Improving Stability of Biodiesel from 20% Free Fatty Acid Palm Oil with Tert-butylhydroquinone at Various Concentrations for 52 Weeks of Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biodiesel Production
2.2.1. Esterification
2.2.2. Transesterification
2.3. Storage Conditions
2.4. Sample Analysis
3. Results and Discussion
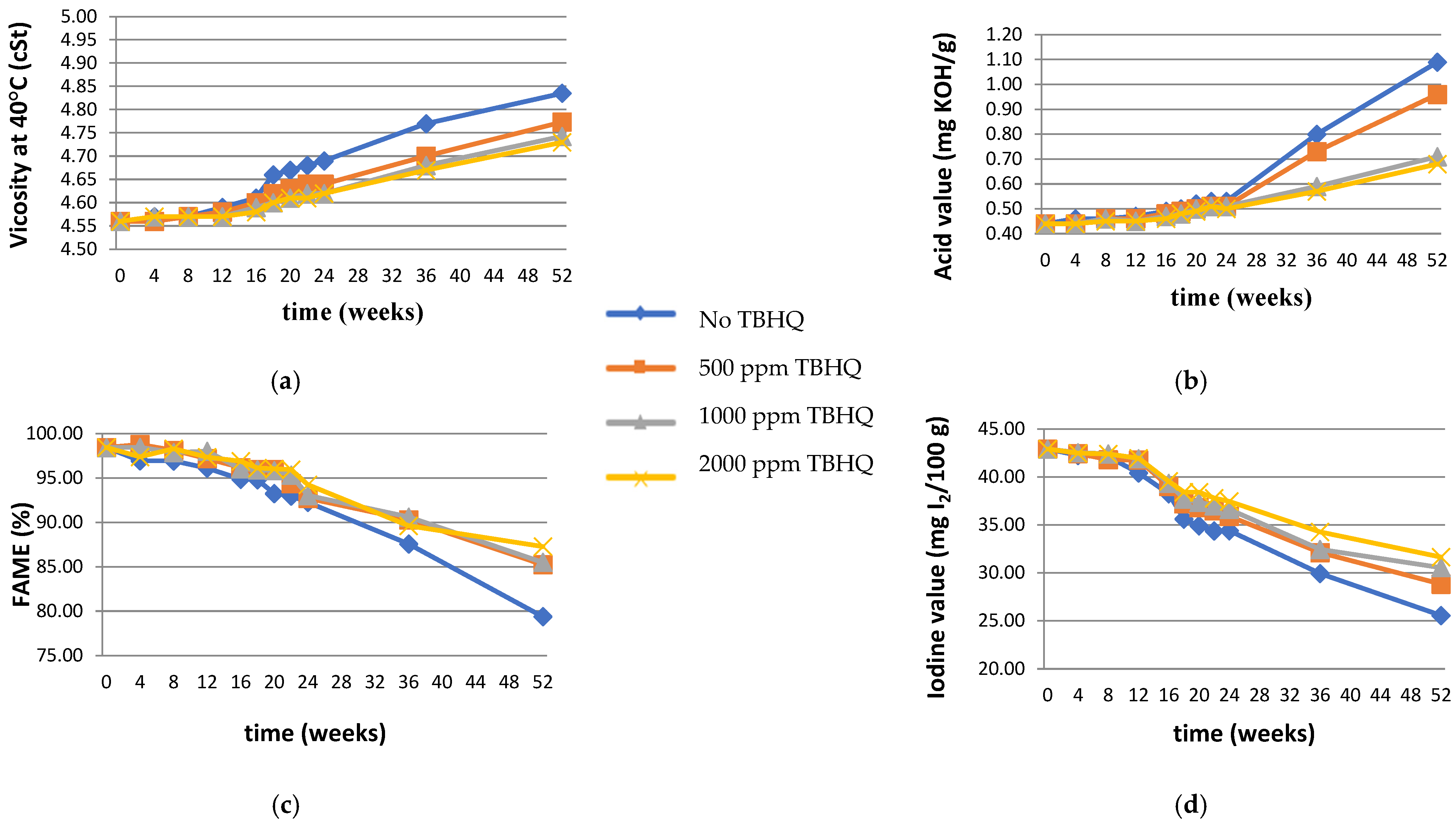
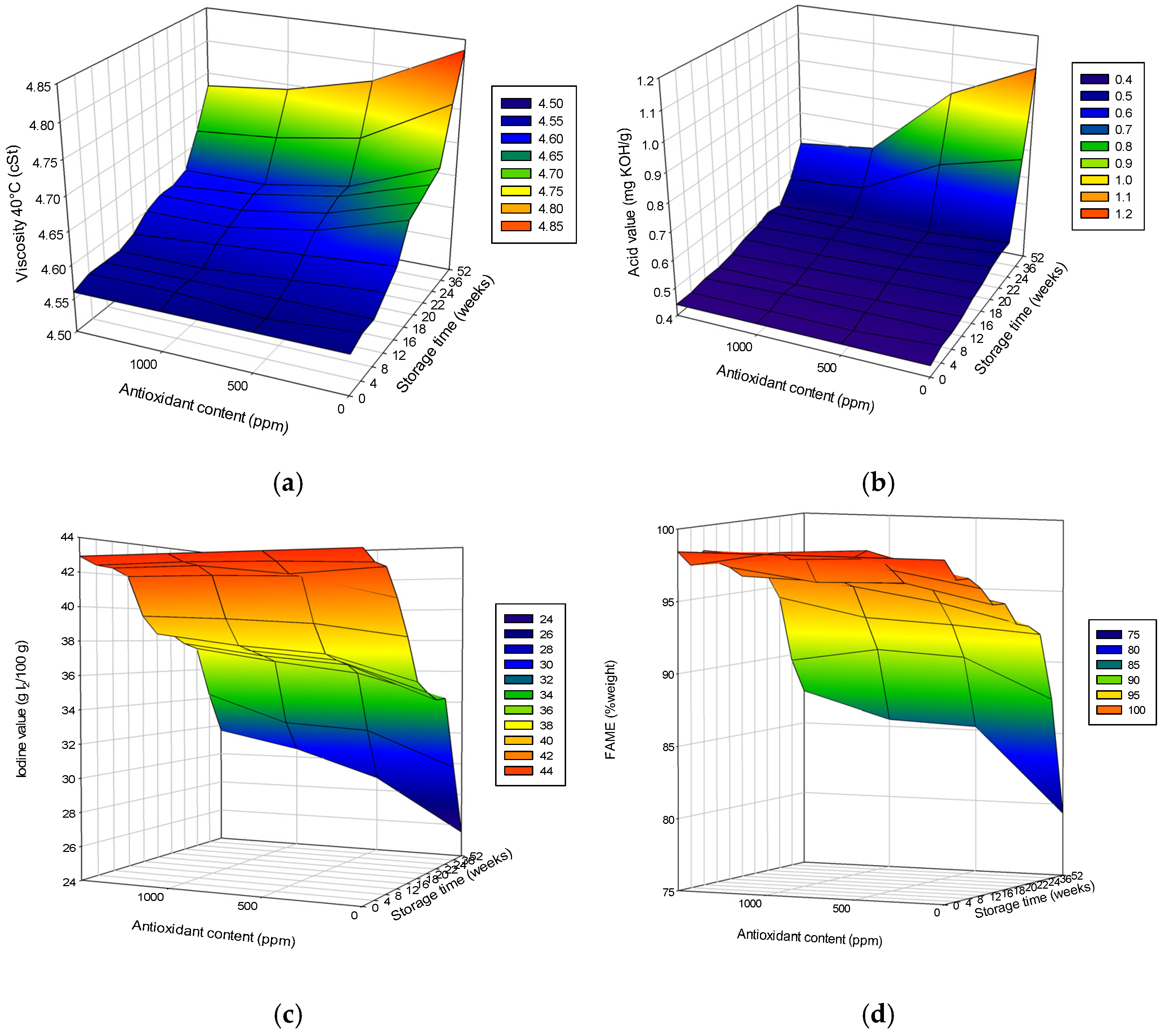
3.1. Effects of Added Antioxidant on Biodiesel Quality
3.2. Effects of Antioxidants on Water-Contaminated Biodiesel
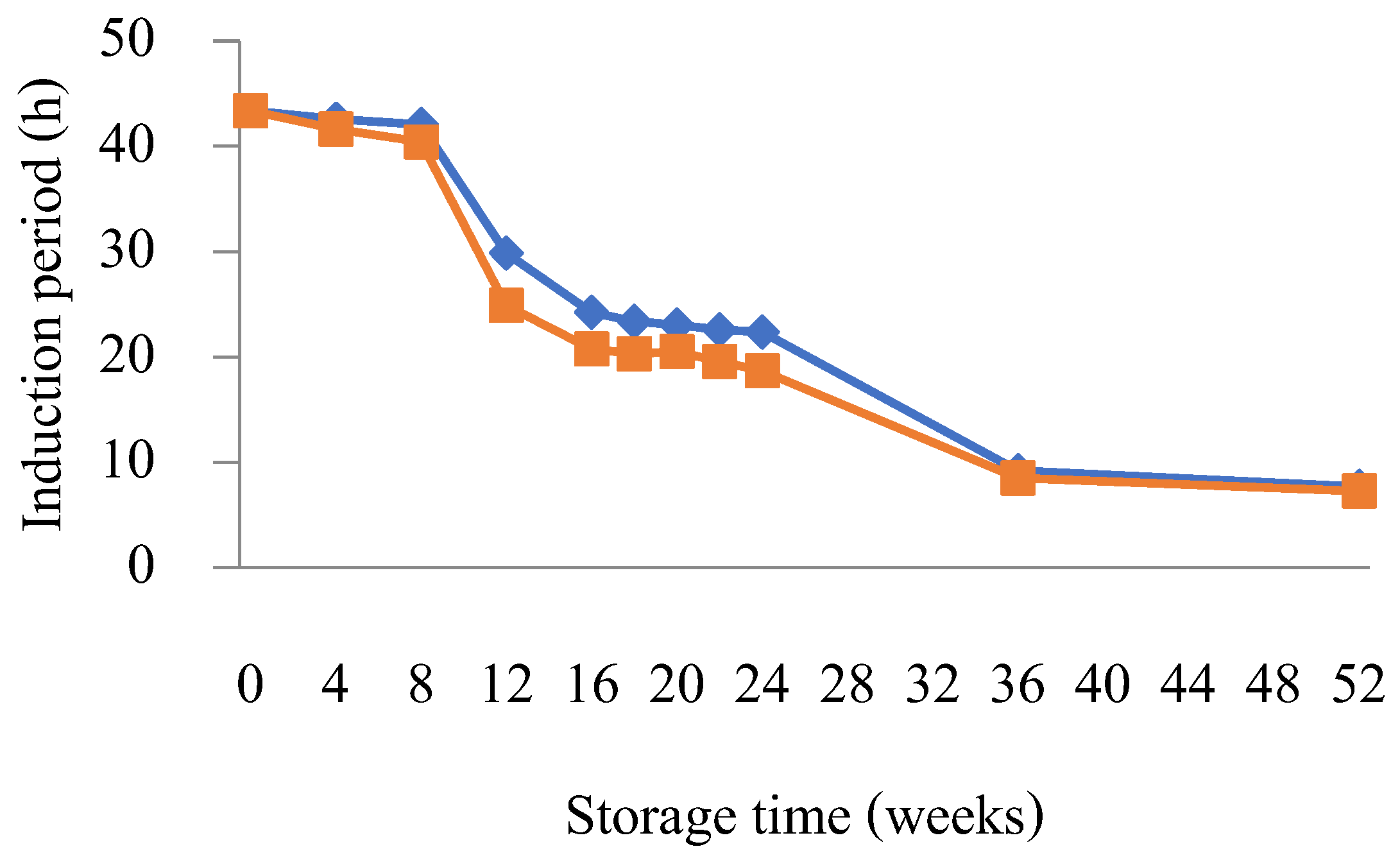
3.3. Evaluation of Storage Stability
Effect of Antioxidant
3.4. Effects of Water Contamination on Biodiesel Oxidation Stability over the Storage
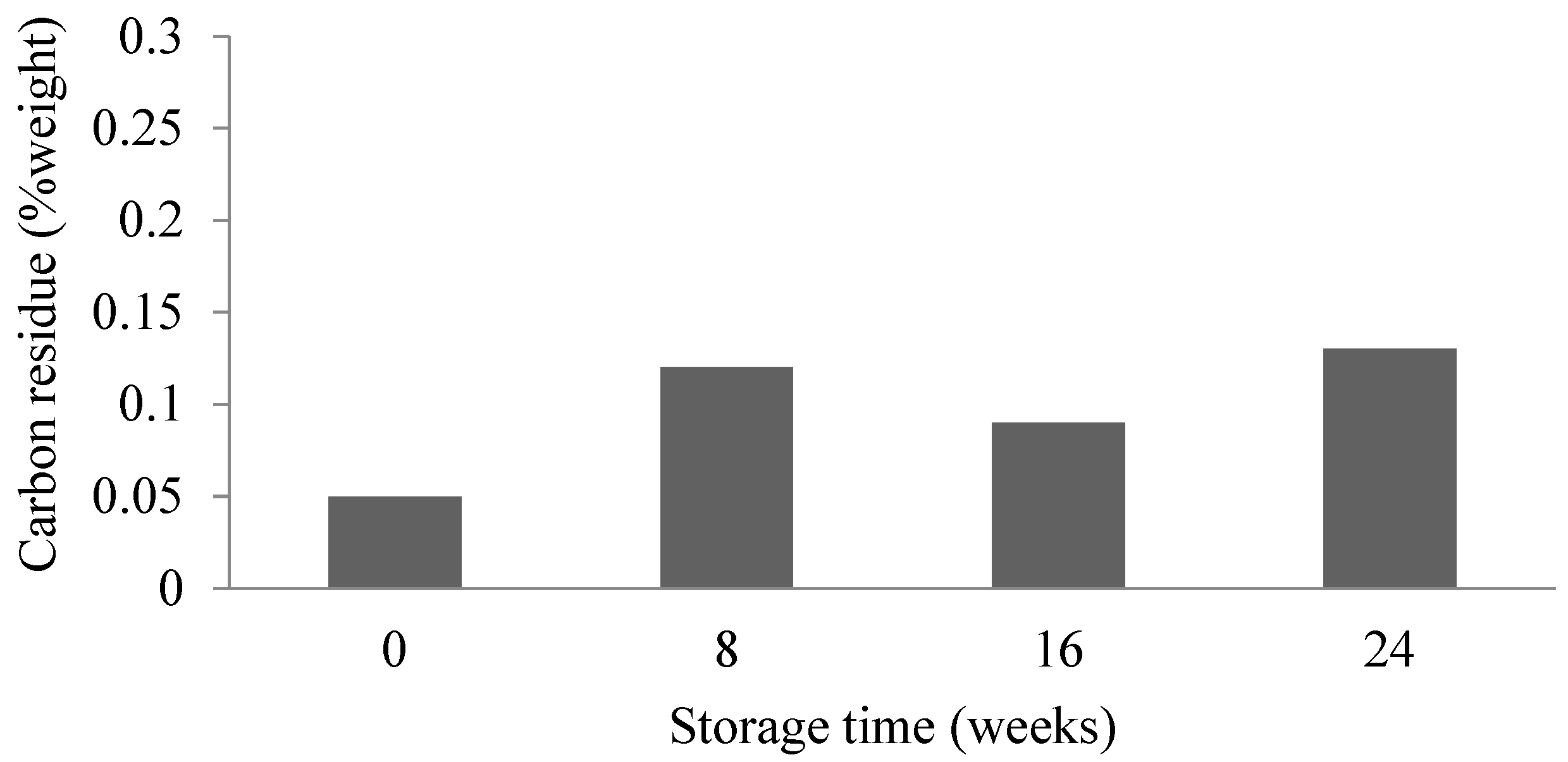
3.5. Carbon Residue Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Azam, W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Qureshi, M.I.; Rasli, A.M.; Zaman, K. Energy crisis, greenhouse gas emissions and sectoral growth reforms: Repairing the fabricated mosaic. J. Clean. Prod. 2016, 112, 3657–3666. [Google Scholar] [CrossRef]
- Ghosh, N.; Halder, G. Current progress and perspective of heterogeneous nanocatalytic transesterification towards biodiesel production from edible and inedible feedstock: A review. Energy Convers. Manag. 2022, 270, 116292. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, V.; Tsai, M.-L.; Chen, C.-W.; Sun, P.-P.; Nargotra, P.; Wang, J.-X.; Dong, C.-D. Development of lignocellulosic biorefineries for the sustainable production of biofuels: Towards circular bioeconomy. Bioresour. Technol. 2023, 381, 129145. [Google Scholar] [CrossRef]
