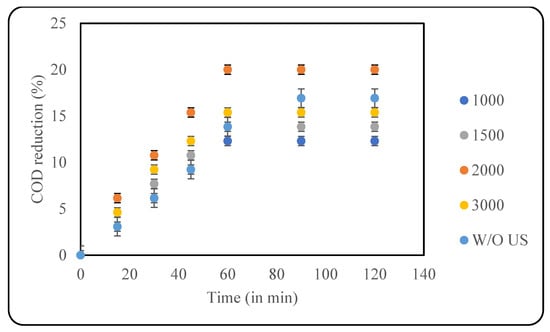
Figure 6.
Effect of US + H2O2 on COD reduction (pH: 2; temp.: 30 °C).
Manikandan et al. [36] studied the effect of UV/H2O2 on the removal of colour from a simulated textile effluent, which contained Reactive blue, direct Red, and Acid Violet dyes, along with other salts and sugar. For their study on the load of H2O2, the authors used the effluent at a 60% pollutant load, and the pH was set to 7. Individually, UV and H2O2 were not effective in colour removal, and the authors found that a hybrid system of UV/ H2O2 resulted in much better colour removals. It was also reported that as the oxidant loading increased from 0.1 to 0.4 M, the removal efficiency increased from 63.76% to 98.77%. The authors found that applying higher oxidant loadings till the optimum level was reached was essential for proper action of the oxidant, and the oxidant was not very effective at lower doses. Increasing the loading beyond 0.4M resulted in a dip in the colour removal, which was attributed to the quenching effect [36]. In another study, Pradhan and Gogate [37] studied the use of hydrodynamic cavitation (HC) and H2O2 for the removal of p-nitrophenol using a pollutant concentration of 5 g/L and orifice and venturi as the cavitating devices. The treatment was carried out for 90 min. The oxidant loading varied from 0.5 g/L to 10 g/L. While only HC with venturi resulted in 53.44% pollutant removal, the HC/H2O2 (5 g/L) system resulted in 59.9% degradation. Any further increase in the oxidant loading caused a scavenging of the available radicals, resulting in lower degradation. In the case of orifice, HC/H2O2 resulted in 52.4% degradation, suggesting the superiority of venturi. To combat the issue of radical scavenging due to an excess amount of the oxidant, the authors applied a step-wise addition of the oxidant. The oxidant loading was timed at 2 g/L every 15 min. The direct addition of 10 g/L of the oxidant resulted in 50.6% degradation, while the step-wise addition resulted in a reduction in the pollutant by 65.5%, which suggests that along with optimised loading, it is also essential to space the loading over a while to allow for it to function efficiently [37]. These studies highlight the efficient performance of H2O2 as an oxidant. Additionally, they also emphasise the need for an activator (UV, ultrasound, or HC) for the peroxide to function efficiently, as also demonstrated in the current work. Finally using an optimum amount of H2O2 is important to achieve best efficiency, and the optimum amount is specific to the system under investigation.
3.2.3. US + Fenton
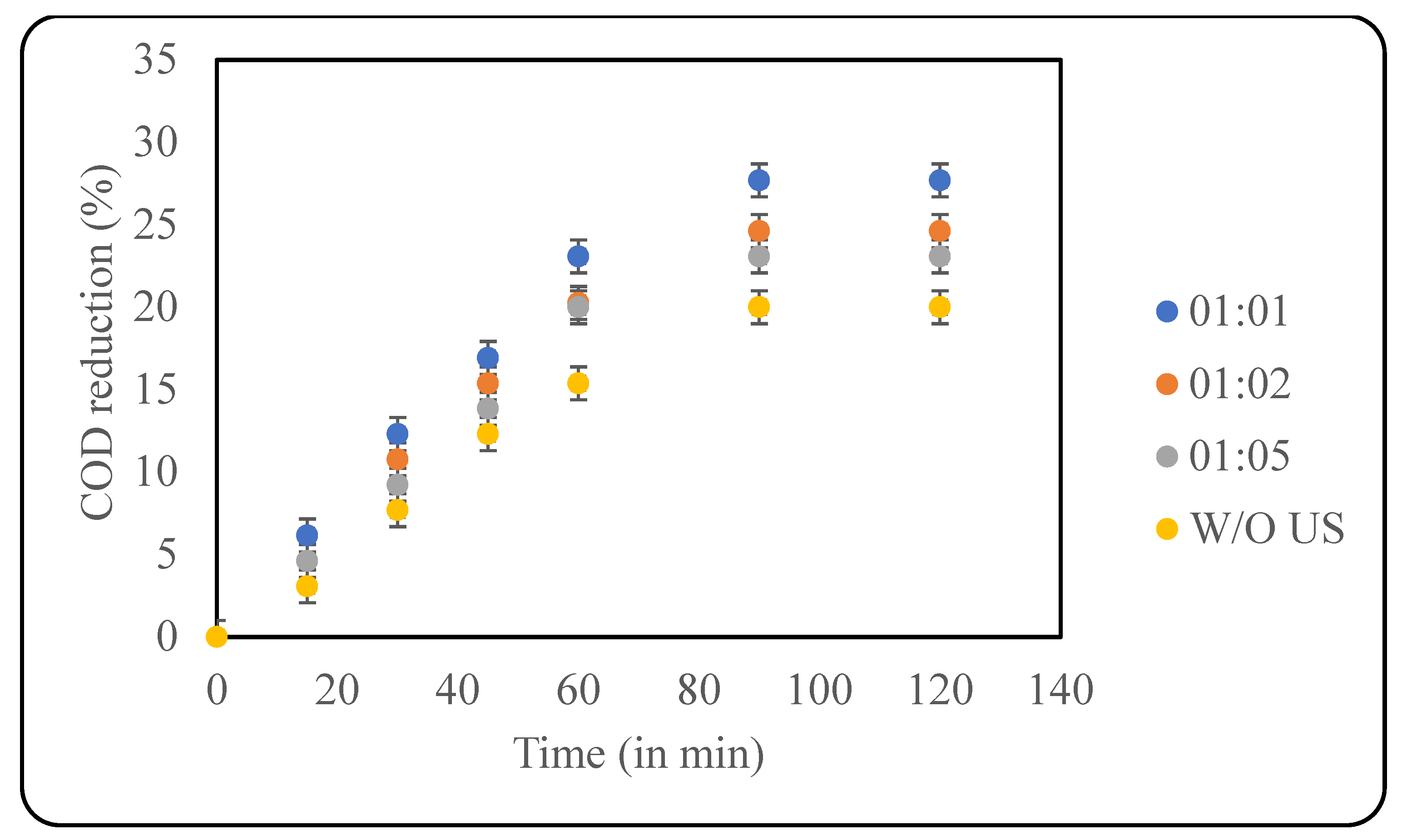
Fenton oxidation is one of the most widely used AOPs that works based on the dissociation of H2O2 using the Fe2+ from the ferrous salts used, resulting in the production of hydroxyl radicals. Fenton chemistry is a multistep and multiway production of radicals using H2O2, Fe2+, Fe3+, and OH2 that creates a pool of OH− that enhances the action of H2O2 [38]. One of the main limitations of the conventional Fenton reaction is the slow regeneration of ferrous ions, which can ultimately lead to wastage of peroxide. Additionally, a high concentration of salt is required, which ultimately can deposit as sludge, resulting in techno-economic difficulties. These problems can be partially addressed by combining US with Fenton, which can work synergistically [39]. In this study, the already optimised loading of H2O2 (2000 ppm) was used to study the effect of the loading of Fe2+ in the treatment of the effluent using the combined Fenton with the ultrasound approach. Ferrous sulphate was the source of ferrous ions. The loading of Fe2+ was chosen based on the ratio between Fe2+ and H2O2, including 1:1, 1:2 and 1:5. The results for the effect of loading are given in Figure 7. A maximum reduction in COD of ~28% after 90 min was observed when the samples were treated with equal concentrations of Fe2+ and H2O2. As the Fe2+ loading decreases, the COD reduction decreases as well, and this can be attributed to the presence of insufficient Fe2+ to activate the peroxide. The effect of Fenton alone was also studied, and it was observed that only 20% COD reduction was observed, clearly elucidating the role of ultrasound in intensification.
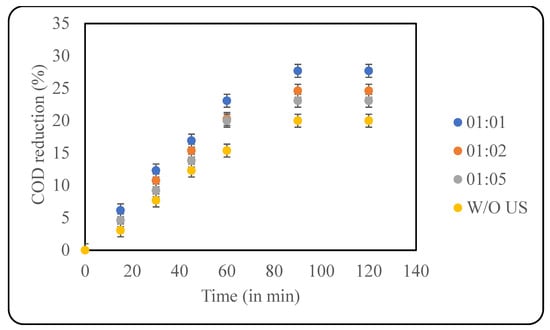
Figure 7.
Effect of Fe2+:H2O2 (in ppm) on COD reduction (pH: 2; temp.: 30 °C).
