Retention and Antimicrobial Activity of Alginate-Encapsulated Bioactive Compounds from Leaves and Fruits of Myrtle (Myrtus communis L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Supercritical Fluid Extraction
2.4. Hydro-Alcoholic Extraction
2.5. Hydrodistillation
2.6. Preparation of the Microcapsules
2.7. Antimicrobial Activity
2.7.1. Determination of Antimicrobial Activity Using the Disc Diffusion Method
2.7.2. Determination of the Minimum Inhibitory Concentration (MIC)
2.8. Retention of Lipid Fraction
2.9. Retention of Phenolic Compounds
2.10. Retention of Essential Oil
2.11. Statistical Analysis
3. Results and Discussion
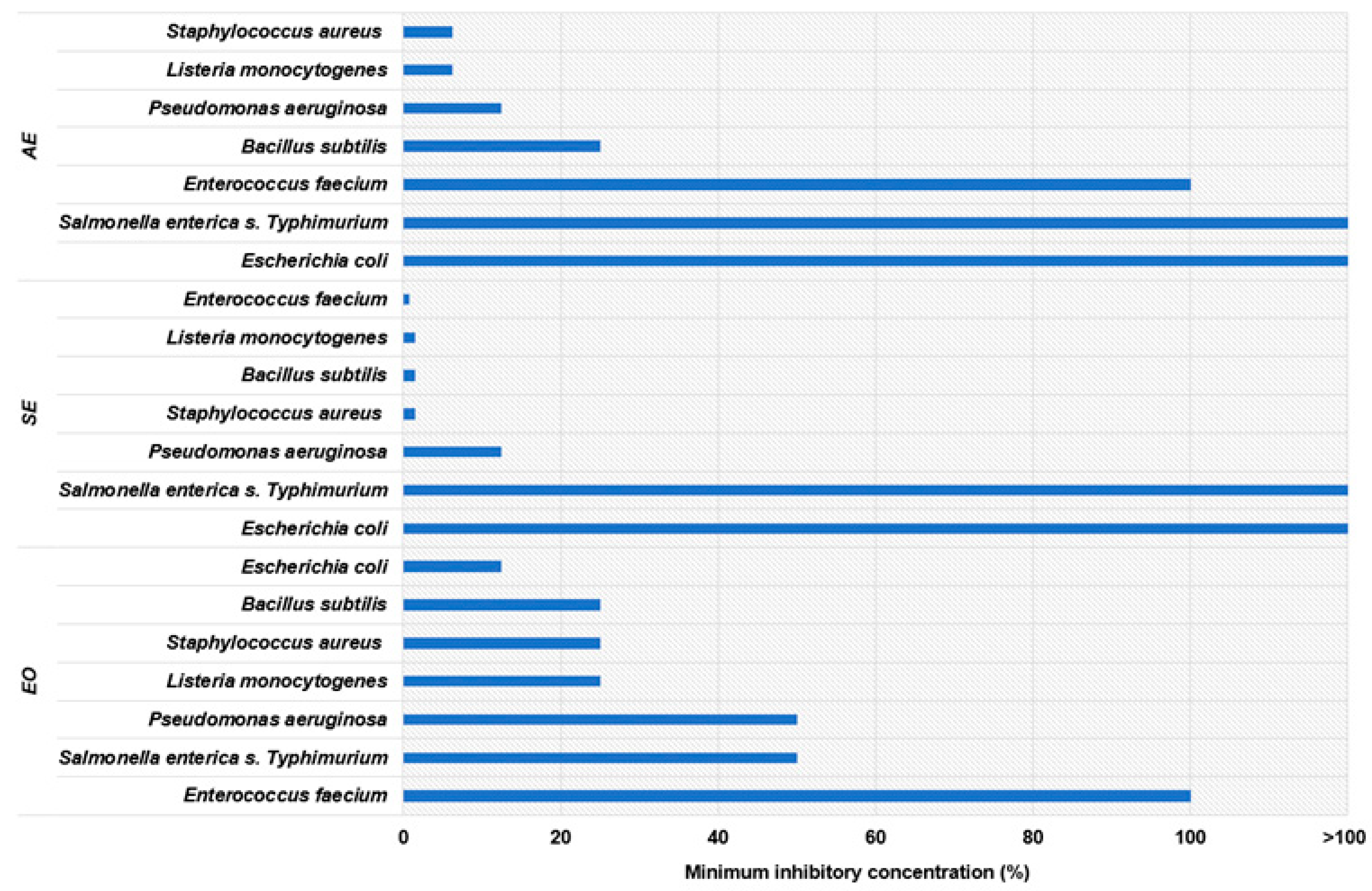
3.1. Antimicrobial Activity of Extracts
3.2. Efficiency of Microencapsulation of Volatiles, Phenolics and Lipids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aleksic, V.; Knezevic, P. Antimicrobial and Antioxidative Activity of Extracts and Essential Oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Cvitković, D.; Lisica, P.; Zorić, Z.; Repajić, M.; Pedisić, S.; Dragović-Uzelac, V.; Balbino, S. Composition and Antioxidant Properties of Pigments of Mediterranean Herbs and Spices as Affected by Different Extraction Methods. Foods 2021, 10, 2477. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Al Juhaimi, F.; Ahmed, I.A.M.; Babiker, E.E.; Ghafoor, K. Antioxidant Activity, Fatty Acid Composition, Phenolic Compounds and Mineral Contents of Stem, Leave and Fruits of Two Morphs of Wild Myrtle Plants. J. Food Meas. Charact. 2020, 14, 1376–1382. [Google Scholar] [CrossRef]
- Öztürk, H.İ.; Demirci, T.; Akın, N. Production of Functional Probiotic Ice Creams with White and Dark Blue Fruits of Myrtus communis: The Comparison of the Prebiotic Potentials on Lactobacillus Casei 431 and Functional Characteristics. LWT Food Sci. Technol. 2018, 90, 339–345. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as Sources of New Antimicrobials and Resistance-Modifying Agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Wagner, H. Synergy Research: Approaching a New Generation of Phytopharmaceuticals. Fitoterapia 2009, 82, 34–37. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The Resistance Mechanisms of Bacteria against Ciprofloxacin and New Approaches for Enhancing the Efficacy of This Antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I.; Owais, M. Evaluation of Anti-Methicillin-Resistant Staphylococcus Aureus (MRSA) Activity and Synergy of Some Bioactive Plant Extracts. Biotechnol. J. 2006, 1, 1093–1102. [Google Scholar] [CrossRef]