- Hsu, H.-W.; Binyet, E.; Nugroho, R.A.A.; Wang, W.-C.; Srinophakun, P.; Chein, R.-Y.; Demafelis, R.; Chiarasumran, N.; Saputro, H.; Alhikami, A.F. Toward sustainability of Waste-to-Energy: An overview. Energy Convers. Manag. 2024, 321, 119063. [Google Scholar] [CrossRef]
- Janaun, J.; Ellis, N. Perspectives on biodiesel as a sustainable fuel. Renew. Sust. Energ. Rev. 2010, 14, 1312–1320. [Google Scholar] [CrossRef]
- Masudi, A.; Muraza, O.; Jusoh, N.W.C.; Ubaidillah, U. Improvements in the stability of biodiesel fuels: Recent progress and challenges. Environ. Sci. Pollut. Res. 2023, 30, 14104–14125. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Chen, K.-S.; Lin, Y.-C.; Hsieh, L.-T.; Lin, L.-F.; Wu, C.-C. Saving energy and reducing pollution by use of emulsified palm-biodiesel blends with bio-solution additive. Energy 2010, 35, 2043–2048. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. An overview of biodiesel oxidation stability. Renew. Sustain. Energ. Rev. 2012, 16, 5924–5950. [Google Scholar] [CrossRef]
- Pullen, J.; Saeed, K. Experimental study of the factors affecting the oxidation stability of biodiesel FAME fuels. Fuel Process. Technol. 2014, 125, 223–235. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.L.; McCormick, R.L. Spectroscopic Study of Biodiesel Degradation Pathways; SAE Technical Paper 2006-01-3300; SAE International: Warrendale, PA, USA, 2006; ISSN 0148-7191. [Google Scholar]
- Hosseinzadeh-Bandbafha, H.; Kumar, D.; Singh, B.; Shahbeig, H.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Biodiesel antioxidants and their impact on the behavior of diesel engines: A comprehensive review. Fuel Process. Technol. 2022, 232, 107264. [Google Scholar] [CrossRef]
