The Impact of Particle Size on the Electrical Resistivity of Burden in the Upper Zone of an EAF During Metallurgical-Grade Silicon Smelting
Abstract
1. Introduction
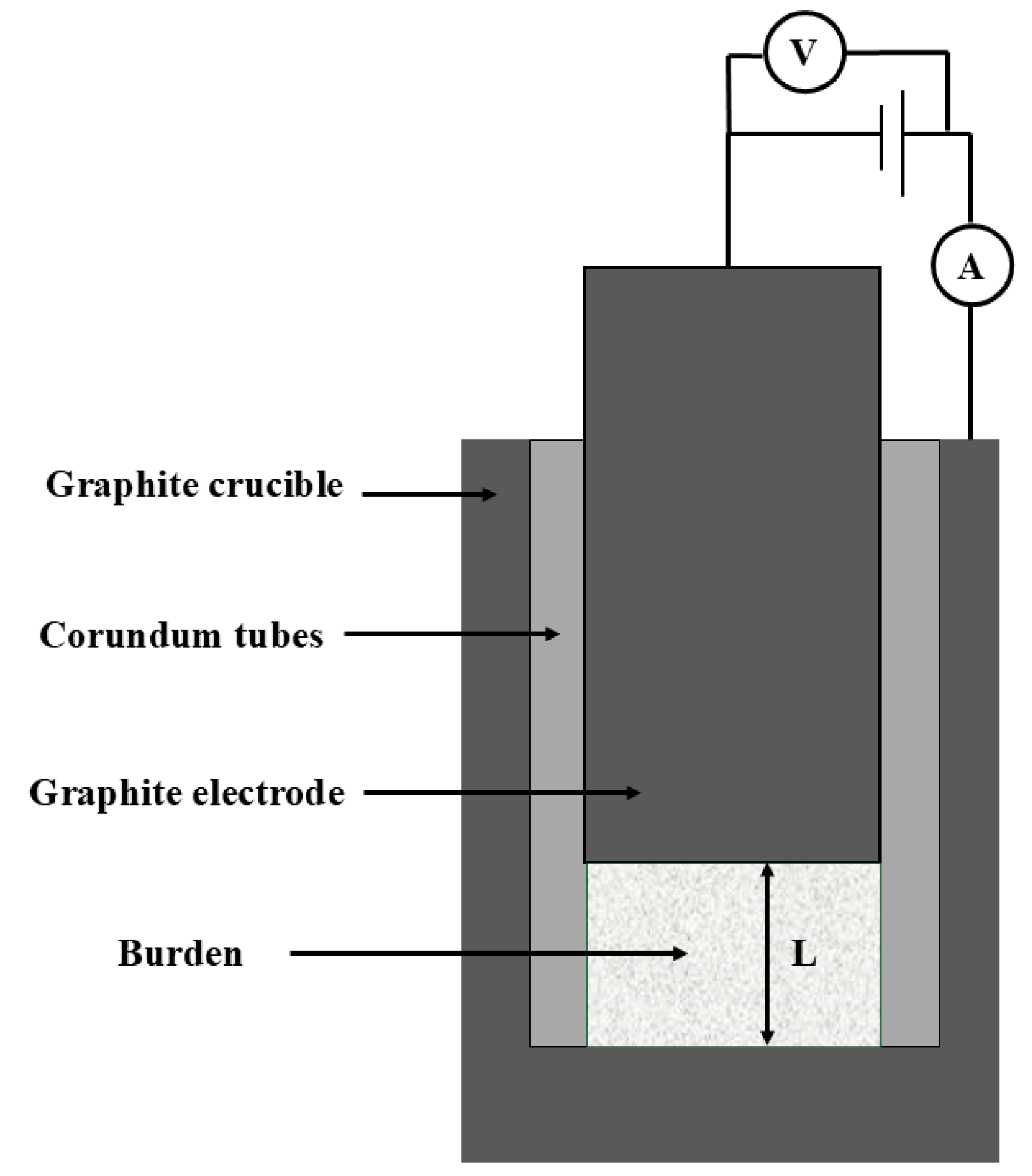
2. Experiment
2.1. Materials