- Long, F.; Yang, H.; Xu, Y.; Hao, H.; Li, P. A Strategy for the Identification of Combinatorial Bioactive Compounds Contributing to the Holistic Effect of Herbal Medicines. Sci. Rep. 2015, 5, srep12361. [Google Scholar] [CrossRef]
- Chawda, P.J.; Shi, J.; Xue, S.; Young Quek, S. Co-Encapsulation of Bioactives for Food Applications. Food Qual. Saf. 2017, 1, 302–309. [Google Scholar] [CrossRef]
- Taheri, A.; Seyfan, A.; Jalalinezhad, S.; Nasery, F. Antibacterial Effect of Myrtus communis Hydro-Alcoholic Extract on Pathogenic Bacteria. Zahedan J. Res. Med. Sci. 2013, 15, 19–24. [Google Scholar]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of Microencapsulated Essential Oils in Cosmetic and Personal Healthcare Products—A Review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of Essential Oils with Biodegradable Polymeric Carriers for Cosmetic Applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Champagne, C.P.; Fustier, P. Microencapsulation for the Improved Delivery of Bioactive Compounds into Foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Okuro, P.K.; Thomazini, M.; Balieiro, J.C.C.; Liberal, R.D.C.O.; Fávaro-Trindade, C.S. Co- Encapsulation of Lactobacillus Acidophilus with Inulin or Polydextrose in Solid Lipid Microparticles Provides Protection and Improves Stability. Food Res. Int. 2013, 53, 96–103. [Google Scholar] [CrossRef]
- Iyer, C.; Kailasapathy, K. Effect of Co-Encapsulation of Probiotics with Prebiotics on Increasing the Viability of Encapsulated Bacteria under In Vitro Acidic and Bile Salt Conditions and in Yogurt. J. Food Sci. 2005, 70, M18–M23. [Google Scholar] [CrossRef]
- Sathyabama, S.; Ranjith kumar, M.; Bruntha devi, P.; Vijayabharathi, R. Brindha priyadharisini Co-Encapsulation of Probiotics with Prebiotics on Alginate Matrix and Its Effect on Viability in Simulated Gastric Environment. LWT Food Sci. Technol. 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Eratte, D.; McKnight, S.; Gengenbach, T.R.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. Co-Encapsulation and Characterisation of Omega-3 Fatty Acids and Probiotic Bacteria in Whey Protein Isolate-Gum Arabic Complex Coacervates. J. Funct. Foods 2015, 19, 882–892. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Marathe, S.J.; Singhal, R.S. Co-Encapsulation of Vitamins B12 and D3 Using Spray Drying: Wall Material Optimization, Product Characterization, and Release Kinetics. Food Chem. 2021, 335, 127642. [Google Scholar] [CrossRef]