Various studies on the use of Fenton for the treatment of effluents have been reported, which also elucidate the advantage of combined ultrasound + Fenton processes, although to a different quantitative extent. Chandak et al. [15] studied the use of the sono-Fenton approach for the treatment of pharmaceutical industrial effluents. While only US resulted in 14% COD removal, the authors observed that the use of the sono-Fenton system at 3:5 Fe2+:H2O2 resulted in 73% COD reduction, clearly demonstrating the benefits of the combined treatment [15]. In another study on the sono-Fenton oxidation of ibuprofen, the authors used two different loadings of Fe2+ to H2O2, including 0.067:3.2 mM and 0.134:6.4 mM. The ratio was constant at 48. The authors observed that using only Fenton at lower loadings resulted in only 62% IBP degradation, while coupling the same with 20 kHz US resulted in complete degradation of the drug after 3h. The use of higher oxidant loadings revealed that the treatment time could be reduced from 180 min to 60 min with 95% drug removal. Conversely, only 40% TOC removal was observed, suggesting that recalcitrant intermediates are formed during the reaction. The authors also checked for synergy by calculating the consumption of H2O2 in the reaction, which was found to be 13% and 44% for Fenton and sono-Fenton, respectively, confirming that the increased oxidant consumption improved the reaction efficiency [39]. The presented analysis of different studies highlights the importance of ferrous ions to activate the peroxide and the significance of the sono-Fenton oxidation treatment, as they show synergy and improve treatment efficiency to a different extent, depending on the specific system.
3.2.4. US + Peroxone
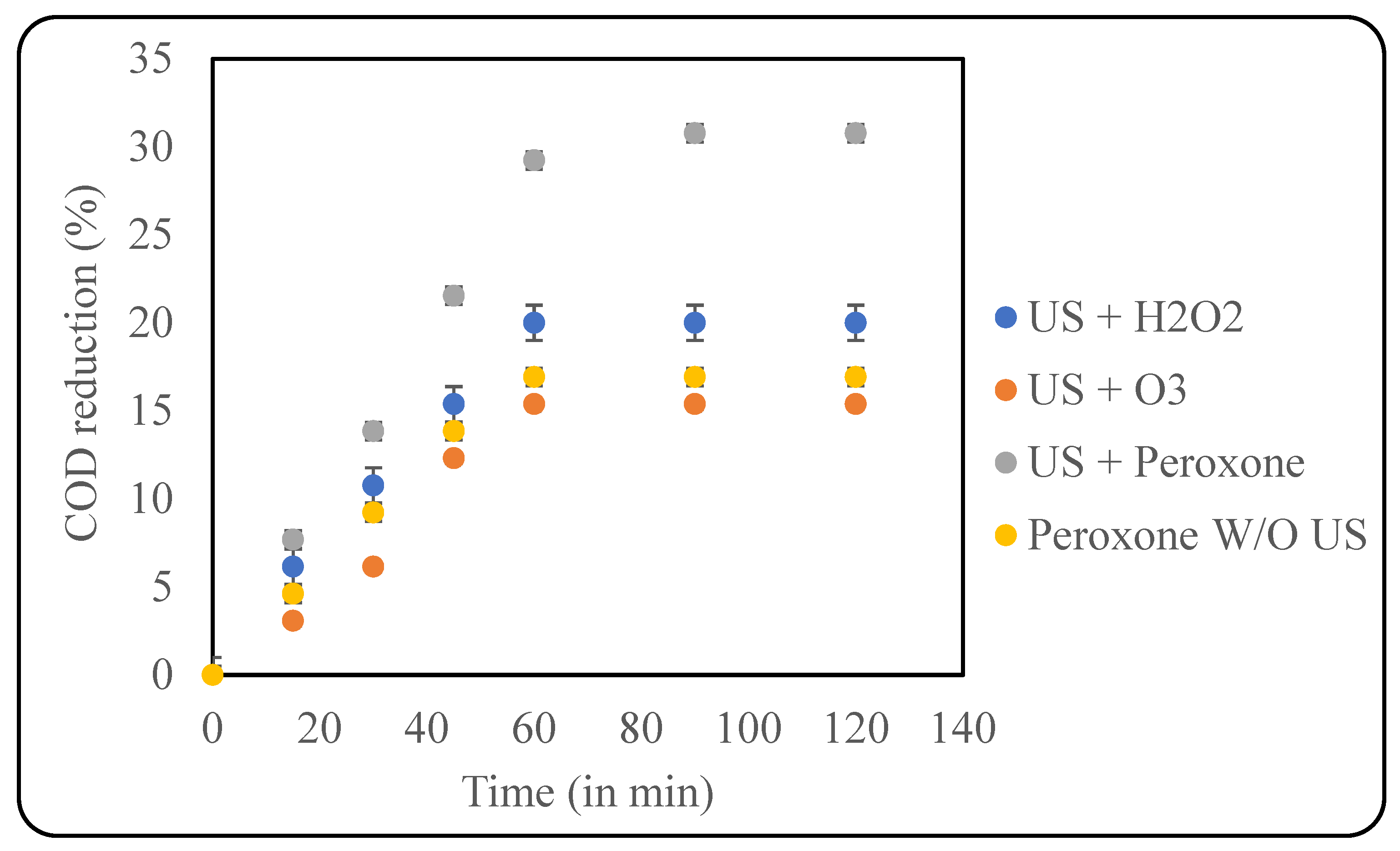
Peroxone uses ozone combined with hydrogen peroxide as an oxidant for effluent treatment. The addition of peroxide to ozone increases the production of hydroxyl radicals by ~50%, thus being an efficient oxidant [40]. The use of peroxone is highly recommended in systems that are refractory to ozone degradation [41]. Additionally, peroxone can also reduce toxic intermediates, like bromate to bromide, thus preventing the exposure of carcinogenic chemicals as well [42]. The current study used the optimised loading of H2O2 along with an ozone loading of 400 mg/h. Initially, the effect of US + ozone was studied, and a COD reduction of only 15.38% was obtained after 60 min of treatment. Subsequently, a US + peroxone treatment was performed, which resulted in ~30% COD reduction. Similarly, treatment using only peroxone was also studied, which produced ~17% COD reduction, which clearly confirms the benefits of using the combination of US + peroxone, as also seen in Figure 8.
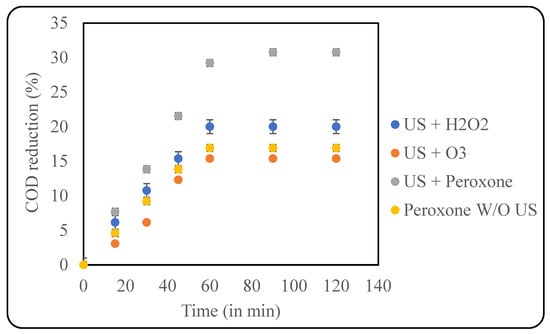
Figure 8.
Effect of US + peroxone on COD reduction (pH: 2; temp.: 30 °C).
Ghanbari et al. [43] studied the degradation of organic compounds using electroperoxone enhanced by US. Electroperoxone is the electrochemical generation of H2O2 while the ozone is supplied externally. Initially, the authors tested a synthetic effluent containing Acid Orange 7 (AO7). The US source was a 20 kHz sonication probe, and the flow for ozone and H2O2 was set to 0.5 l/min. US/O3 was able to degrade 68.5% of the dye after 60 min, whereas the electroperoxone process resulted in 100% dye degradation at the same time of treatment. Combining US and electroperoxone resulted in 100% degradation after only 15 min, exhibiting the synergy between the two applied processes. To check for the efficiency of the process in a complex system, the authors tested it for the treatment of a textile industrial effluent. The COD of the effluent was ~550 ppm, and the pH was 5.8. The colour removal achieved for the EP and US/EP processes was 93% and 99%, while the COD removal was 72% and 85%, respectively, again confirming the benefits of combining EP and US. The combined process also improved the biodegradability of the effluent [43]. In another study by Dey and Gogate [44], related to the sono-peroxone treatment of a coke-industry effluent with an initial pH of ~9 and COD of 3360 mg/L, the authors initially tested the effects of US + H2O2 (20–60 mL/L) and US + ozone (0.5–2 L/min) under the ultrasonic parameters of 130W of power, a 70% duty cycle, and a 20 kHz frequency. The COD reduction at an optimised H2O2 (40 mL/L) and ozone (1 L/min) loading were 81.96% and 81.75%, respectively. Subsequently, the authors tested US + peroxone at optimised oxidant loading, which produced a COD reduction of 90.48%. The peroxone-treated sample was also tested for toxicity, and a mild increase in microbial toxicity was seen, confirming that the intermediates are not highly toxic [44]. The presented studies confirm the efficiency of using peroxone-based treatments for diverse effluents and the impact of combining peroxone with other methods, like US. The studies prove that peroxone can degrade the effluent to produce less or non-toxic intermediates, which can be biodegraded, and also, the treatment efficacy is specific to the effluent in question, which elucidates the importance of the current work.