2.2. Experimental Methods
2.3. Contact Resistance
2.4. Characterization Methods
2.4.1. Industrial Analyses
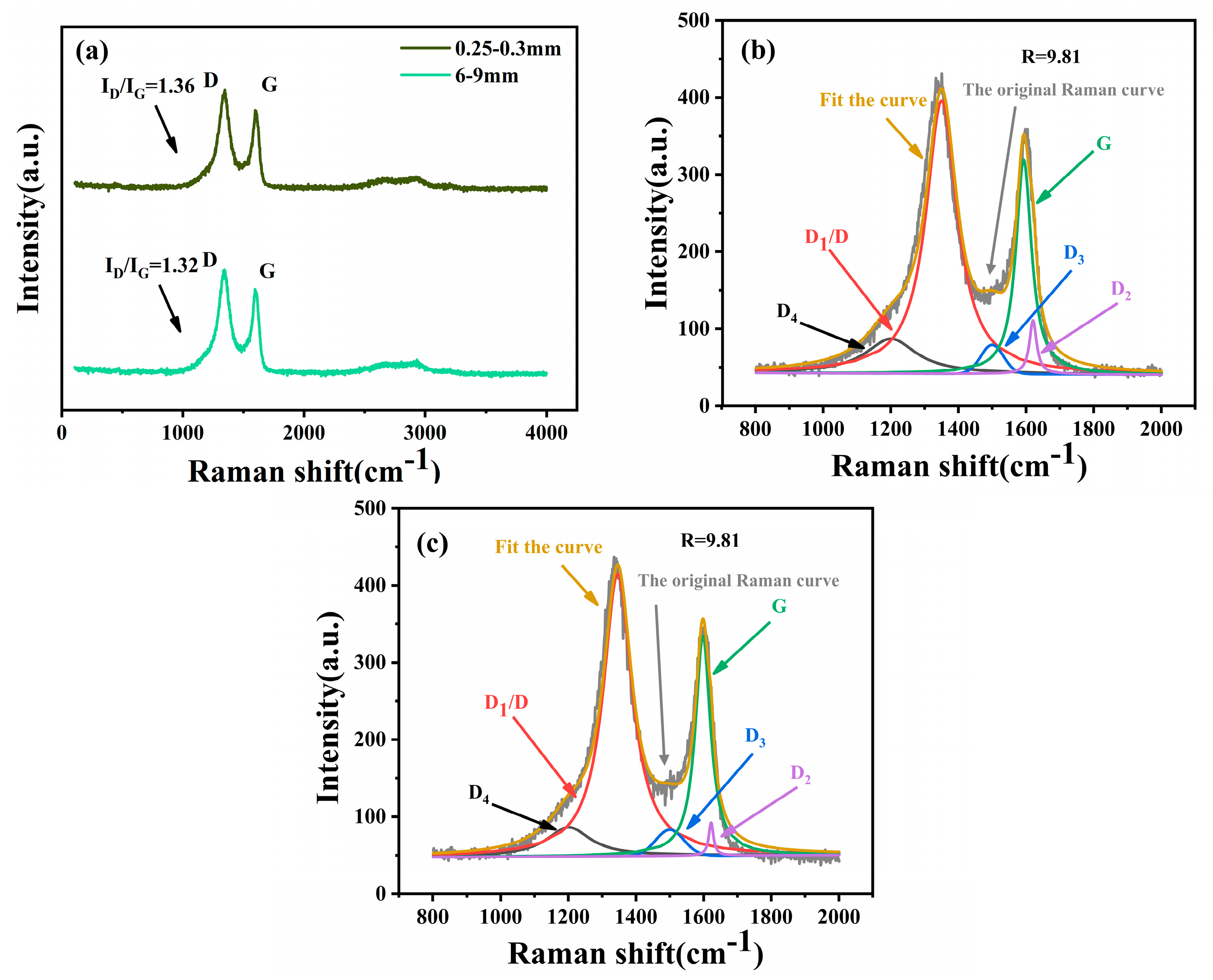
2.4.2. Raman Spectral Analysis
2.4.3. Infrared Spectral Analysis