- Yaakob, Z.; Narayanan, B.N.; Padikkaparambil, S. A review on the oxidation stability of biodiesel. Renew. Sust. Energ. Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- El-Araby, R.; Amin, A.; El Morsi, A.; El-Ibiari, N.; El-Diwani, G. Study on the characteristics of palm oil–biodiesel–diesel fuel blend. Egypt. J. Pet. 2018, 27, 187–194. [Google Scholar] [CrossRef]
- Rathmann, R.; Szklo, A.; Schaeffer, R. Targets and results of the Brazilian biodiesel incentive program–has it reached the promised land? Appl. Energy 2012, 97, 91–100. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Christensen, E.; McCormick, R.L. Long-term storage stability of biodiesel and biodiesel blends. Fuel Process. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef]
- Serrano, M.; Martínez, M.; Aracil, J. Long term storage stability of biodiesel: Influence of feedstock, commercial additives and purification step. Fuel Process. Technol. 2013, 116, 135–141. [Google Scholar] [CrossRef]
- Lau, C.H.; Lau, H.L.N.; Ng, H.K.; Thangalazhy-Gopakumar, S.; Lee, L.Y.; Gan, S. Evaluation of synthetic and bio-based additives on the oxidation stability of palm biodiesel: Parametric, kinetics and thermodynamics studies. Sustain. Energy Technol. Assess. 2024, 64, 103738. [Google Scholar] [CrossRef]
- Paw, J.K.S.; Kiong, T.S.; Kamarulzaman, M.K.; Adam, A.; Hisham, S.; Kadirgama, K.; Ramasamy, D.; Yaw, C.T.; Yusop, A.F.; Yusaf, T. Advancing renewable fuel integration: A comprehensive response surface methodology approach for internal combustion engine performance and emissions optimization. Heliyon 2023, 9, e22238. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, L.; Matteo, R.; Lazzeri, L.; Malaguti, L.; Folegatti, L.; Bondioli, P.; Pochi, D.; Grilli, R.; Fornaciari, L.; Benigni, S. Technical performance and chemical–physical property assessment of safflower oil tested in an experimental hydraulic test rig. Lubricants 2023, 11, 39. [Google Scholar] [CrossRef]