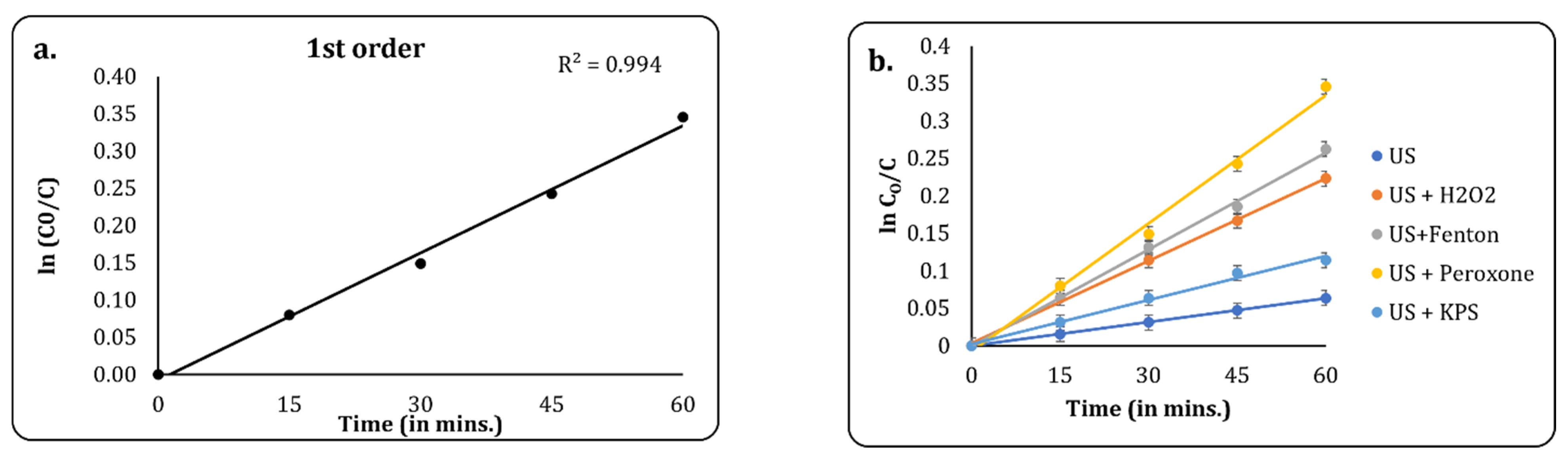
3.2.5. Kinetic Studies
Kinetic analyses were performed to understand the rate of reactions and thus finalise the best treatment plan for scale-up. For hybrid treatment techniques based on advanced oxidation processes, most studies have reported either first-order or second-order kinetics as the best fit, with the majority having found success with first-order kinetics [45,46]. In this study, we attempted to fit first-order kinetics to the best treatment method: US + peroxone. It was observed that the best fit was obtained for the first-order plot, with a correlation coefficient (R2) of 0.994. Thus, first order was chosen as the best kinetic model, and it was used for the analyses of other methods. A comparative plot is given in Figure 9. A linear graph was plotted for ln (C0/C) against time based on the following equation:
where C0 = the initial COD of the effluent, C = the final COD of the effluent, k = the rate constant, and t = the time of reaction. The rate constants and correlation coefficient obtained from the graphs are given in Table 2.
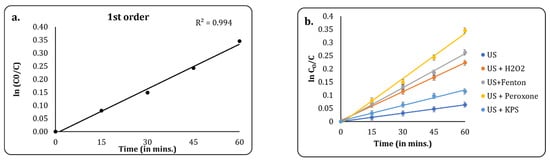
Figure 9.
(a) First-order kinetic fit for US + peroxone process; (b) first-order kinetic fit for several USs and AOPs.

Table 2.
Comparison of rate constants and correlation coefficients of various treatment methods.
3.3. Toxicity Analysis
For any pretreatment approach applied before biological oxidation, it is important to test for toxicity against biological agents, like microbes, cell lines, model animals, etc. Any pretreatment aims to facilitate better BO by reducing the recalcitrancy of the effluent and making it amenable to the action of microbes and, at the same time, not introducing any toxicity due to any residual oxidants, and this is checked by various parameters, like an analysis of intermediates, the biodegradability index (BI), and toxicity. For systems with a matrix, a toxicity analysis is one of the simpler methods to perform. The current study used the agar well diffusion method for the analysis of microbial toxicity. The test enabled a check for the elimination of recalcitrant compounds using the pretreatment and was also used to prove the absence of any toxic intermediates formed by the pretreatment methods. Both the raw and pretreated samples were tested to check for any change in the zone of clearance. Doxycycline was the positive control (PC), which showed a zone of clearance of 25 mm, while the sterile distilled water (NC) showed no zone of clearance against S. aureus and E. coli as expected. The data on the zone of clearance are given in Table 3. As seen from the table, there is a marginal decrease in the zone of clearance between the untreated and the pretreated samples, which shows that recalcitrancy was reduced by the pretreatment, and there is no formation of any toxic intermediates. The value of the clearance zone (<10 mm) suggests that the organisms are resistant to the sample, and the samples are non-toxic.

Table 3.
Zone of clearance data in toxicity analysis.
Various studies on the need for a toxicity analysis before biodegradation and related analyses are available in the literature. Somensi et al. [47] used ozonation for the treatment of textile industrial wastewater and subjected the samples to a toxicity analysis using bacterial luminescence studies against Vibrio fischeri. The authors stated that an optimised pretreatment technology can be decided upon using these tests, as they ascertain the viability of the samples for biodegradation. The effluent had an initial COD of 1500 mg/L, which was reduced to 1125 mg/L after 4 h of ozonation treatment. The samples were diluted to various levels (90.4–50%) before a toxicity analysis. It was observed that there was a decrease in toxicity of 1.5% to 15%, based on the sample dilutions and applied treatment, suggesting a partial success in removing the recalcitrancy from the effluent. The authors suggested that any more toxicity removal would require an increased treatment time, which would, in turn, increase the treatment cost. Another input from the authors was that if the samples are to be subjected to microbiological treatment, microbial toxicity is a promising method compared to animal or cell line toxicity studies [47].
In another study by Changotra et al. [48], the researchers worked on the use of Fenton-based treatment methods, like dark Fenton, photo-Fenton, and electro-Fenton, for the treatment of real pharmaceutical effluents (two streams, including low strength wastewater—LSW, with an initial COD of ~8500 mg/L, and high strength wastewater—HSW, with an initial COD of ~37,500 mg/L). Of the three treatment methods, photo-Fenton was the most successful, with ~60% COD reduction for both the effluents. Similarly, dark Fenton resulted in ~50% COD reduction, and electro-Fenton caused ~40% COD reduction for both the effluents. A toxicity analysis of the samples was performed using a disc diffusion assay against P. aeruginosa, B. subtilis, and E. coli. The results show that pretreatment using dark Fenton and photo-Fenton eliminated toxicity against the test microbes, while the electro-Fenton-treated samples exhibited a minimal zone of clearance. Initial samples of both effluents (LSW and HSW) showed a large clearance zone, clearly confirming that the initial microbial toxicity was significantly or completely eliminated after treatment [48]. The importance of a toxicity analysis is clearly understood through these studies, as the importance of understanding microbial toxicity before aerobic oxidation proves essential for deciding further treatment plans.
3.4. Biological Oxidation
The microbial-assisted oxidation of molecules in effluents is known as biological oxidation (BO). It is the most commonly used method for effluent treatment, as it can be performed on a large scale and does not require any complex setup. Additionally, BO works across effluents from various industries. With recent challenges of effluents with recalcitrant chemicals, BO can be used as a secondary treatment technology with any of the oxidation approaches as the initial treatment option. The current study also applied BO as a secondary treatment to various pretreated effluents. Cow dung was used as a treatment source for microbes. A comparative study on direct BO and BO after pretreatment was conducted to check for improvements in the efficiency of hybrid systems.
3.4.1. Sludge Preparation
Cow dung, one of the commonly used microbial sources for sludge, contains a variety of organisms, like Acinetobacter, Bacillus, Pseudomonas, Serratia, and Alcaligenes spp., with diverse metabolic abilities to degrade complex organic pollutants [49]. Cow dung, obtained from a local cattle farm, was used for the treatment. The procured cow dung was diluted with water to form a uniform slurry and mixed and filtered with a 0.5 mm sieve to remove large particles. The pH of the slurry was adjusted to 7 and maintained at 37 °C, with the periodic addition of nutrients. A microbial analysis of the sludge was conducted.