- Koushki, V.; Babaei, A.; Mehraban Sangatash, M.; Safari, O. The Efficacy of Co-Encapsulation with Herbal Extracts on Viability of Probiotic Bacteria During Storage in Fruit Juices. J. Innov. Food Sci. Technol. 2021, 13, 1. [Google Scholar]
- Le Priol, L.; Gmur, J.; Dagmey, A.; Morandat, S.; El Kirat, K.; Saleh, K.; Nesterenko, A. Co-Encapsulation of Vegetable Oils with Phenolic Antioxidants and Evaluation of Their Oxidative Stability under Long-Term Storage Conditions. LWT 2021, 142, 111033. [Google Scholar] [CrossRef]
- Chen, Q.; McGillivray, D.; Wen, J.; Zhong, F.; Quek, S.Y. Co-Encapsulation of Fish Oil with Phytosterol Esters and Limonene by Milk Proteins. J. Food Eng. 2013, 50, 505–512. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of Bioactive Compounds by “Extrusion” Technologies: A Review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3100–3118. [Google Scholar] [CrossRef]
- Poshadri, A.; Kuna, A. Microencapsulation Technology: A Review. J. Res. ANGRAU 2010, 38, 86–102. [Google Scholar] [CrossRef]
- Manojlovic, V.; Rajic, N.; Djonlagic, J.; Obradovic, B.; Nedovic, V.; Bugarski, B. Application of Electrostatic Extrusion—Flavour Encapsulation and Controlled Release. Sensors 2008, 8, 1488–1496. [Google Scholar] [CrossRef]
- Aguilar, N.N. Study of the Influence of Buchi Encapsulator Input Variables on Properties of Formed Alginate Beads. Ph.D. Thesis, Universitat de Barcelona, Barcelona, Spain, 2018. [Google Scholar]
- Choudhury, N.; Meghwal, M.; Das, K. Microencapsulation: An Overview on Concepts, Methods, Properties and Applications in Foods. Food Front. 2021, 2, 426–442. [Google Scholar] [CrossRef]
- Cvitković, D.; Lisica, P.; Zorić, Z.; Pedisić, S.; Repajić, M.; Dragović-Uzelac, V.; Balbino, S. The Influence of Cryogrinding on Essential Oil, Phenolic Compounds and Pigments Extraction from Myrtle (Myrtus communis L.) Leaves. Processes 2022, 10, 2716. [Google Scholar] [CrossRef]
- HRN EN ISO 659; Oilseeds—Determination of Oil Content (Reference Method). Croatian Standards Institute: Zagreb, Croatia, 2010.