- Ramos, T.; Santos, E.; Ventura, M.; Pina, J.; Cavalheiro, A.; Fiorucci, A.; Silva, M. Eugenol and TBHQ antioxidant actions in commercial biodiesel obtained by soybean oil and animal fat. Fuel 2021, 286, 119374. [Google Scholar] [CrossRef]
- Rajamohan, S.; Gopal, A.H.; Muralidharan, K.R.; Huang, Z.; Paramasivam, B.; Ayyasamy, T.; Nguyen, X.P.; Le, A.T.; Hoang, A.T. Evaluation of oxidation stability and engine behaviors operated by Prosopis juliflora biodiesel/diesel fuel blends with presence of synthetic antioxidant. Sustain. Energy Technol. Assess. 2022, 52, 102086. [Google Scholar] [CrossRef]
- Laemthong, T.; Chiarasamran, N.; Saisriyoot, M.; Thanapimmetha, A.; Ooi, C.W.; Chang, Y.K.; Srinophakun, P. Effects of oxidation inducers on palm, jatropha, and sunflower biodiesel properties during 22-week storage and improvements with antioxidants. Biofuels Bioprod. Biorefining 2025. [Google Scholar] [CrossRef]
- Urrutia, C.; Sangaletti-Gerhard, N.; Cea, M.; Suazo, A.; Aliberti, A.; Navia, R. Two step esterification–transesterification process of wet greasy sewage sludge for biodiesel production. Bioresour. Technol. 2016, 200, 1044–1049. [Google Scholar] [CrossRef]
- Goto, S.; Oguma, M.; Chollacoop, N. Benchmarking of Biodiesel Fuel Standardization in East Asia Working Group. In Current Status of Biodiesel Fuel in East-Asia and ASEAN Countries, EAS-ERIA Biodiesel Fuel Trade Handbook; ERIA: Jakarta, Indonesia, 2010; pp. 96–169. [Google Scholar]
- Nadkarni, R.; Nadkarni, R. Guide to ASTM Test Methods for the Analysis of Petroleum Products and Lubricants; ASTM International: West Conshohocken, PA, USA, 2007; Volume 44. [Google Scholar]