3.4.2. Conventional Biological Oxidation
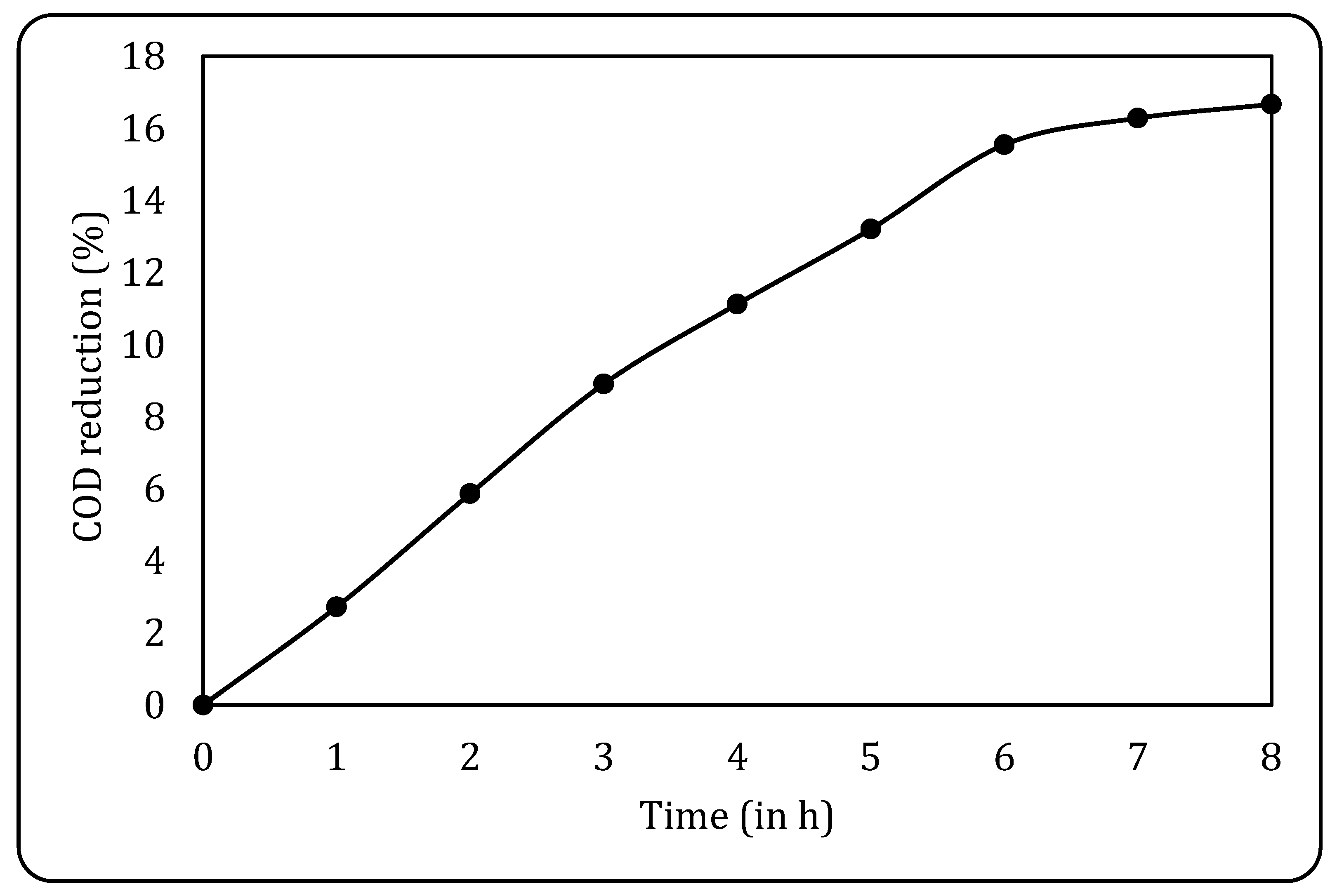
Initially, the raw effluent was subjected to biological oxidation using non-acclimatised sludge to check its efficiency and optimise the treatment parameters. The treatment was carried out for 8 h with 180 mL of the effluent and 60 mL of the sludge (a 1:3 sludge-to-effluent ratio), and aeration was supplied using an aquarium pump. The results obtained for the same are given in Figure 10, where it can be seen that a maximum COD reduction of 16.67% was achieved after 8 h. It is also observed that the COD reduction remained fairly constant after 8 h, and hence, this was finalised as the optimum treatment time for all the subsequent studies.
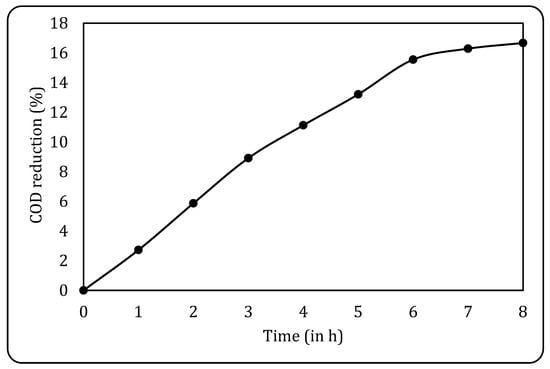
Figure 10.
COD reduction using conventional BO.
Studies on using raw sludge for effluent treatment reflect the low efficiency of this treatment, as only about 15% COD removal could be obtained. An analysis of the literature revealed acclimatisation as one of the avenues for enhancing the efficacy of BO. For example, Kamali et al. [50] studied the use of activated sludge for the treatment of phenolic wastewater. On the use of non-acclimatised sludge (1 g/L MLVSS) for effluent treatment, it was observed that for a lower phenolic loading (25 mg/L), complete degradation was observed, while for a higher loading (250 mg/L), the degradation rate is very low (0.0065 g phenol/g VSS/h), almost 10 times less than the results obtained for treatment with acclimatised sludge [50]. Similarly, Jimenez-Silva et al. [51] studied bioaugmentation as the acclimatisation process for ibuprofen (IBP) removal under microbial action. The authors collected activated sludge from three different wastewater treatment plants, enriched in the same way in terms of IBP-degrading communities. It was reported that non-acclimatised POOL organisms could degrade about 75% of IBP after 96 h of treatment, which was found to be slower and less efficient than the treatment with acclimatised sludge, which resulted in complete IBP removal after 72 h of treatment [51]. The presented analysis of studies helps in understanding that acclimatisation not only improves degradation efficiency but also reduces treatment time, which impacts the economy of treatment. Based on this observation, the next set of studies targeted the use of acclimatised sludge in the current work as well.
3.4.3. Sludge Acclimatisation
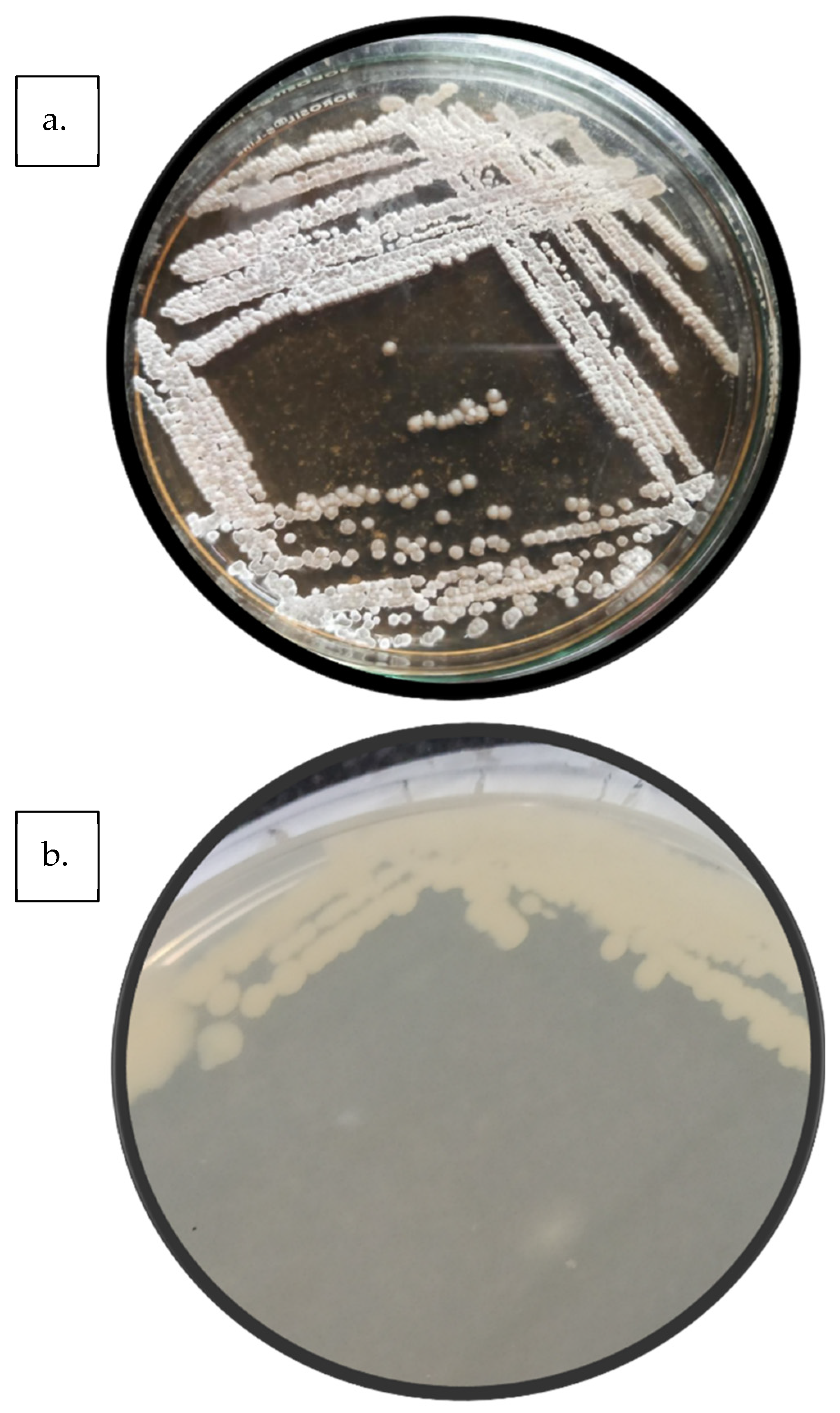
Sludge acclimatisation was carried out to improve the efficiency of the BO treatment. Acclimatisation refers to the method by which microbial systems are gradually adapted to environmental conditions, chemical loads, and wastewater compositions [52]. It essentially induces effective stress to the organism for longer sustenance, and the organism thrives in the conditions [53]. Industrially, the effluent and the sludge used for the treatment come from the same plant. Thus, the microbes are accustomed to the conditions. Conversely, in the case of treatment in a CETP or otherwise, when acclimatised sludge is not readily available, acclimatization must be carried out. The current study used cow dung from a local farm, and thus, the sludge was acclimatised for the effluent. The sludge acclimatisation process was performed for 21 days, after which it was ready for BO. To check for the specific presence of organisms, plating was performed. Two major colonies were identified, whose characteristics are detailed in Table 4, whereas image of the colonies is depicted in Figure 11.