- Amensour, M.; Bouhdid, S.; Fernández-López, J.; Idaomar, M.; Senhaji, N.S.; Abrini, J. Antibacterial Activity of Extracts of Myrtus communis against Food-Borne Pathogenic and Spoilage Bacteria. Int. J. Food Prop. 2010, 13, 1215–1224. [Google Scholar] [CrossRef]
- Özcan, M.M.; Uyar, B.; Ünver, A. Antibacterial Effect of Myrtle (Myrtus communis L.) Leaves Extract on Microorganisms. J. Food Saf. Food Qual. 2015, 66, 18–21. [Google Scholar] [CrossRef]
- Pereira, P.; Bernardo-Gil, M.G.; Cebola, M.J.; Mauricio, E.; Romano, A. Supercritical Fluid Extracts with Antioxidant and Antimicrobial Activities from Myrtle (Myrtus communis L.) Leaves. Response Surface Optimization. J. Supercrit. Fluids 2013, 83, 57–64. [Google Scholar] [CrossRef]
- Pereira, P.; Mauricio, E.M.; Duarte, M.P.; Lima, K.; Fernandes, A.S.; Bernardo-Gil, G.; Cebola, M.-J. Potential of Supercritical Fluid Myrtle Extracts as an Active Ingredient and Co-Preservative for Cosmetic and Topical Pharmaceutical Applications. Sustain. Chem. Pharm. 2022, 28, 100739. [Google Scholar] [CrossRef]
- Hsouna, A.; Ben; Hamdi, N.; Miladid, R.; Abdelkafid, S. Myrtus communis Essential Oil: Chemical Composition and Antimicrobial Activities against Food Spoilage Pathogens. Chem. Biodivers. 2014, 11, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Cherrat, L.; Espina, L.; Bakkali, M.; García-Gonzalo, D.; Pagán, R.; Laglaoui, A. Chemical Composition and Antioxidant Properties of Laurus nobilis L. and Myrtus communis L. Essential Oils from Morocco and Evaluation of Their Antimicrobial Activity Acting Alone or in Combined Processes for Food Preservation. J. Sci. Food Agric. 2014, 94, 1197–1204. [Google Scholar] [CrossRef]
- Alyousef, A.A. Antifungal Activity and Mechanism of Action of Different Parts of Myrtus communis Growing in Saudi Arabia against Candida Spp. J. Nanomater. 2021, 2021, 3484125. [Google Scholar] [CrossRef]
- Bonjar, G.H.S.; Aghighi, S.; Nik, A.K. Antibacterial and Antifungal Survey in Plants Used in Indigenous Herbal-Medicine of South East Regions of Iran. J. Biol. Sci. 2004, 4, 405–412. [Google Scholar] [CrossRef]
- Hassan, A.A.; Abd-Elazis, G.O. Genotoxicity and Antimicrobial Activity of Myrtus communis L., Ziziphus spina-Christi (L.) Willd and Cassia Angustifolia Vahl Extracts. Bangladesh J. Bot. 2020, 49, 557–566. [Google Scholar] [CrossRef]
- Nejad, B.S.; Nejad, M.E.; Naanaie, S.Y.; Zarrin, M. Antifungal Efficacy of Myrtus communis Linn. Jentashapir J. Health Res. 2014, 5, e21879. [Google Scholar] [CrossRef]
- Ghasemi, P.A.; Jahanbazi, P.; Enteshari, S.; Malekpoor, F.; Hamedi, B. Antimicrobial Activity of Some Iranian Medicinal Plants. Arch. Biol. Sci. 2010, 62, 633–641. [Google Scholar] [CrossRef]
- Rasooli, I.; Moosavi, M.L.; Rezaee, M.B.; Jaimand, K. Susceptibility of Microorganisms to Myrtus communis L. Essential Oil and Its Chemical Composition. J. Agric. Sci. Technol. 2002, 4, 127–133. [Google Scholar]
- Mahboubi, M.; Bidgoli, F.G. In Vitro Synergistic Efficacy of Combination of Amphotericin B with Myrtus communis Essential Oil against Clinical Isolates of Candida albicans. Phytomedicine 2010, 17, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Curini, M.; Bianchi, A.; Epifano, F.; Bruni, R.; Torta, L.; Zambonelli, A. Composition and in Vitro Antifungal Activity of Essential Oils of Erigeron Canadensis and Myrtus communis from France. Chem. Nat. Compd. 2003, 39, 191–194. [Google Scholar] [CrossRef]