- Ruppel, T.; Huybrighs, T.; Shelton, C. Fatty Acid Methyl Esters in B100 Biodiesel by Gas Chromatography (Modified EN 14103); Perkin Elmer: Shelton, CT, USA, 2008; Volume 3, pp. 804–818. [Google Scholar]
- Souza, V.M.G.D.; d’Avila, L.A. Kinetic modeling of the effect of antioxidant additives on biodiesel oxidation stability. Ind. Eng. Chem. Res. 2023, 62, 15428–15433. [Google Scholar] [CrossRef]
- Varatharajan, K.; Pushparani, D.S. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Yang, S.; Bi, Y. Inhibitory effect of antioxidants on biodiesel crystallization: Revealing the role of antioxidants. Fuel 2021, 297, 120782. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Guiberteau, A.; Encinar, J.M. Effect of tert-butylhydroquinone on biodiesel properties during extreme oxidation conditions. Fuel 2022, 310, 122339. [Google Scholar] [CrossRef]
- Obadiah, A.; Kannan, R.; Ramasubbu, A.; Kumar, S.V. Studies on the effect of antioxidants on the long-term storage and oxidation stability of Pongamia pinnata (L.) Pierre biodiesel. Fuel Process. Technol. 2012, 99, 56–63. [Google Scholar] [CrossRef]
- Almeida, E.S.; Portela, F.M.; Sousa, R.M.; Daniel, D.; Terrones, M.G.; Richter, E.M.; Muñoz, R.A. Behaviour of the antioxidant tert-butylhydroquinone on the storage stability and corrosive character of biodiesel. Fuel 2011, 90, 3480–3484. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Guiberteau, A.; Márquez, S. The effect of antioxidants on corn and sunflower biodiesel properties under extreme oxidation conditions. J. Am. Oil. Chem. Soc. 2020, 97, 201–212. [Google Scholar] [CrossRef]