2.4.4. Specific Surface and Porosity Analysis
2.4.5. X-Ray Fluorescence Spectrometer
3. Results and Analyses
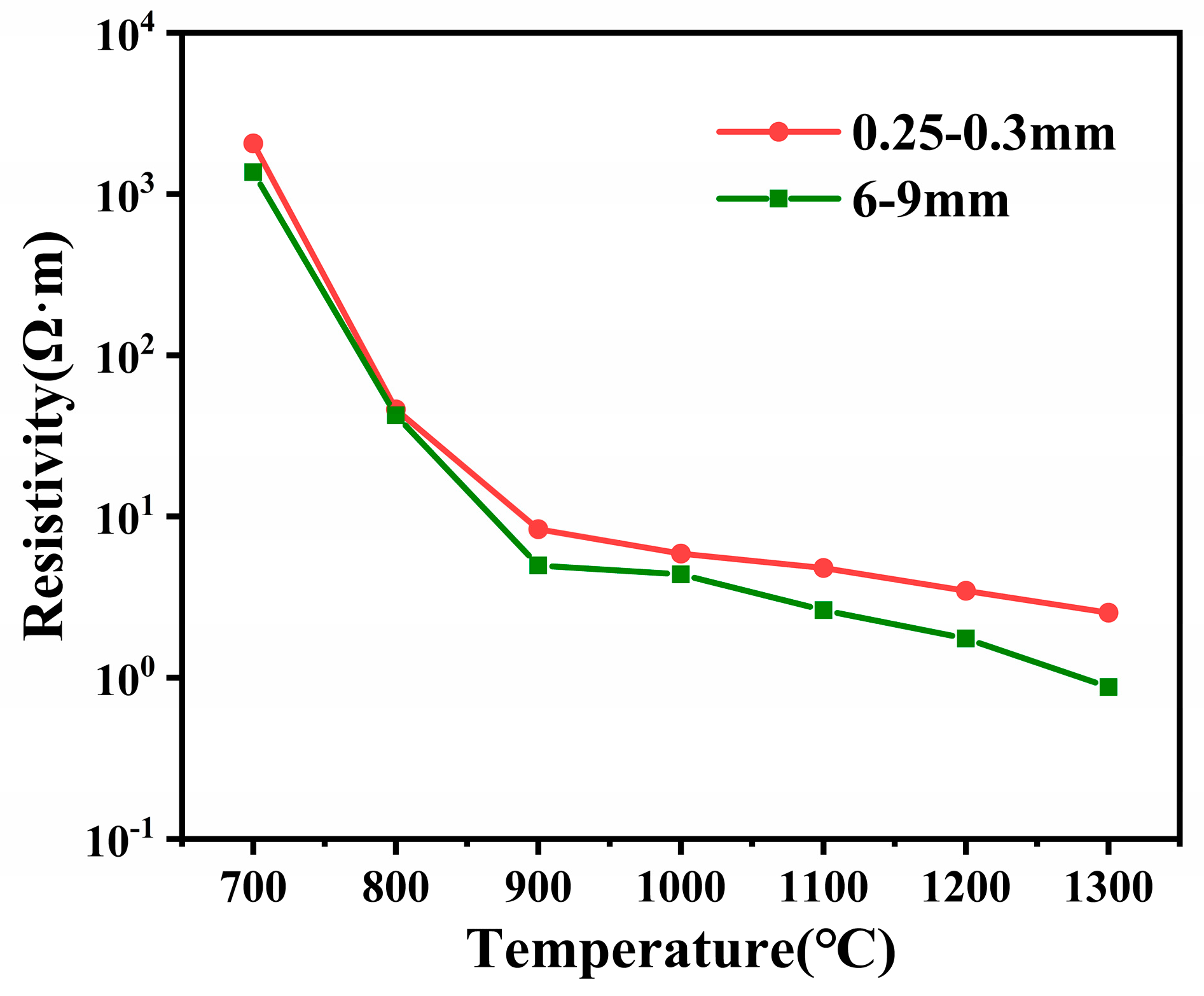
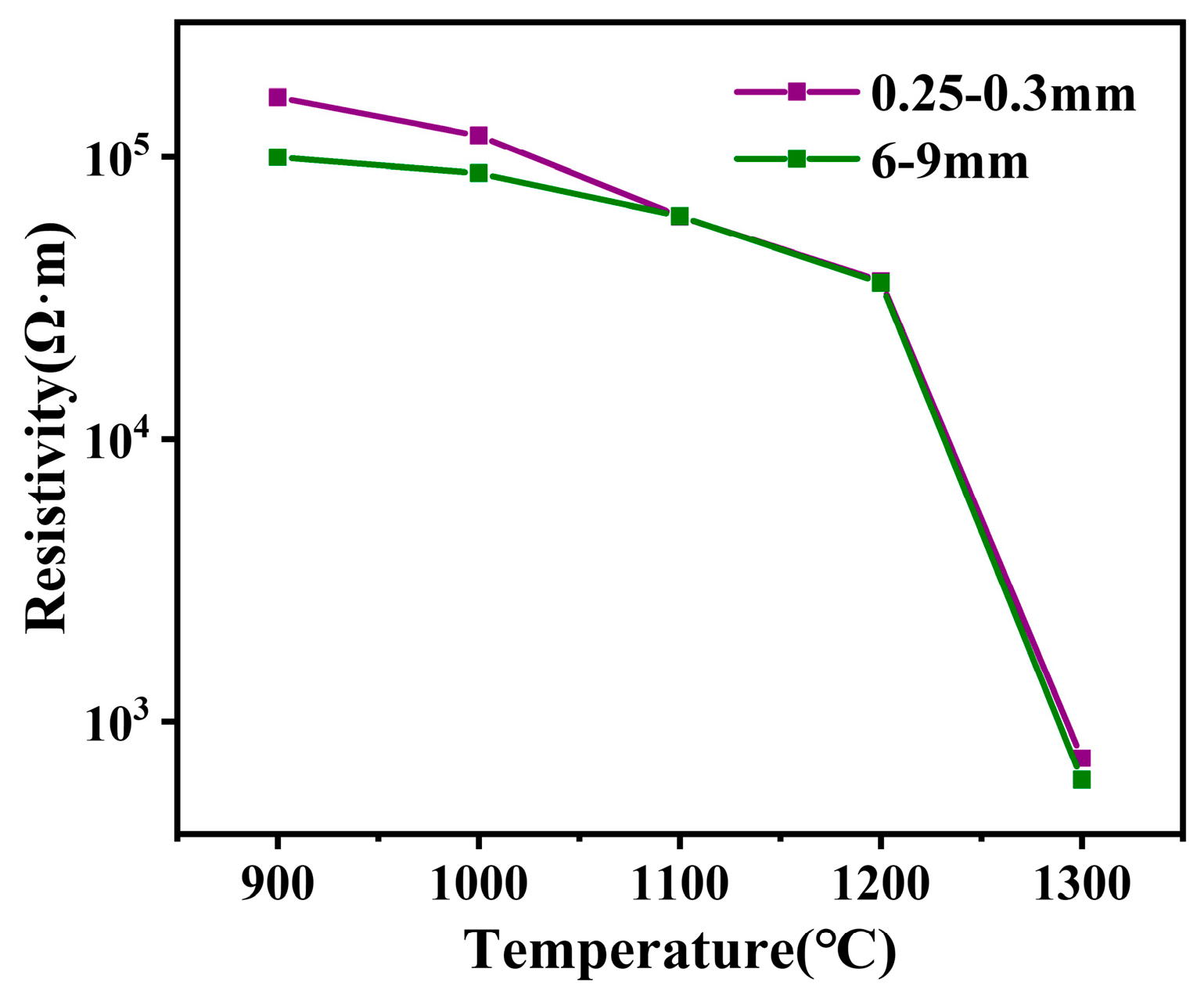
3.1. Results of Resistivity of Different Grain Sizes of Charge at High Temperature