- Kordali, S.; Kotan, R.; Cakir, A. Screening of Antifungal Activities of 21 Oxygenated Monoterpenes In-Vitro as Plant Disease Control Agents. Allelopath. J. 2007, 19, 373–392. [Google Scholar]
- Berendika, M.; Domjanić Drozdek, S.; Odeh, D.; Oršolić, N.; Dragičević, P.; Sokolović, M.; Garofulić, I.E.; Đikić, D.; Jurčević, I.L. Beneficial Effects of Laurel (Laurus nobilis L.) and Myrtle (Myrtus communis L.) Extract on Rat Health. Molecules 2022, 27, 581. [Google Scholar] [CrossRef]
- Ghazanfari, S.; Moradi, M.A.; Bardzardi, M.M. Intestinal Morphology and Microbiology of Broiler Chicken Fed Diets Containing Myrtle (Myrtus communis) Essential Oil Supplementation. Iran. J. Appl. Anim. Sci. 2014, 4, 549–554. [Google Scholar]
- Mir, M.A.; Bashir, N.; Alfaify, A.; Oteef, M.D.Y. GC-MS Analysis of Myrtus communis Extract and Its Antibacterial Activity against Gram-Positive Bacteria. BMC Complement. Med. Ther. 2020, 20, 86. [Google Scholar] [CrossRef]
- Mohamadi, Y.; Lograda, T.; Ramdani, M.; Figueredo, G.; Chalard, P. Chemical Composition and Antimicrobial Activity of Myrtus communis Essential Oils from Algeria. Biodiversitas J. Biol. Divers. 2021, 22, 933–946. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Hamedi, B.; Mehravar, L.; Firouznejhad, M. Diversity in Chemical Composition and Antibacterial Activity of the Essential Oils of Wild Populations of Myrtle from Natural Habitats in Southwestern Iran. Indian. J. Tradit. Knowl. 2014, 13, 484–489. [Google Scholar]
- Yadegarinia, D.; Gachkar, L.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Biochemical Activities of Iranian Mentha piperita L. and Myrtus communis L. Essential Oils. Phytochemistry 2006, 67, 1249–1255. [Google Scholar] [CrossRef]
- Anges, H.M. Development of Novel Nanoemulsions as Delivery Systems. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2016. [Google Scholar]
- Shen, W.; Koirala, N.; Mukherjee, D.; Lee, K.; Zhao, M.; Li, J. Tween 20 Stabilized Conventional Heavy Crude Oil-In-Water Emulsions Formed by Mechanical Homogenization. Front. Environ. Sci. 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Noppakundilograt, S.; Piboon, P.; Graisuwan, W.; Nuisin, R.; Kiatkamjornwong, S. Encapsulated Eucalyptus Oil in Ionically Cross-Linked Alginate Microcapsules and Its Controlled Release. Carbohydr. Polym. 2015, 131, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, L.; Eddy, D.R.; Al Anshori, J.; Harja, A.; Wahyudi, T.; Mulyawan, A.S.; Julaeha, E. Microencapsulation of Citrus Aurantifolia Essential Oil with the Optimized CaCl2 Crosslinker and Its Antibacterial Study for Cosmetic Textiles. RSC Adv. 2022, 12, 30682–30690. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, R.; Belscak-Cvitanovic, A.; Manojlovic, V.; Komes, D.; Nedovic, V.; Bugarski, B. Encapsulation of Thyme (Thymus Serpyllum L.) Aqueous Extract in Calcium Alginate Beads. J. Sci. Food Agric. 2012, 92, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Dobroslavić, E.; Cegledi, E.; Robić, K.; Elez Garofulić, I.; Dragović-Uzelac, V.; Repajić, M. Encapsulation of Fennel Essential Oil in Calcium Alginate Microbeads via Electrostatic Extrusion. Appl. Sci. 2024, 14, 3522. [Google Scholar] [CrossRef]