Table 4.
Colony characteristics of (a) Organism 1 and (b) Organism 2.

Figure 11.
Isolated organisms: (a) Organism 1; (b) Organism 2.
Various authors have used different methods for sludge acclimatisation. In a study on the use of sludge for the treatment of ibuprofen, the authors isolated and found different microbial communities for the treatment. The first stage of acclimatisation was to mix the various communities and create a “POOL” that had better efficiency in the treatment than individual communities. The second stage of acclimatisation was through bioaugmentation. The obtained POOL was centrifuged and suspended into synthetic wastewater with fixed doses of IBP to provide a toxic shock to the microbes at manageable levels. The growth of the microbes in this environment caused it to perform better with a real effluent [51]. Similarly, Kamali et al. [50] used acclimatisation for the treatment of phenolic wastewater. The authors used two modes of acclimatisation where two reactor tanks were filled with the sludge and operated on batch mode. In one tank, there was daily charging with a known concentration of phenol through which acclimatisation was carried out. In the second tank, along with the sludge, a biochar prepared from pinewood was also added and then acclimatised, similar to the first tank. Thus, it can be said that the acclimatisation process has been used across all effluent types and forms an important part of improving treatment efficiency [50], which was the main reason for carrying out the acclimatisation study in our work.
3.4.4. Effect of Pretreatment on Biological Oxidation Using Acclimatised Sludge
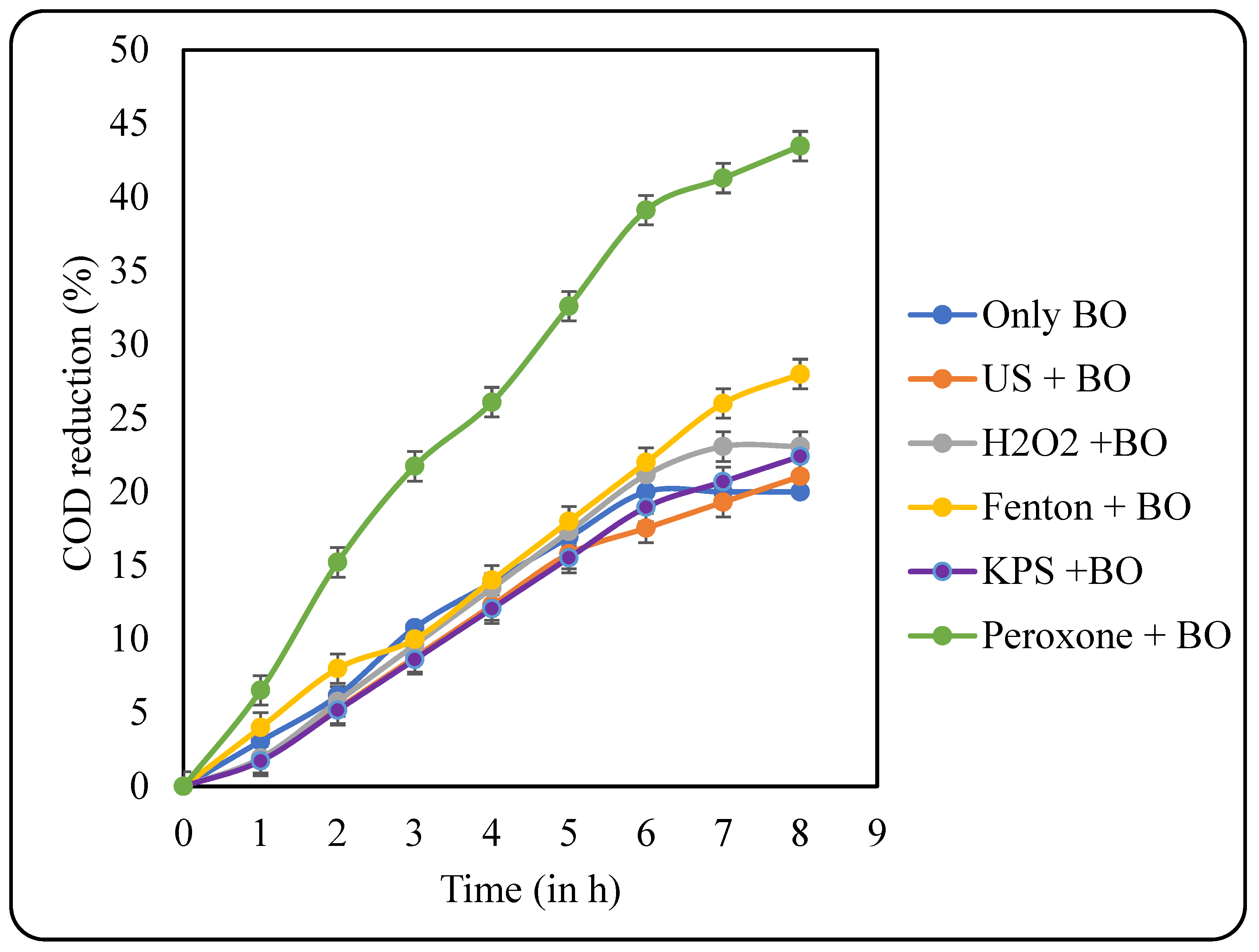
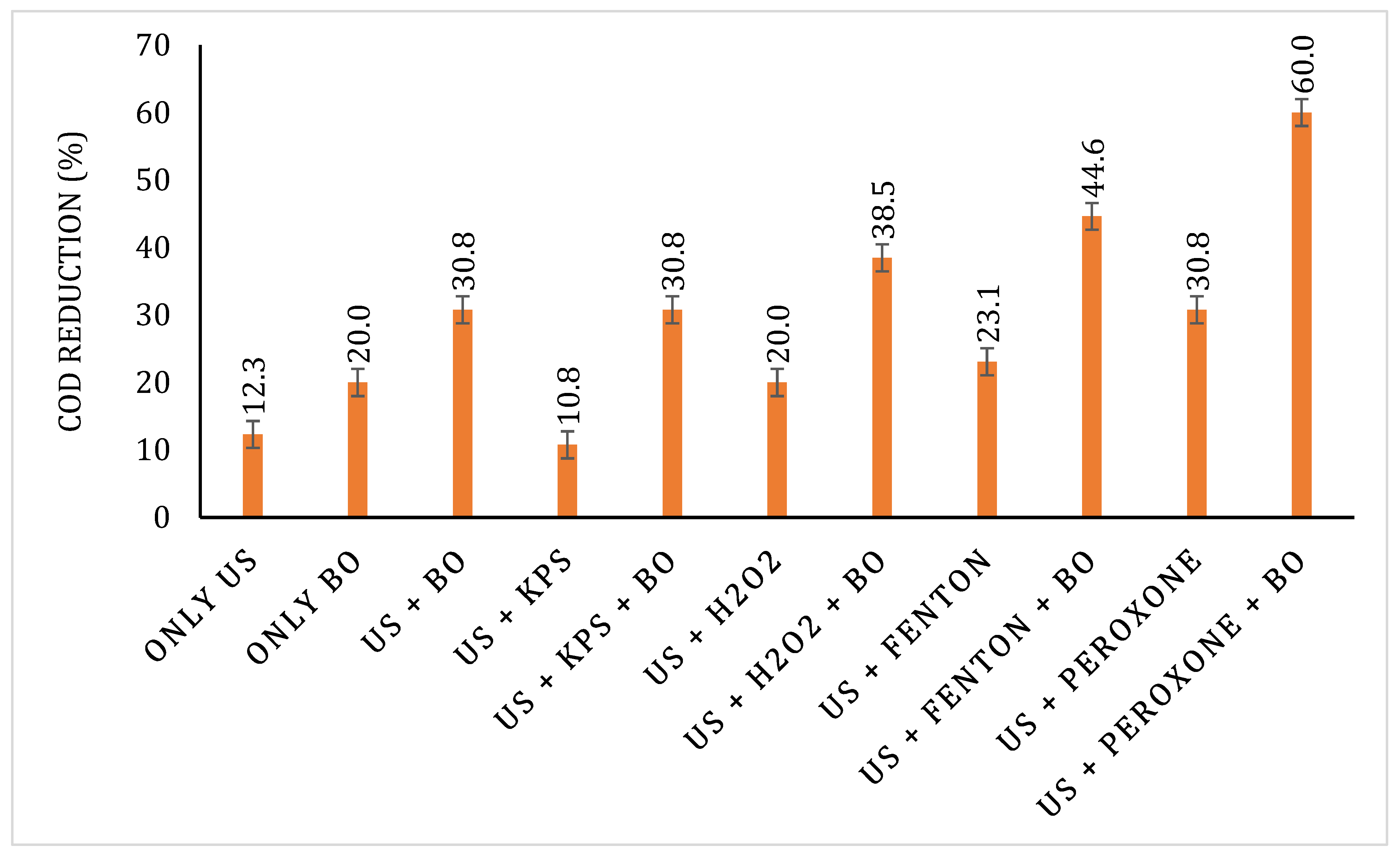
After sludge acclimatisation, the sludge was used to treat the pretreated effluents. The samples were filtered to remove any particulate matter, and then, the pH was adjusted to 7 for the efficient action of microbes. COD reduction data after the BO treatment is shown in Figure 12. An increase in the COD reduction was observed after sludge acclimatisation. The highest COD reduction of 43.48% was observed after BO for the US + peroxone-pretreated sample, which was followed by US + Fenton, US + H2O2, US + KPS, US, and only BO with their respective COD reductions of 28, 23.08, 22.41, 21.05, and 20%. The overall COD reduction of pretreatment + BO also follows the same trend as for the BO with US + peroxone-treated samples, displaying the highest overall COD reduction of 60%. The results for overall COD reduction are displayed in Figure 13.
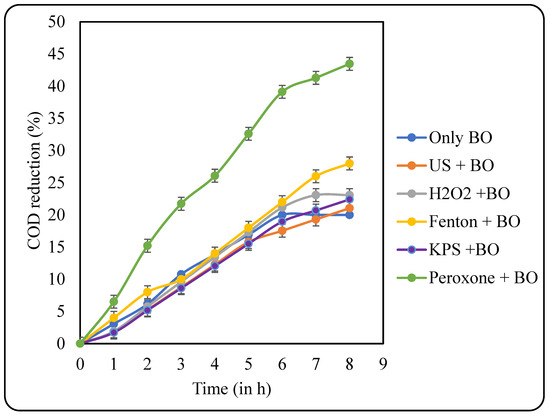
Figure 12.
Effects of various pretreatments on COD reduction at the stage of BO.
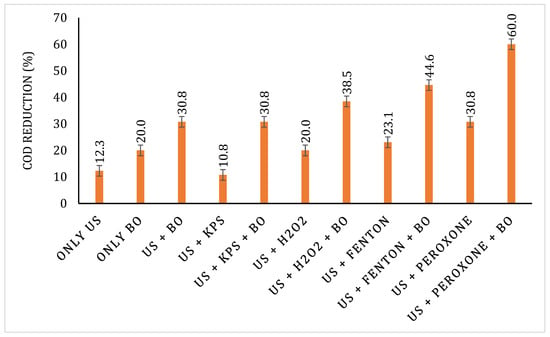
Figure 13.
Overall COD reduction for various treatment strategies explored in the work.
The effect of pretreatment on BO and the optimised treatment scheme can vary greatly depending on the effluent under study. In a study on the use of a membrane bioreactor (MBR), a moving bed biofilm reactor (MBBR), and an AOP for the treatment of a pharmaceutical effluent, the authors used two schemes, including MBBR-MBR-AOP and MBR-AOP, operated as a continuous system with a 272 L reactor and 78 L separation tank. Connected to this tank was a photochemical batch reactor with 0.8 L of working volume for AOPs. The reaction conditions included 10 h of HRT, 6.5 g/L of MLSS, and 25 mg/L of H2O2 loading for a UV/H2O2 approach. The effluent was also spiked with carbamazepine, ciprofloxacin, and ibuprofen to check for their degradation, as they are the most commonly found drugs in effluents. From the results, it was observed that MBR can be both a pretreatment or a secondary treatment scheme, and