- Jakeria, M.; Fazal, M.; Haseeb, A. Influence of different factors on the stability of biodiesel: A review. Renew. Sustain. Energy Rev. 2014, 30, 154–163. [Google Scholar] [CrossRef]
- Yang, Z.; Hollebone, B.P.; Wang, Z.; Yang, C.; Landriault, M. Factors affecting oxidation stability of commercially available biodiesel products. Fuel Process. Technol. 2013, 106, 366–375. [Google Scholar] [CrossRef]
- Christensen, E.D.; McCormick, R.L. Water contamination impacts on biodiesel antioxidants and storage stability. Energy Fuels 2023, 37, 5179–5188. [Google Scholar] [CrossRef]
- Borsato, D.; de Moraes Cini, J.R.; da Silva, H.C.; Coppo, R.L.; Angilelli, K.G.; Moreira, I.; Maia, E.C.R. Oxidation kinetics of biodiesel from soybean mixed with synthetic antioxidants BHA, BHT and TBHQ: Determination of activation energy. Fuel Process. Technol. 2014, 127, 111–116. [Google Scholar] [CrossRef]
- Huang, Y.; Li, F.; Bao, G.; Li, M.; Wang, H. Qualitative and quantitative analysis of the influence of biodiesel fatty acid methyl esters on iodine value. Environ. Sci. Pollut. Res. 2022, 29, 2432–2447. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Lipid Oxidation and Measuring Oxidative Rancidity in Oils and Foods. In Oxidation of Foods and Beverages; Elsevier: Amsterdam, The Netherlands, 2010; pp. 337–368. [Google Scholar]
- Riyadi, T.W.; Spraggon, M.; Herawan, S.; Idris, M.; Paristiawan, P.; Putra, N.; Silambarasan, R.; Veza, I. Biodiesel for HCCI engine: Prospects and challenges of sustainability biodiesel for energy transition. Results Eng. 2023, 17, 100916. [Google Scholar] [CrossRef]
- Santos, N.D.S.A.; Roso, V.R.; Malaquias, A.C.T.; Baeta, J.G.C. Internal combustion engines and biofuels: Examining why this robust combination should not be ignored for future sustainable transportation. Renew. Sustain. Energ. Rev. 2021, 148, 111292. [Google Scholar]