3.2. Effect of Particle Size on Coal Resistivity
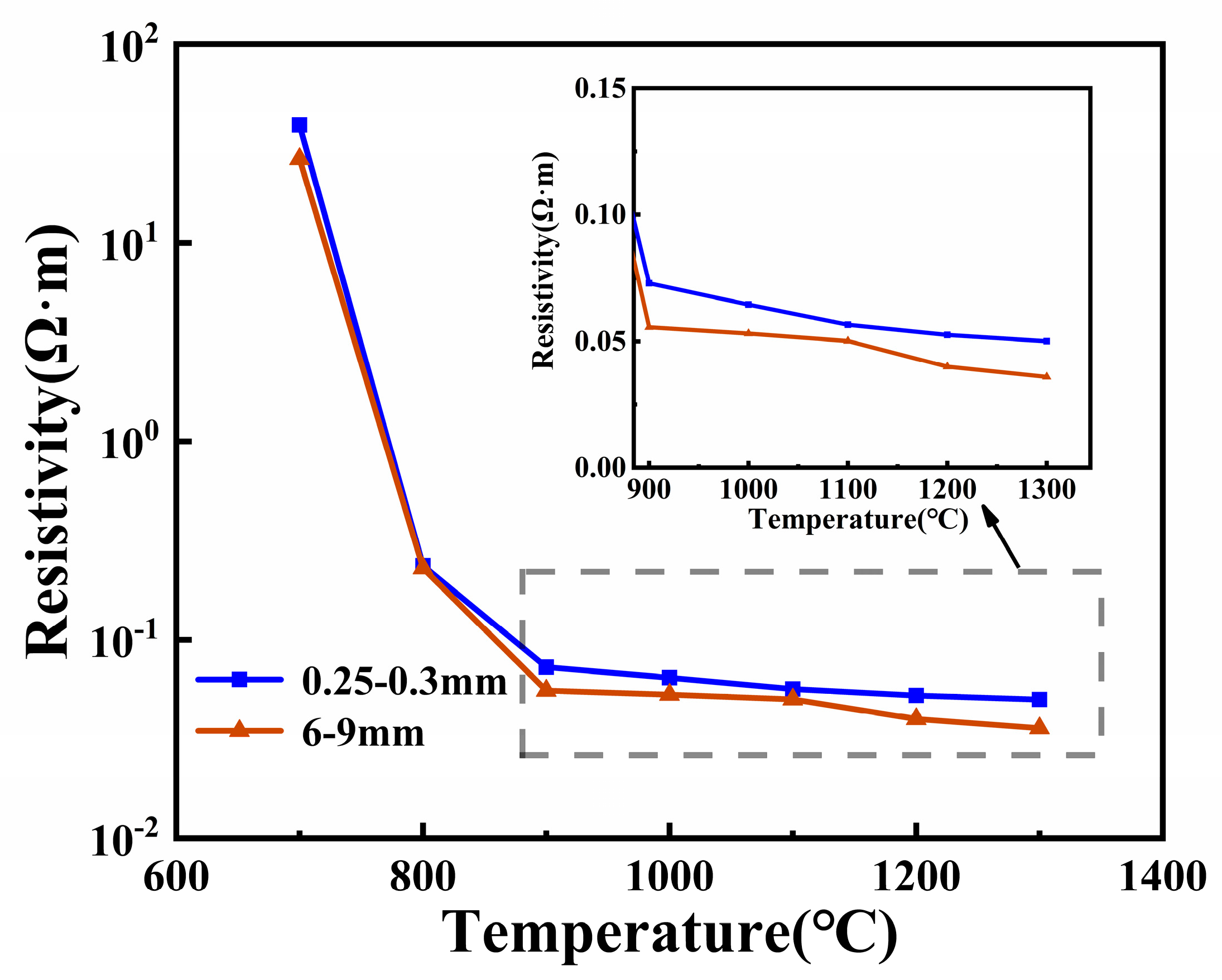
3.3. Effect of Particle Size on the Resistivity of Silica Ores
3.4. Mechanism of Particle Size Effect on High-Temperature Resistivity of the Furnace Charge
3.4.1. Mechanisms of Inherent Resistivity of the Furnace Charge After Furnace Charge Mixing
3.4.2. Mechanism of Furnace Charge in Furnace Charge Contact Resistance
4. Conclusions
- 1.
