A Novel Fe(III)-Complex with 1,10-Phenanthroline and Succinate Ligands: Structure, Intermolecular Interactions, and Spectroscopic and Thermal Properties for Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
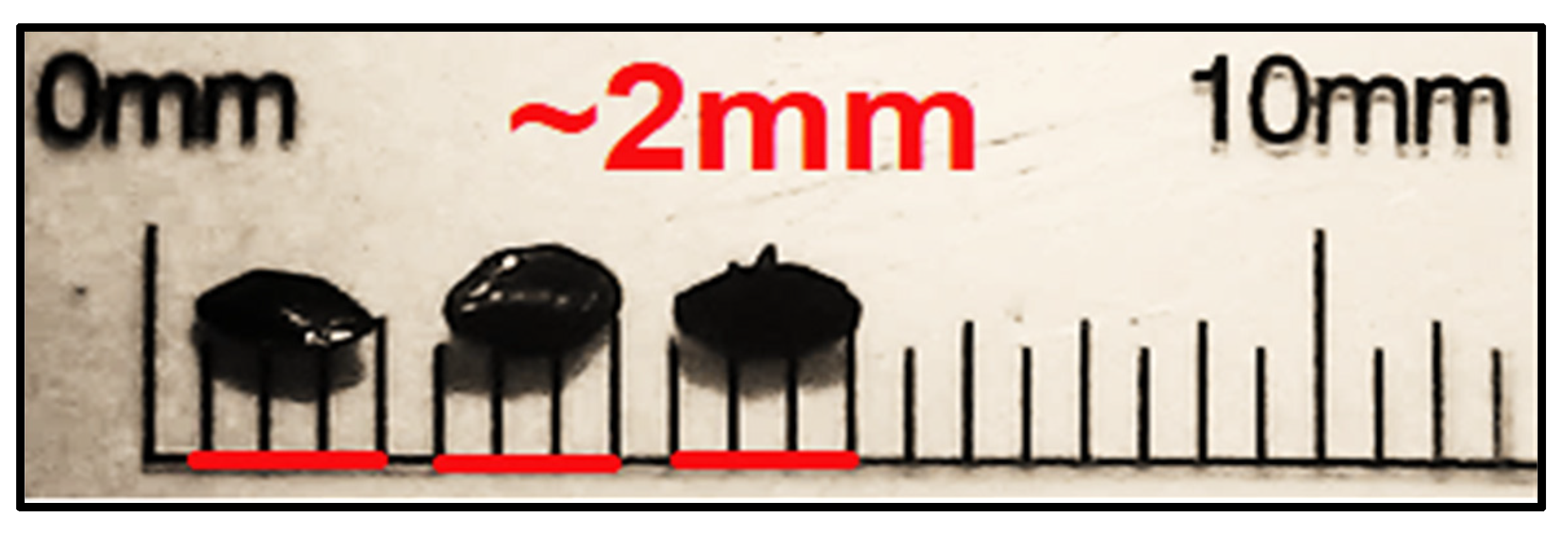
2.2. Preparation of Crystals