- Tutak, W.; Grab-Rogaliński, K.; Jamrozik, A. Combustion and emission characteristics of a biodiesel-hydrogen dual-fuel engine. Appl. Sci. 2020, 10, 1082. [Google Scholar] [CrossRef]
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Suzihaque, M.; Alwi, H.; Ibrahim, U.K.; Abdullah, S.; Haron, N. Biodiesel production from waste cooking oil: A brief review. Mater. Today 2022, 63, S490–S495. [Google Scholar] [CrossRef]
- Maheshwari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]

| Antioxidant | Usage Details | Minimum Concentration (ppm) Required by the EU Standard |
|---|---|---|
| THBQ (tert-butylhydroquinone) | More effective in vegetable oil or animal fats with low-free fatty acids feedstocks. Additionally, it can be employed in storage environments, like high temperatures or air insufflation. | 500 |
| PG (propyl gallate) | Moderate to high antioxidant activity for biodiesel | 500 |
| PY (pyrogallol) | High antioxidant activity on biodiesel with high-free fatty acid feedstock like animal fats | 500 |
| BHT (butylated hydroxytoluene) | More effective in animal fats | 1000 |
| BHA (butylated hydroxyanisole) | More effective in animal fats | 2000 |
| Property | Unit | Test Method [28] | Standard Limit | Palm Biodiesel |
|---|---|---|---|---|
| Acid value | mg KOH/g | ASTM D664 | <0.5 | 0.45 |
| Carbon residue value | wt% | ASTM D4530 | <0.3 | 0.05 |
| Kinematic viscosity (at 40 °C) | cSt | ASTM D445 | 3.5–5 | 4.56 |
| Oxidative stability (at 110 °C) | h | EN 15751 | >10 | 43.37 |
| Iodine value | g I2/100 g sample | EN 14111 | <120 | 42.92 |
| Flash point | °C | ASTM D 93 | >52 | 165 |