- An increase in particle sizes from 0.25–0.3mm to 6–9 mm and a 64% reduction in resistivity were observed. The effect of particle size on the resistivity of the furnace charge can be divided into two parts: one is to change the physical and chemical properties of coal through the particle size to affect the inherent resistivity of the furnace charge, and the other is to change the height of the particle stack and the number of contact points to affect the contact resistance between the furnace charge.
- 2.
- Particle size changes the inherent resistivity of coal by affecting its degree of graphitization-like behavior. When the particle size of coal decreases from 6–9 mm to 0.25–0.3 mm, the degree of graphitization-like behavior (ID/IG) increases from 1.32 to 1.36 and the structural ordering decreases. This resulted in an increase in the electrical resistivity of the coal of about 29 percent.
- 3.
- An increase in particle size from 0.25–0.3 mm to 6–9 mm and a 2-fold reduction in the contact resistance of the furnace charge were observed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bye, G.; Ceccaroli, B. Solar grade silicon: Technology status and industrial trends. Sol. Energy Mater. Sol. Cells 2014, 130, 634–646. [Google Scholar] [CrossRef]
- Ballif, C.; Haug, F.J.; Boccard, M.; Verlinden, P.J.; Hahn, G. Status and perspectives of crystalline silicon photovoltaics in research and industry. Nat. Rev. Mater. 2022, 7, 597–616. [Google Scholar] [CrossRef]
- Ringdalen, E.; Tangstad, M. Reaction mechanisms in carbothermic production of silicon, study of selected reactions. Miner. Met. Mater. Soc. 2012, 17, 195–203. [Google Scholar] [CrossRef]
- Neuschutz, D. State and trends of the electric arc furnace technology. High Temp. Mater. Process. Int. Q. High-Technol. Plasma Process. 2000, 4, 12. [Google Scholar] [CrossRef]
- Tesfahunegn, Y.A.; Magnusson, T.; Tangstad, M.; Saevarsdottir, G. Comparative study of AC and DC solvers based on current and power distributions in a submerged arc furnace. Metall. Mater. Trans. B 2020, 51, 510–518. [Google Scholar] [CrossRef]
- Xie, R.; Chen, Z.; Ma, W.; Wang, X.; Gan, X.; Tao, C.; Qu, J. High efficient and clean utilization of renewable energy for the process of industrial silicon. Renew. Energy 2024, 231, 120902. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, W.; Wu, J.; Wei, K.; Yang, X.; Lv, G.; Xie, K.; Yu, J. Influence of carbothermic reduction on submerged arc furnace energy efficiency during silicon production. Energy 2016, 116, 687–693. [Google Scholar] [CrossRef]
- Hasannezhad, H.; Meysami, A. Effects of porosity and electrical resistance of metallurgical coke and semicoke on silicon recovery in an electric arc furnace. JOM 2019, 71, 336–341. [Google Scholar] [CrossRef]
- Tong, X.; You, G.; Ding, Y.; Xue, H.; Wang, Y.; Guo, W. Effect of grain size on low-temperature electrical resistivity and thermal conductivity of pure magnesium. Mater. Lett. 2018, 229, 261–264. [Google Scholar] [CrossRef]