2.3. Characterization Techniques and Theoretical Methods
3. Results and Discussion
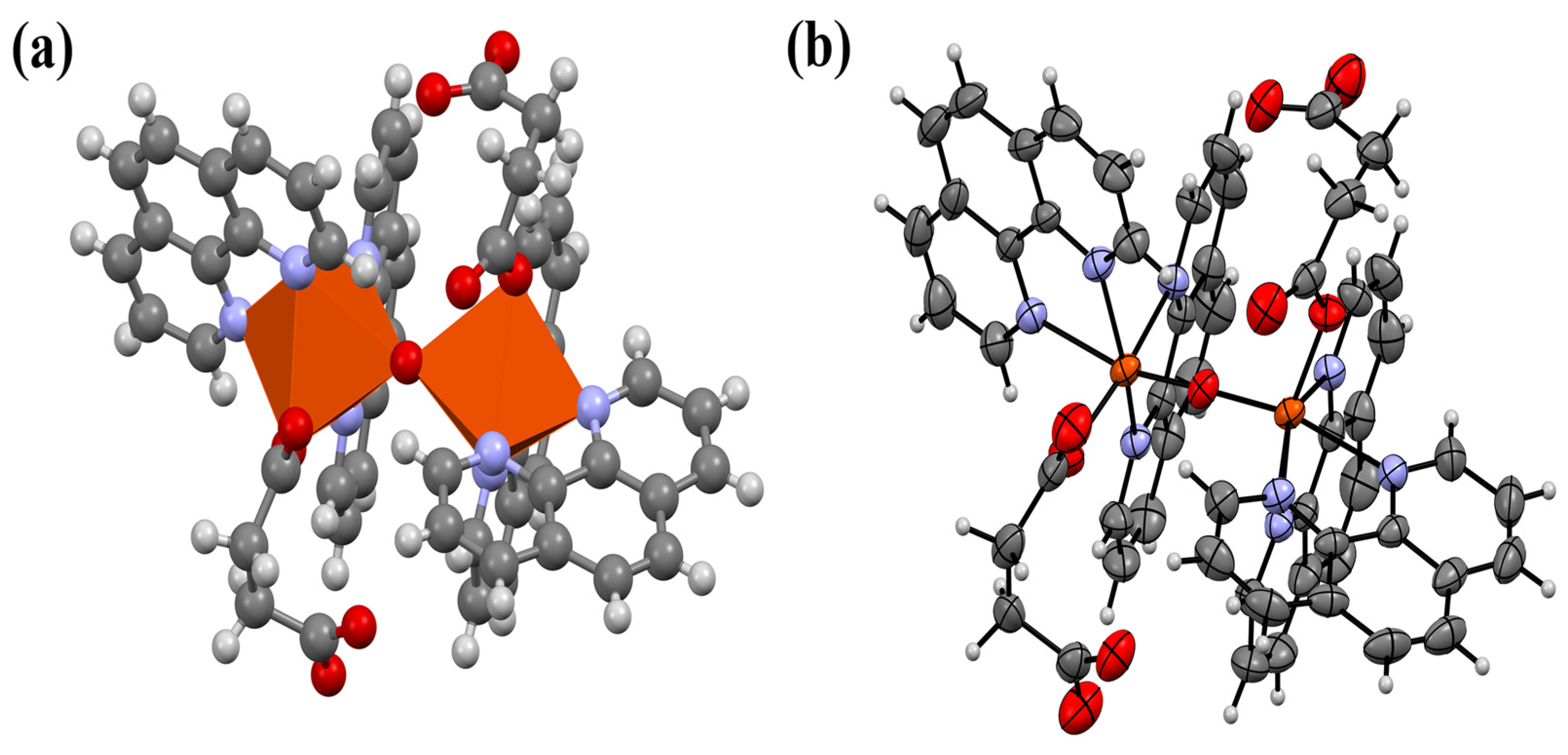
3.1. Structural Analysis