| Pour point | °C | ASTM D 97 | Not specified | 19 |
| Ester content | wt% | EN 14103 | >96.5 | 98.88 |
| Saturated | 59.67 | |||
| Unsaturated | 39.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laemthong, T.; Triwittayayont, S.; Sakulshah, N.; Khomlaem, C.; Chiarasumran, N.; Thanapimmetha, A.; Saisriyoot, M.; Wang, W.-C.; Chiang, Y.-Y.; Srinophakun, P. Improving Stability of Biodiesel from 20% Free Fatty Acid Palm Oil with Tert-butylhydroquinone at Various Concentrations for 52 Weeks of Storage. Processes 2025, 13, 1237. https://doi.org/10.3390/pr13041237
Laemthong T, Triwittayayont S, Sakulshah N, Khomlaem C, Chiarasumran N, Thanapimmetha A, Saisriyoot M, Wang W-C, Chiang Y-Y, Srinophakun P. Improving Stability of Biodiesel from 20% Free Fatty Acid Palm Oil with Tert-butylhydroquinone at Various Concentrations for 52 Weeks of Storage. Processes. 2025; 13(4):1237. https://doi.org/10.3390/pr13041237
Chicago/Turabian StyleLaemthong, Tunyaboon, Sarun Triwittayayont, Netipon Sakulshah, Chanin Khomlaem, Nutchapon Chiarasumran, Anusith Thanapimmetha, Maythee Saisriyoot, Wei-Cheng Wang, Ya-Yu Chiang, and Penjit Srinophakun. 2025. "Improving Stability of Biodiesel from 20% Free Fatty Acid Palm Oil with Tert-butylhydroquinone at Various Concentrations for 52 Weeks of Storage" Processes 13, no. 4: 1237. https://doi.org/10.3390/pr13041237
APA StyleLaemthong, T., Triwittayayont, S., Sakulshah, N., Khomlaem, C., Chiarasumran, N., Thanapimmetha, A., Saisriyoot, M., Wang, W.-C., Chiang, Y.-Y., & Srinophakun, P. (2025). Improving Stability of Biodiesel from 20% Free Fatty Acid Palm Oil with Tert-butylhydroquinone at Various Concentrations for 52 Weeks of Storage. Processes, 13(4), 1237. https://doi.org/10.3390/pr13041237