both methods could produce high-quality reclaimed water with low BOD, TSS, and turbidity and no E. coli. In the MBBR-MBR-AOP method, complete degradation was achieved with 5 min of AOP treatment. The authors observed that although the use of AOP did not improve water quality for reclamation, it was effective in the removal of pharmaceuticals. Additionally, it did not add any toxic by-products at the last stage. The authors concluded that MBR alone is sufficient to reclaim water, but to improve the stability of the system, MBBR is required, and AOP treatment is essential for the complete removal of pharmaceutical drugs [54]. In another study, Marinez et al. [55] used the Fenton method as a pretreatment to biodegradation using a Sequential Batch Reactor (SBR) for the treatment of a pharmaceutical effluent with an initial TOC of 1.5 g/L. For the pretreatment studies, the treatment was carried out at 70 °C with a peroxide loading of 7.5 mgH2O2/mgTOC, and 35% reduction in TOC was observed. Subsequently, the effluent was subjected to biodegradation using SBR with an HRT of 2 days, and >90% TOC reduction was observed. The authors also noted that traditionally, the industry used only Fenton treatment, and the peroxide loading required to achieve complete mineralisation of organics was 21.4 mgH2O2/mgTOC at 120 °C, respectively. Thus, the coupling of treatments effectively reduced the chemical load and treatment energy, which lowered the cost by 36% [55]. The presented comparative analysis of various studies highlights the importance of coupling chemical and biological treatment methods, as this improves the overall efficiency of the treatment by having different modes of oxidation mechanisms. Hybrid methods also help in reducing the overall treatment cost, making them more efficient and suitable for commercial exploitation.
3.5. A Comparative Analysis of the Various Treatment Strategies
Energy and cost efficiency are important parameters that govern any treatment method’s success and viability. Using multiple treatments may give better results, but this adds to the cost and energy input, which is crucial at an industrial level. Thus, the best treatment scheme is one that gives the maximum output with the minimal possible input. Cavitation combined with AOP is expected to be efficient in the removal of organic compounds in both synthetic and real effluents. Researchers have also claimed that when considering the process economics of a cavitation-based treatment, it should be assumed that it precedes BO, as it improves the reduction in sludge and enhances the expected treatment [56]. This study involved two processes, and thus two yields could be estimated, including the Cavitational yield and the BO yield. The total of these yields contributes to the energy and ultimately the cost of the treatment. The detailed process economics, in terms of the cost of the treatment, is tabulated in Table 5. The calculation was performed according to the detailed methodology presented in our previous work [11]. The electricity cost was considered to be 8.78 INR/kWh, and the cost of chemicals was considered based on their commercial prices, namely, H2O2: 760 INR/L and FeSO4·7H2O: 10 INR/kg. The energy consumption for the ozone generator was 0.24 kWh. The table shows that the treatment cost for all the methods is ~10 INR/L, making the process selection more driven by the obtained degradation efficiency. Another observation is the higher energy cost for all the studies compared to the chemical costs. The literature is divided in this aspect, as some studies have shown lower energy costs, while others have shown higher chemical costs [57].

Table 5.
Process comparison based on yield and costs.