- Napiórkowska, A.; Szpicer, A.; Wojtasik-Kalinowska, I.; Perez, M.D.T.; González, H.D.; Kurek, M.A. Microencapsulation of Juniper and Black Pepper Essential Oil Using the Coacervation Method and Its Properties after Freeze-Drying. Foods 2023, 12, 4345. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Đorđević, V.; Karlović, S.; Pavlović, V.; Komes, D.; Ježek, D.; Bugarski, B.; Nedović, V. Protein-Reinforced and Chitosan-Pectin Coated Alginate Microparticles for Delivery of Flavan-3-Ol Antioxidants and Caffeine from Green Tea Extract. Food Hydrocoll. 2015, 51, 361–374. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Hubinger, M.D. Hydrophilic Food Compounds Encapsulation by Ionic Gelation. Curr. Opin. Food Sci. 2017, 15, 50–55. [Google Scholar] [CrossRef]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Retention and Release of Oil-in-Water Emulsions from Filled Hydrogel Beads Composed of Calcium Alginate: Impact of Emulsifier Type and PH. Soft Matter 2015, 11, 2228–2236. [Google Scholar] [CrossRef]
- Lupo, B.; Maestro, A.; Porras, M.; Gutiérrez, J.M.; González, C. Preparation of Alginate Microspheres by Emulsification/Internal Gelation to Encapsulate Cocoa Polyphenols. Food Hydrocoll. 2014, 38, 56–65. [Google Scholar] [CrossRef]
- Won, K.; Kim, S.; Kim, K.-J.; Park, H.W.; Moon, S.-J. Optimization of Lipase Entrapment in Ca-Alginate Gel Beads. Process Biochem. 2005, 40, 2149–2154. [Google Scholar] [CrossRef]
- Tunsirikongkon, A.; Pyo, Y.-C.; Kim, D.-H.; Tran, P.; Park, J.-S. Effect of Calcium Chloride on the Protein Encapsulation and Stability of Proliposomal Granules. J. Drug Deliv. Sci. Technol. 2020, 57, 101672. [Google Scholar] [CrossRef]

| Exp. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 28 | 38 | 48 | 48 | 48 | 38 | 28 | 38 | 38 | 38 | 38 | 28 | 38 | 38 | 38 | 48 | 28 |
| Emulsifier (g) | 1 | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | 1.5 | 0.5 | 1.5 | 1 | 0.5 | 1.5 | 1.5 |
| CaCl2 (%) | 3 | 6 | 9 | 3 | 6 | 9 | 9 | 6 | 6 | 6 | 9 | 6 | 3 | 6 | 3 | 6 | 6 |
| MO | AE | SE | EO | DMSO 10 µL | Kanamycin (10 mg L−1)/5 µL |
|---|---|---|---|---|---|
| Staphylococcus aureus * | 19.75 b ± 3.18 | 12.25 a ± 0.35 | 18.75 b ± 0.35 | nd | 30.25 c ± 1.77 |
| Bacillus subtilis * | 11.50 b ± 4.94 | 8.75 a ± 0.35 | 12.00 b ± 2.12 | nd | 22.25 c ± 5.67 |
| Enterococcus faecium * | 25.00 c ± 2.12 | 10.00 b ± 4.24 | 7.75 a ± 0.35 | nd | 21.50 c ± 2.83 |
| Listeria monocytogenes * | 35.25 c ± 0.35 | 7.75 a ± 1.77 | 20.75 b ± 4.60 | nd | 32.50 c ± 4.95 |
| Pseudomonas aeruginosa * | 8.75 a ± 3.18 | 8.00 a ± 2.83 | 16.00 b ± 5.65 | nd | 32.00 c ± 4.24 |
| Escherichia coli * | 6.50 a ± 1.13 | 6.50 a ± 0.71 | 28.00 b ± 2.83 | nd | 40.50 c ± 0.71 |
| Salmonella enterica s. Typhimurium * | 6.50 a ± 0.71 | 6.00 a ± 1.41 | 8.50 b ± 0.71 | nd | 23.25 c ± 2.47 |
| MO | AE | SE | EO | DMSO 10 µL | Nystatin (5 mg mL−1)/10 µL |
| Candida albicans * | nd | 19.5 c ± 3.54 | 15.25 a,b ± 4.60 | nd | 17.00 b ± 0.00 |
| Saccharomyces cerevisiae * | nd | 11.25 a ± 3.18 | 18.00 b ± 5.31 | nd | 25.00 c ± 0.00 |
| Candida utilis * | nd | 20.00 a ± 0.00 | 20.5 a ± 2.12 | nd | 25.00 b ± 0.00 |
| Rhodotorula sp. * | nd | 21.25 b ± 3.89 | 10.25 a ± 6.01 | nd | 21.00 b ± 0.00 |
| MO | AE | SE | EO | DMSO 10 µL | Kanamycin (50 mg L−1)/5 µL |
| Lactobacillus brevis * | nd | 9.00 a ± 1.50 | nd | nd | 17.00 b ± 2.10 |
| Lactobacillus plantarum | nd | nd | nd | nd | 10.00 ± 2.34 |