- Ivanov, O.; Maradudina, O.; Lyubushkin, R. Grain size effect on electrical resistivity of bulk nanograined Bi2Te3 material. Mater. Charact. 2015, 99, 175–179. [Google Scholar] [CrossRef]
- Surup, G.R.; Pedersen, T.A.; Chaldien, A.; Beukes, J.P.; Tangstad, M. Electrical resistivity of carbonaceous bed material at high temperature. Processes 2020, 8, 933. [Google Scholar] [CrossRef]
- Eidem, P.; Tangstad, M.; Bakken, J. Measurement of material resistivity and contact resistance of metallurgical coke. In Proceedings of the International Ferro-Alloys Congress XI, New Delhi, India, 18–21 February 2007; pp. 561–571. [Google Scholar]
- Xu, C.; Wei, K.; Du, Z.; Ma, W. Effect of particle size on the properties of biomass gasification residue pellets used as a metallurgical-grade silicon reducing agent. Powder Technol. 2024, 435, 119406. [Google Scholar] [CrossRef]
- Zobnin, N.N.; Torgovets, A.K.; Pikalova, I.A.; Yussupova, Y.S.; Atakishiyev, S.A. Influence of thermal stability of quartz and the particle size distribution of burden materials on the process of electrothermal smelting of metallurgical silicon. Orient. J. Chem. 2018, 34, 1120. [Google Scholar] [CrossRef]
- Kök, M.V.; Özbas, E.; Karacan, O.; Hicyilmaz, C. Effect of particle size on coal pyrolysis. J. Anal. Appl. Pyrolysis 1998, 45, 103–110. [Google Scholar] [CrossRef]
- Parkhomenko, E.I. Electrical resistivity of minerals and rocks at high temperature and pressure. Rev. Geophys. 1982, 20, 193–218. [Google Scholar] [CrossRef]
- Tsybul’kin, G.A. On the calculation of the electrical resistivity of conducting loose materials. Russ. Metall. 2011, 2011, 594–598. [Google Scholar] [CrossRef]
- Greenwood, J.A.; Williamson, J. Contact of nominally flat surfaces. Proceedings of the royal society of London. Ser. A Math. Phys. Sci. 1966, 295, 300–319. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, Y.; Feng, Z. A contact model for electrical contact resistance between a rigid plane and a cylindrical surface. J. Mech. Sci. Technol. 2021, 35, 4125–4134. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Hu, J.; Si, Y.; Yang, H.; Shao, J.; Wang, X.; Lei, T.; Agblevor, F.A.; Chen, H. Influence of volatiles-char interactions between coal and biomass on the volatiles released, resulting char structure and reactivity during co-pyrolysis. Energy Convers. Manag. 2017, 152, 229–238. [Google Scholar] [CrossRef]
- Babu, S.S.; Santella, M.L.; Feng, Z.; Riemer, B.W.; Cohron, J.W. Empirical model of effects of pressure and temperature on electrical contact resistance of metals. Sci. Technol. Weld. Join. 2001, 6, 126–132. [Google Scholar] [CrossRef]
- Bi, D.; Chen, H.; Ye, T. Influences of temperature and contact pressure on thermal contact resistance at interfaces at cryogenic temperatures. Cryogenics 2012, 52, 403–409. [Google Scholar] [CrossRef]
- Liu, S.; Gao, D.; Sang, S.; Wang, H. Characteristics of apparent resistivity of coals with different particle sizes. Coal Sci. Technol. 2022, 50, 162–169. [Google Scholar] [CrossRef]