Studies understanding the importance of cost analysis for optimising a treatment method have been conducted in the past. Segura et al. [57] tried different on-site hospital wastewater treatment methods, like catalytic wet air oxidation (CWO), intensified Fenton, and catalytic photo-Fenton. While CWO had an ~85% reduction in pharmaceuticals, Fenton and photo-Fenton showed a reduction of 99.8% and 94.5%, respectively. For CWO, the energy cost was 1.74 EUR/m3, and the catalyst cost was 4.93 EUR/m3 (total cost: 6.67 EUR/m3). The energy cost for the Fenton treatment was 0.5 EUR/m3, and the chemical cost amounted to 0.35 EUR/m3 (total cost: 0.85 EUR/m3). For the photo-Fenton treatment, the energy and chemical costs were 9.94 EUR/m3 and 0.42 EUR/m3 (total cost: 10.36 EUR/m3). Based on the degradation and costs, intensified Fenton was reported as the most suitable method [57]. In another study, authors used ozone-based treatment methods for the treatment of synthetic and real textile industry effluents. The authors targeted Reactive Black 5 (RB5) and its industrial form Setazol Black DPT. The methods used were O3, UV/O3, O3/H2O2, and O3/UV/H2O2, and H2O2/UV. While the ozone-based methods could eliminate colour, H2O2/UV was ineffective for an industrial effluent. The COD reduction was very low for an industrial effluent (10%), while the COD for a synthetic effluent reduced significantly (~90%). The ozone-based methods increased biodegradability and decreased toxicity, making them suitable for application before BO. A cost analysis showed that the major investment cost comes from the ozonation unit and the UV source. The operating cost for O3 and O3/H2O2 was 50% of the cost of discharged water and freshwater, thus making treatment process industrially viable. Conversely, the cost for O3/UV and O3/UV/H2O2 was 80% of the discharged effluent cost and thus not viable industrially. At the lab scale, the ozone was too excessive to counter mass transfer issues. Industrially, ozone mass transfer would be better. Thus, less ozone could be used, which may bring down the cost. Based on these findings, the authors recommended O3 and O3/H2O2 for industrial textile effluent treatment [58]. The presented results and comparison with the literature show that a cost analysis is an important aspect of process optimization at both lab and industrial scales, as it ensures the economic viability of the treatment and ensures efficient scale-up possibilities.
4. Conclusions
The current work used US-based pretreatment for the removal of toxic compounds from a real pharmaceutical effluent. The reaction conditions were optimised, and various AOPSs in combination with US were applied as the pretreatment to biological oxidation. US-assisted peroxone was the best treatment approach, with a COD removal of about 30% after 60 min of treatment. The pretreatment methods did not introduce any toxic by-products into the system, which was confirmed by a toxicity analysis. BO, performed using non-acclimatised sludge based on cow dung, resulted in a lower COD reduction, and the application of acclimatised sludge and the pretreated samples resulted in increased COD reduction. The BO of the US + peroxone-pretreated effluent gave the best COD reduction of 60% in 8 h, serving as the optimized treatment time. This study demonstrated the beneficial effects of a US-based peroxone treatment in reducing the COD of a real effluent with a complex matrix to facilitate better biodegradation.
Author Contributions
Conceptualization, P.R.G.; Methodology, A.M.I. and A.V.K.; Data curation, A.M.I.; Writing—original draft, A.M.I. and A.V.K.; Writing—review & editing, P.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the funding of the All India Council of Technical Education (AICTE) for the AICTE Doctoral Fellowship, awarded to Akshara Iyer.
Institutional Review Board Statement
All authors declare adherence to the standard ethics related to research publication and writing of the manuscript.
Data Availability Statement
Data will be made available on request from the authors.
Acknowledgments
The authors would like to thank Herbert Brown Pharmaceuticals, Mumbai, for supplying them with the effluent.
Conflicts of Interest
The authors declare no conflicts of interest or any funding that could have influenced the outcomes of the work.
References
- Naddeo, V.; Belgiorno, V.; Kassinos, D.; Mantzavinos, D.; Meric, S. Ultrasonic degradation, mineralization and detoxification of diclofenac in water: Optimization of operating parameters. Ultrason. Sonochemistry 2010, 17, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kerc, A.; Bekbolet, M.; Saatci, A.M. Effect of partial oxidation by ozonation on the photocatalytic degradation of humic acids. Int. J. Photoenergy 2003, 5, 497421. [Google Scholar] [CrossRef]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Sakumoto, A.; Miyata, T. Treatment of waste water by a combined technique of radiation and conventional method. Radiat. Phys. Chem. (1977) 1984, 24, 99–115. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef]
- An, T.; Yang, H.; Li, G.; Song, W.; Cooper, W.J.; Nie, X. Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B Environ. 2010, 94, 288–294. [Google Scholar] [CrossRef]
- Lin, D.; Fu, Y.; Li, X.; Wang, L.; Hou, M.; Hu, D.; Li, Q.; Zhang, Z.; Xu, C.; Qiu, S.; et al. Application of persulfate-based oxidation processes to address diverse sustainability challenges: A critical review. J. Hazard. Mater. 2022, 440, 129722. [Google Scholar] [CrossRef]
- Eren, Z.; Ince, N.H. Sonolytic and sonocatalytic degradation of azo dyes by low and high frequency ultrasound. J. Hazard. Mater. 2010, 177, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, D.; Hamdaoui, O.; Vasseghian, Y.; Momotko, M.; Boczkaj, G.; Kyzas, G.Z.; Wang, C. Bibliometric analysis and literature review of ultrasound-assisted degradation of organic pollutants. Sci. Total Environ. 2023, 876, 162551. [Google Scholar] [CrossRef]
- Raut-Jadhav, S.; Badve, M.P.; Pinjari, D.V.; Saini, D.R.; Sonawane, S.H.; Pandit, A.B. Treatment of the pesticide industry effluent using hydrodynamic cavitation and its combination with process intensifying additives (H2O2 and ozone). Chem. Eng. J. 2016, 295, 326–335. [Google Scholar] [CrossRef]
- Lakshmi, N.J.; Iyer, A.M.; Gogate, P.R. Treatment of wastewater containing ciprofloxacin using the hybrid treatment approach based on acoustic cavitation. Can. J. Chem. Eng. 2024, 102, 2403–2417. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Torres-Palma, R.A.; Pétrier, C.; Esplugas, S.; Gimenez, J.; Pulgarin, C. Mineralization enhancement of a recalcitrant pharmaceutical pollutant in water by advanced oxidation hybrid processes. Water Res. 2009, 43, 3984–3991. [Google Scholar] [CrossRef]
- Al Momani, F.; Sans, C.; Esplugas, S. A comparative study of the advanced oxidation of 2,4-dichlorophenol. J. Hazard. Mater. 2004, 107, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Rajoriya, S.; Saharan, V.K.; George, S. An advanced pretreatment strategy involving hydrodynamic and acoustic cavitation along with alum coagulation for the mineralization and biodegradability enhancement of tannery waste effluent. Ultrason. Sonochemistry 2018, 44, 299–309. [Google Scholar] [CrossRef]
- Chandak, S.; Ghosh, P.K.; Gogate, P.R. Treatment of real pharmaceutical wastewater using different processes based on ultrasound in combination with oxidants. Process Saf. Environ. Prot. 2020, 137, 149–157. [Google Scholar] [CrossRef]
- Antes, F.G.; Diehl, L.O.; Pereira, J.S.F.; Guimarães, R.C.L.; Guarnieri, R.A.; Ferreira, B.M.S.; Dressler, V.L.; Flores, E.M.M. Feasibility of low frequency ultrasound for water removal from crude oil emulsions. Ultrason. Sonochemistry 2015, 25, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Antes, F.G.; Diehl, L.O.; Pereira, J.S.F.; Guimarães, R.C.L.; Guarnieri, R.A.; Ferreira, B.M.S.; Flores, E.M.M. Effect of ultrasonic frequency on separation of water from heavy crude oil emulsion using ultrasonic baths. Ultrason. Sonochemistry 2017, 35, 541–546. [Google Scholar] [CrossRef]
- Shajeelammal, J.; Mohammed, S.; Prathish, K.P.; Jeeva, A.; Asok, A.; Shukla, S. Treatment of real time textile effluent containing azo reactive dyes via ozonation, modified pulsed low frequency ultrasound cavitation, and integrated reactor. J. Hazard. Mater. Adv. 2022, 7, 100098. [Google Scholar] [CrossRef]
- Zampeta, C.; Bertaki, K.; Triantaphyllidou, I.-E.; Frontistis, Z.; Vayenas, D.V. Treatment of real industrial-grade dye solutions and printing ink wastewater using a novel pilot-scale hydrodynamic cavitation reactor. J. Environ. Manag. 2021, 297, 113301. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Hilal, N. Pharmaceuticals removal from wastewater: Ultrasound technology and its potential amalgamation with membrane processes. J. Water Process Eng. 2023, 53, 103810. [Google Scholar] [CrossRef]
- Iyer, A.M.; Lakshmi, N.J.; Gogate, P.R. Intensified Biological Oxidation of Ciprofloxacin using Acoustic Cavitation based Pretreatment. Environ. Qual. Manag. 2025, 34, e70030. [Google Scholar] [CrossRef]
- ISO 15705:2002; Water Quality—Determination of the Chemical Oxygen Demand Index (ST-COD)—Small-Scale Sealed-Tube Method. International Organization for Standardization: Vernier, Geneva, Switzerland, 2021.