| Lactobacillus kimchi | nd | nd | nd | nd | nd |
| Exp. | Temperature (°C) | Emulsifier (g) | CaCl2 (%) | Volatiles Retention (%) | Phenolics Retention (%) | Lipids Retention (%) |
|---|---|---|---|---|---|---|
| 1 | 28 | 1 | 3 | 77.07 | 37.65 | 48.73 |
| 2 | 38 | 1 | 6 | 74.36 | 42.75 | 22.21 |
| 3 | 48 | 1 | 9 | 77.22 | 46.99 | 27.86 |
| 4 | 48 | 1 | 3 | 77.25 | 47.65 | 40.64 |
| 5 | 48 | 0.5 | 6 | 69.03 | 23.66 | 16.21 |
| 6 | 38 | 0.5 | 9 | 69.15 | 47.24 | 14.69 |
| 7 | 28 | 1 | 9 | 83.66 | 55.16 | 25.15 |
| 8 | 38 | 1 | 6 | 72.56 | 47.49 | 20.17 |
| 9 | 38 | 1 | 6 | 74.34 | 40.03 | 23.73 |
| 10 | 38 | 1 | 6 | 72.58 | 45.51 | 17.79 |
| 11 | 38 | 1.5 | 9 | 71.27 | 61.41 | 29.79 |
| 12 | 28 | 0.5 | 6 | 75.45 | 33.14 | 23.68 |
| 13 | 38 | 1.5 | 3 | 62.89 | 62.66 | 62.80 |
| 14 | 38 | 1 | 6 | 71.97 | 48.59 | 22.62 |
| 15 | 38 | 0.5 | 3 | 71.46 | 12.89 | 26.16 |
| 16 | 48 | 1.5 | 6 | 64.96 | 62.96 | 36.17 |
| 17 | 28 | 1.5 | 6 | 66.24 | 43.01 | 37.32 |
| Source of Variation | Volatiles Retention (%) | Phenolics Retention (%) | Lipids Retention (%) | |||
|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | |
| A | 15.37 | 0.006 * | 0.90 | 0.364 | 2.36 | 0.168 |
| B | 30.76 | 0.001 * | 76.56 | <0.001 * | 87.47 | <0.001 * |
| C | 12.57 | 0.009 * | 14.92 | 0.003 * | 78.51 | <0.001 * |
| AB | 4.16 | 0.081 | 10.37 | 0.009 * | 0.96 | 0.360 |
| AC | 6.92 | 0.034 * | 3.95 | 0.075 | 2.80 | 0.138 |
| BC | 18.03 | 0.004 * | 15.17 | 0.003 * | 11.14 | 0.012 |
| A2 | 22.86 | 0.002 * | - | - | 8.71 | 0.021 |
| B2 | 136.85 | <0.001 * | - | - | 2.34 | 0.170 |
| C2 | 19.44 | 0.003 * | - | - | 37.70 | <0.001 * |
| Lack of fit | 0.313 | 0.236 | 0.154 | |||
| R2 | 0.974 | 0.924 | 0.971 | |||
| Model | Volatiles retention (%) = 113.90 − 2.33 × A + 32.02 × B − 2.76 × C + 0.26 × A × B − 0.06 × A × C + 1.78 × B × C + 0.03 × A2− 28.70 × B2 + 0.30 × C2 | Phenolics retention (%) = −16.17 − 0.41 × A + 7.95 × B + 13.77 × C + 1.47 × A × B − 0.15 × A × C − 5.93 × B × C | Lipids retention (%) = 153.03 − 4.56 × A + 11.65 × B − 16.07 × C + 0.32 × A × B + 0.09 × A × C − 3.59 × B × C + 0.05 × A2 + 9.61 × B2+ 1.07 × C2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cvitković, D.; Mrvčić, J.; Dobroslavić, E.; Dragović-Uzelac, V.; Balbino, S. Retention and Antimicrobial Activity of Alginate-Encapsulated Bioactive Compounds from Leaves and Fruits of Myrtle (Myrtus communis L.). Processes 2025, 13, 1220. https://doi.org/10.3390/pr13041220
Cvitković D, Mrvčić J, Dobroslavić E, Dragović-Uzelac V, Balbino S. Retention and Antimicrobial Activity of Alginate-Encapsulated Bioactive Compounds from Leaves and Fruits of Myrtle (Myrtus communis L.). Processes. 2025; 13(4):1220. https://doi.org/10.3390/pr13041220
Chicago/Turabian StyleCvitković, Daniela, Jasna Mrvčić, Erika Dobroslavić, Verica Dragović-Uzelac, and Sandra Balbino. 2025. "Retention and Antimicrobial Activity of Alginate-Encapsulated Bioactive Compounds from Leaves and Fruits of Myrtle (Myrtus communis L.)" Processes 13, no. 4: 1220. https://doi.org/10.3390/pr13041220
APA StyleCvitković, D., Mrvčić, J., Dobroslavić, E., Dragović-Uzelac, V., & Balbino, S. (2025). Retention and Antimicrobial Activity of Alginate-Encapsulated Bioactive Compounds from Leaves and Fruits of Myrtle (Myrtus communis L.). Processes, 13(4), 1220. https://doi.org/10.3390/pr13041220