- Xu, X.; Cao, D.; Wei, Y.; Wang, A.; Chen, G.; Wang, T.; Wang, G.; Chen, X. Impact of graphitization degree on the electrochemical and thermal properties of coal. ACS Omega 2024, 9, 2443–2456. [Google Scholar] [CrossRef]
- Zhu, H.; Yu, G.; Guo, Q.; Wang, X. In situ Raman spectroscopy study on catalytic pyrolysis of a bituminous coal. Energy Fuels 2017, 31, 5817–5827. [Google Scholar] [CrossRef]
- Mochidzuki, K.; Soutric, F.; Tadokoro, K.; Antal, M.J.; Tóth, M.; Zelei, B.; Várhegyi, G. Electrical and physical properties of carbonized charcoals. Ind. Eng. Chem. Res. 2003, 42, 5140–5151. [Google Scholar] [CrossRef]
- Possokhov, Y.M.; Butakova, V.I.; Popov, V.K. Decomposition of IR spectra of coal. Coke Chem. 2015, 58, 49–57. [Google Scholar] [CrossRef]
- Sun, S.; Xu, D.; Wei, Y.; Zhi, Y.; Jiang, G.; Guo, Y. Influence laws of operating parameters on coal pyrolysis characteristics. J. Anal. Appl. Pyrolysis 2022, 167, 105684. [Google Scholar] [CrossRef]
- Zhu, W.; Song, W.; Lin, W. Effect of the coal particle size on pyrolysis and char reactivity for two types of coal and demineralized coal. Energy Fuels 2008, 22, 2482–2487. [Google Scholar] [CrossRef]
- Stubington, J.F. Release of volatiles from large coal particles in a hot fluidized bed. Fuel 1984, 63, 1013–1019. [Google Scholar] [CrossRef]
- Parkhomenko, E.I. Electrical Properties of Rocks; Springer: Berlin/Heidelberg, Germany, 1967. [Google Scholar] [CrossRef]
- Saeed, A.; Adewuyi, S.O.; Ahmed, H.A.; Alharbi, S.R.; Al Garni, S.E.; Abolaban, F. Electrical and dielectric properties of the natural calcite and quartz. Silicon 2022, 14, 5265–5276. [Google Scholar] [CrossRef]
- Hashin, Z.; Shtrikman, S. A variational approach to the theory of the effective magnetic permeability of multiphase materials. J. Appl. Phys. 1962, 33, 3125–3131. [Google Scholar] [CrossRef]


| Sample | Mad (%) | Aad (%) | Vad (%) | FCad (%) |
|---|---|---|---|---|
| Coal | 3.21 | 1.05 | 40.08 | 54.94 |
| Ingredient | Fe2O3 | Al2O3 | CaO | SiO2 |
|---|---|---|---|---|
| Content/% | 0.042 | 0.283 | 0.003 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Wei, K.; Deng, X.; Ma, W. The Impact of Particle Size on the Electrical Resistivity of Burden in the Upper Zone of an EAF During Metallurgical-Grade Silicon Smelting. Processes 2025, 13, 1227. https://doi.org/10.3390/pr13041227
Guo W, Wei K, Deng X, Ma W. The Impact of Particle Size on the Electrical Resistivity of Burden in the Upper Zone of an EAF During Metallurgical-Grade Silicon Smelting. Processes. 2025; 13(4):1227. https://doi.org/10.3390/pr13041227
Chicago/Turabian StyleGuo, Weifang, Kuixian Wei, Xiaocong Deng, and Wenhui Ma. 2025. "The Impact of Particle Size on the Electrical Resistivity of Burden in the Upper Zone of an EAF During Metallurgical-Grade Silicon Smelting" Processes 13, no. 4: 1227. https://doi.org/10.3390/pr13041227
APA StyleGuo, W., Wei, K., Deng, X., & Ma, W. (2025). The Impact of Particle Size on the Electrical Resistivity of Burden in the Upper Zone of an EAF During Metallurgical-Grade Silicon Smelting. Processes, 13(4), 1227. https://doi.org/10.3390/pr13041227