- Wei, Z.; Spinney, R.; Ke, R.; Yang, Z.; Xiao, R. Effect of pH on the sonochemical degradation of organic pollutants. Environ. Chem. Lett. 2016, 14, 163–182. [Google Scholar] [CrossRef]
- Naddeo, V.; Meriç, S.; Kassinos, D.; Belgiorno, V.; Guida, M. Fate of pharmaceuticals in contaminated urban wastewater effluent under ultrasonic irradiation. Water Res. 2009, 43, 4019–4027. [Google Scholar] [CrossRef]
- Saharan, V.K.; Badve, M.P.; Pandit, A.B. Degradation of Reactive Red 120 dye using hydrodynamic cavitation. Chem. Eng. J. 2011, 178, 100–107. [Google Scholar] [CrossRef]
- Entezari, M.H.; Kruus, P. Effect of frequency on sonochemical reactions II. Temperature and intensity effects. Ultrason. Sonochemistry 1996, 3, 19–24. [Google Scholar] [CrossRef]
- Ince, N.H. Ultrasound-assisted advanced oxidation processes for water decontamination. Ultrason. Sonochemistry 2018, 40, 97–103. [Google Scholar] [CrossRef]
- Al-Bsoul, A.; Al-Shannag, M.; Tawalbeh, M.; Al-Taani, A.A.; Lafi, W.K.; Al-Othman, A.; Alsheyab, M. Optimal conditions for olive mill wastewater treatment using ultrasound and advanced oxidation processes. Sci. Total Environ. 2020, 700, 134576. [Google Scholar] [CrossRef]
- Im, J.-K.; Heo, J.; Boateng, L.K.; Her, N.; Flora, J.R.V.; Yoon, J.; Zoh, K.-D.; Yoon, Y. Ultrasonic degradation of acetaminophen and naproxen in the presence of single-walled carbon nanotubes. J. Hazard. Mater. 2013, 254–255, 284–292. [Google Scholar] [CrossRef]
- Fedorov, K.; Sun, X.; Boczkaj, G. Combination of hydrodynamic cavitation and SR-AOPs for simultaneous degradation of BTEX in water. Chem. Eng. J. 2021, 417, 128081. [Google Scholar] [CrossRef]
- Daware, G.B.; Gogate, P.R. Intensified sonochemical degradation of 2-Picoline in combination with advanced oxidizing agents. Ultrason. Sonochemistry 2021, 77, 105702. [Google Scholar] [CrossRef]
- Daware, G.B.; Pangarkar, B.L.; Kayande, U.P.; Shinde, P.R.; Kolhe, M.J.; Dabhade, G.B.; Rajesh, Y.; Joshi, P.P. Intensified removal of 4-Methylpyridine by ultrasonication in presence of advanced oxidants. J. Indian Chem. Soc. 2023, 100, 100810. [Google Scholar] [CrossRef]
- Hou, S.; Ling, L.; Shang, C.; Guan, Y.; Fang, J. Degradation kinetics and pathways of haloacetonitriles by the UV/persulfate process. Chem. Eng. J. 2017, 320, 478–484. [Google Scholar] [CrossRef]
- Wu, Q. Wastewater Treatment by Enhanced H2O2—Based Advanced Oxidation Process (AOP) Methods: A Review. J. Phys. Conf. Ser. 2022, 2152, 012011. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, P.; Palanisamy, P.N.; Baskar, R.; Sivakumar, P.; Sakthisharmila, P. Optimization of treatment efficiency of UV/H2O2 process on simulated textile industry wastewater. Desalination Water Treat. 2016, 57, 27169–27180. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Gogate, P.R. Removal of p-nitrophenol using hydrodynamic cavitation and Fenton chemistry at pilot scale operation. Chem. Eng. J. 2010, 156, 77–82. [Google Scholar] [CrossRef]
- Pawar, V.; Gawande, S. An overview of the Fenton Process for Industrial Wastewater. J. Mech. Civ. Eng. 2015, 2, 127–136. [Google Scholar]
- Adityosulindro, S.; Barthe, L.; González-Labrada, K.; Jáuregui Haza, U.J.; Delmas, H.; Julcour, C. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste)water. Ultrason. Sonochemistry 2017, 39, 889–896. [Google Scholar] [CrossRef]
- Flyunt, R.; Leitzke, A.; Mark, G.; Mvula, E.; Reisz, E.; Schick, R.; von Sonntag, C. Determination of •OH, O2•-, and Hydroperoxide Yields in Ozone Reactions in Aqueous Solution. J. Phys. Chem. B 2003, 107, 7242–7253. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298. [Google Scholar] [CrossRef]
- Li, Y.; Shen, W.; Fu, S.; Yang, H.; Yu, G.; Wang, Y. Inhibition of bromate formation during drinking water treatment by adapting ozonation to electro-peroxone process. Chem. Eng. J. 2015, 264, 322–328. [Google Scholar] [CrossRef]
- Ghanbari, F.; Zirrahi, F.; Lin, K.-Y.A.; Kakavandi, B.; Hassani, A. Enhanced electro-peroxone using ultrasound irradiation for the degradation of organic compounds: A comparative study. J. Environ. Chem. Eng. 2020, 8, 104167. [Google Scholar] [CrossRef]
- Dey, A.; Gogate, P.R. Comparative study of different ultrasound based hybrid oxidation approaches for treatment of real effluent from coke oven plant. J. Environ. Manag. 2024, 352, 120095. [Google Scholar] [CrossRef]
- Darsinou, B.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Mantzavinos, D. Sono-activated persulfate oxidation of bisphenol A: Kinetics, pathways and the controversial role of temperature. Chem. Eng. J. 2015, 280, 623–633. [Google Scholar] [CrossRef]
- Son, H.-S.; Choi, S.-B.; Khan, E.; Zoh, K.-D. Removal of 1,4-dioxane from water using sonication: Effect of adding oxidants on the degradation kinetics. Water Res. 2006, 40, 692–698. [Google Scholar] [CrossRef]
- Somensi, C.A.; Simionatto, E.L.; Bertoli, S.L.; Wisniewski, A.; Radetski, C.M. Use of ozone in a pilot-scale plant for textile wastewater pre-treatment: Physico-chemical efficiency, degradation by-products identification and environmental toxicity of treated wastewater. J. Hazard. Mater. 2010, 175, 235–240. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Dhir, A. Treatment of real pharmaceutical wastewater using combined approach of Fenton applications and aerobic biological treatment. J. Photochem. Photobiol. A Chem. 2019, 376, 175–184. [Google Scholar] [CrossRef]
- Passatore, L.; Rossetti, S.; Juwarkar, A.A.; Massacci, A. Phytoremediation and bioremediation of polychlorinated biphenyls (PCBs): State of knowledge and research perspectives. J. Hazard. Mater. 2014, 278, 189–202. [Google Scholar] [CrossRef]
- Kamali, M.; Aminabhavi, T.M.; Tarelho, L.A.C.; Hellemans, R.; Cuypers, J.; Capela, I.; Costa, M.E.V.; Dewil, R.; Appels, L. Acclimatized activated sludge for enhanced phenolic wastewater treatment using pinewood biochar. Chem. Eng. J. 2022, 427, 131708. [Google Scholar] [CrossRef]
- Jiménez-Silva, V.A.; Santoyo-Tepole, F.; Ruiz-Ordaz, N.; Galíndez-Mayer, J. Study of the ibuprofen impact on wastewater treatment mini-plants with bioaugmented sludge. Process Saf. Environ. Prot. 2019, 123, 140–149. [Google Scholar] [CrossRef]
- Basheer, F.; Farooqi, I.H. Biodegradation of p-cresol by aerobic granules in sequencing batch reactor. J. Environ. Sci. 2012, 24, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M. Nonbiodegradable and Other Recalcitrant Molecules. Biotechnol. Bioeng. 1973, 15, 611–647. [Google Scholar] [CrossRef]
- Monteoliva-García, A.; Martín-Pascual, J.; Muñío, M.M.; Poyatos, J.M. Effects of carrier addition on water quality and pharmaceutical removal capacity of a membrane bioreactor—Advanced oxidation process combined treatment. Sci. Total Environ. 2020, 708, 135104. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Molina, R.; Rodríguez, I.; Pariente, M.I.; Segura, Y.; Melero, J.A. Techno-economical assessment of coupling Fenton/biological processes for the treatment of a pharmaceutical wastewater. J. Environ. Chem. Eng. 2018, 6, 485–494. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Segura, Y.; Cruz del Álamo, A.; Munoz, M.; Álvarez-Torrellas, S.; García, J.; Casas, J.A.; De Pedro, Z.M.; Martínez, F. A comparative study among catalytic wet air oxidation, Fenton, and Photo-Fenton technologies for the on-site treatment of hospital wastewater. J. Environ. Manag. 2021, 290, 112624. [Google Scholar] [CrossRef]
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Comparison between industrial and simulated textile wastewater treatment by AOPs—Biodegradability, toxicity and cost assessment. Chem. Eng. J. 2016, 306, 550–559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).