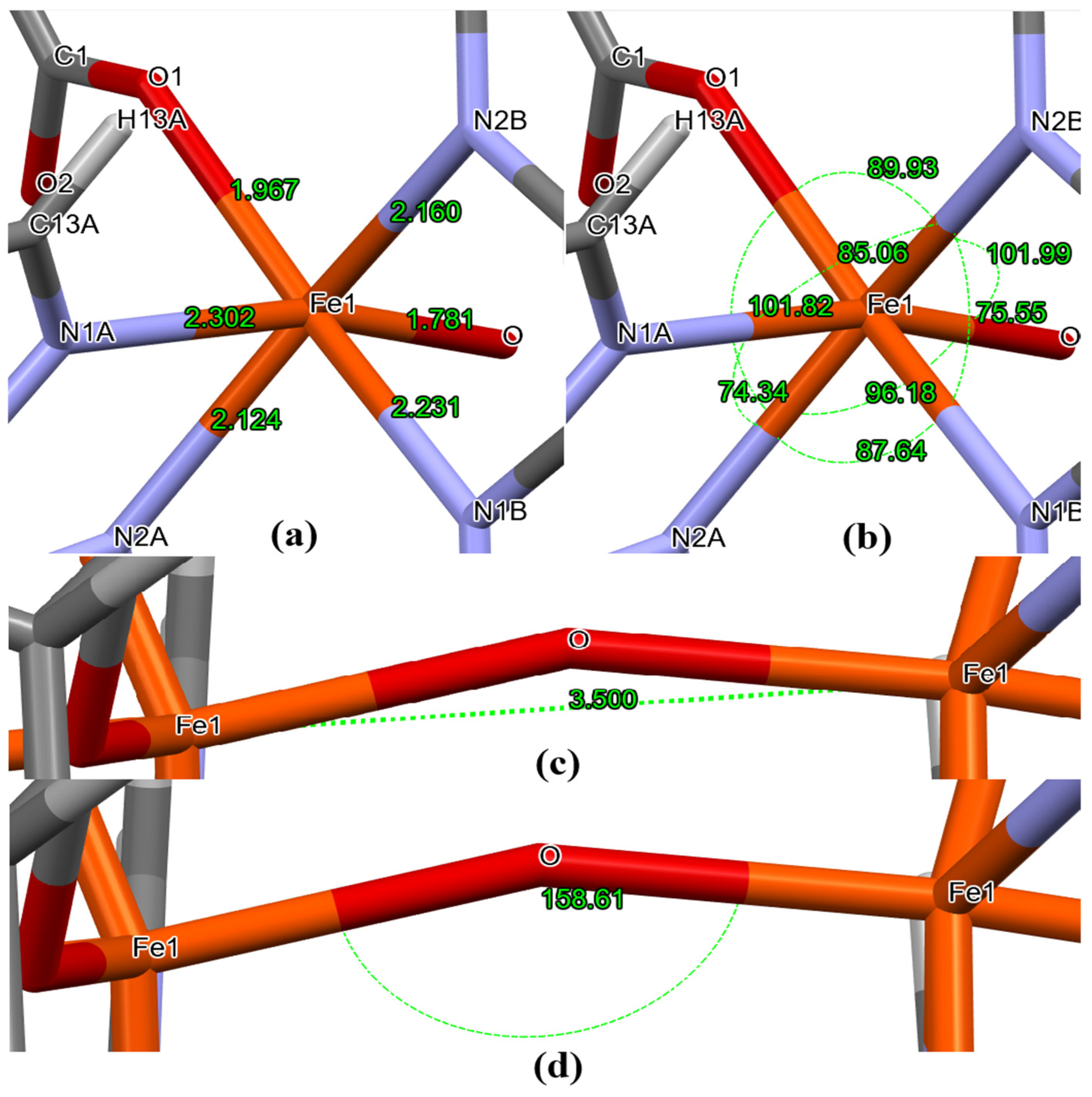
3.2. Insights on Coordination Mechanisms, Crystal Packing and Bonding Aspects
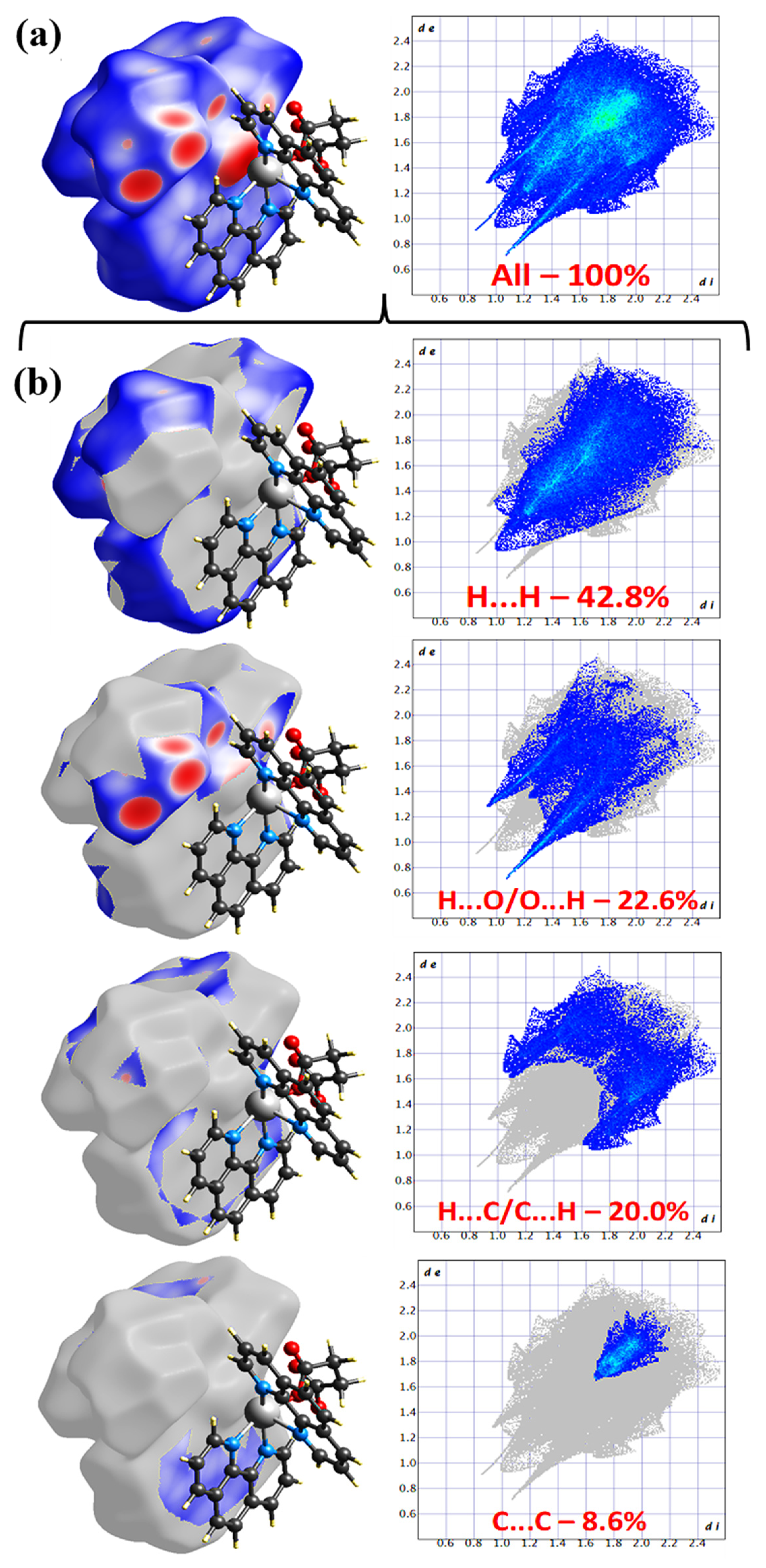
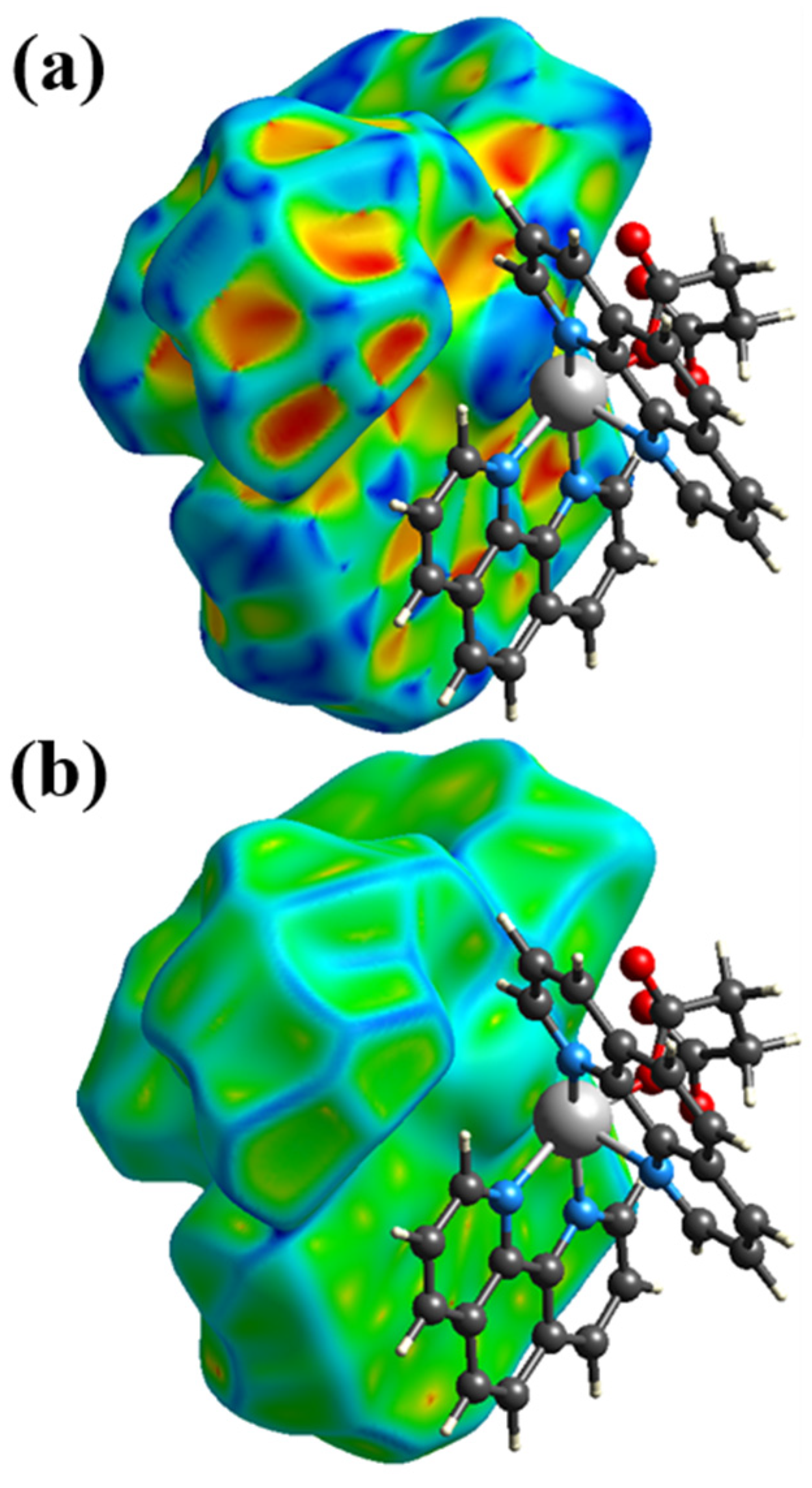
3.3. Hirshfeld Surface Analysis
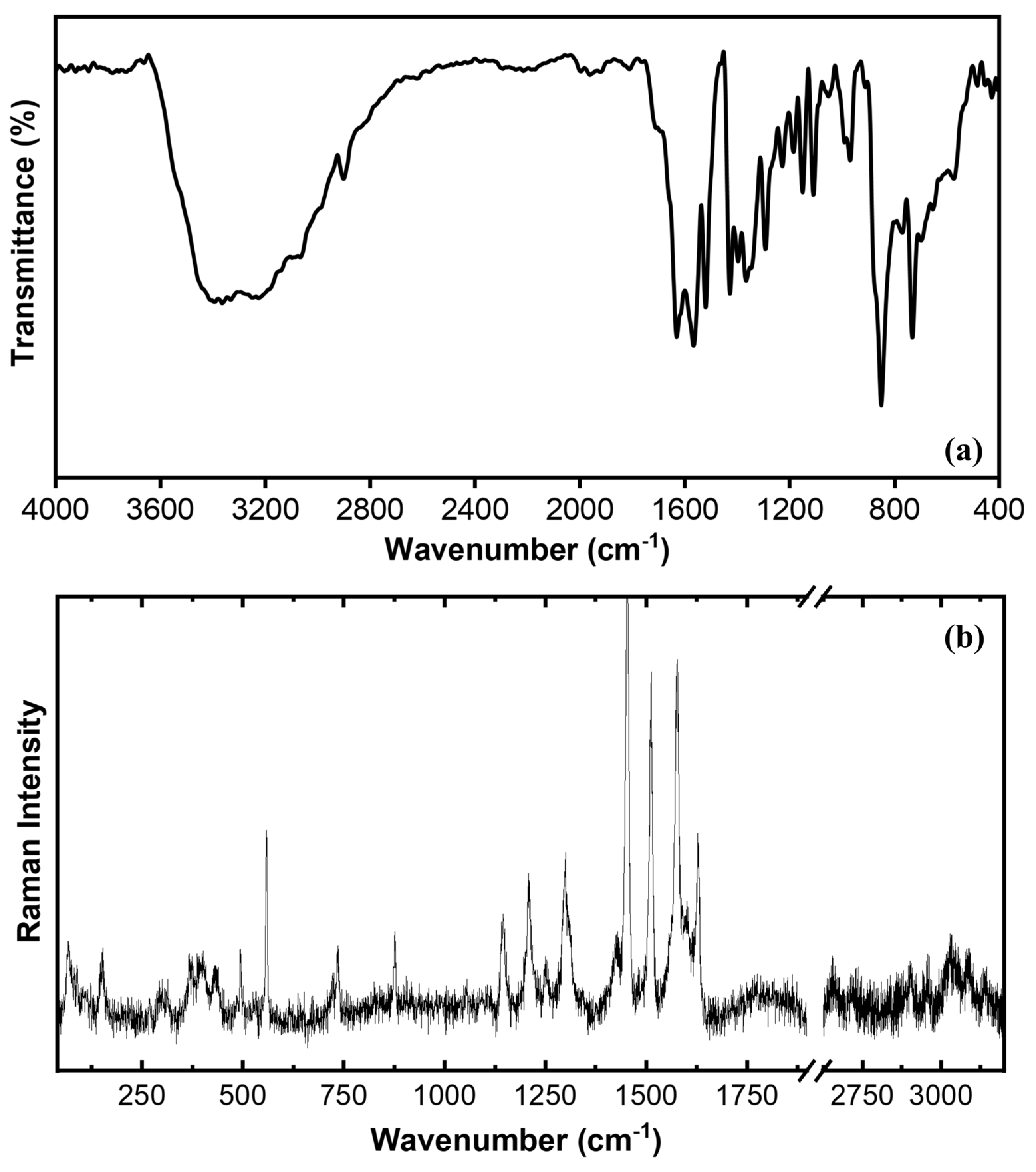
3.4. Vibrational Study
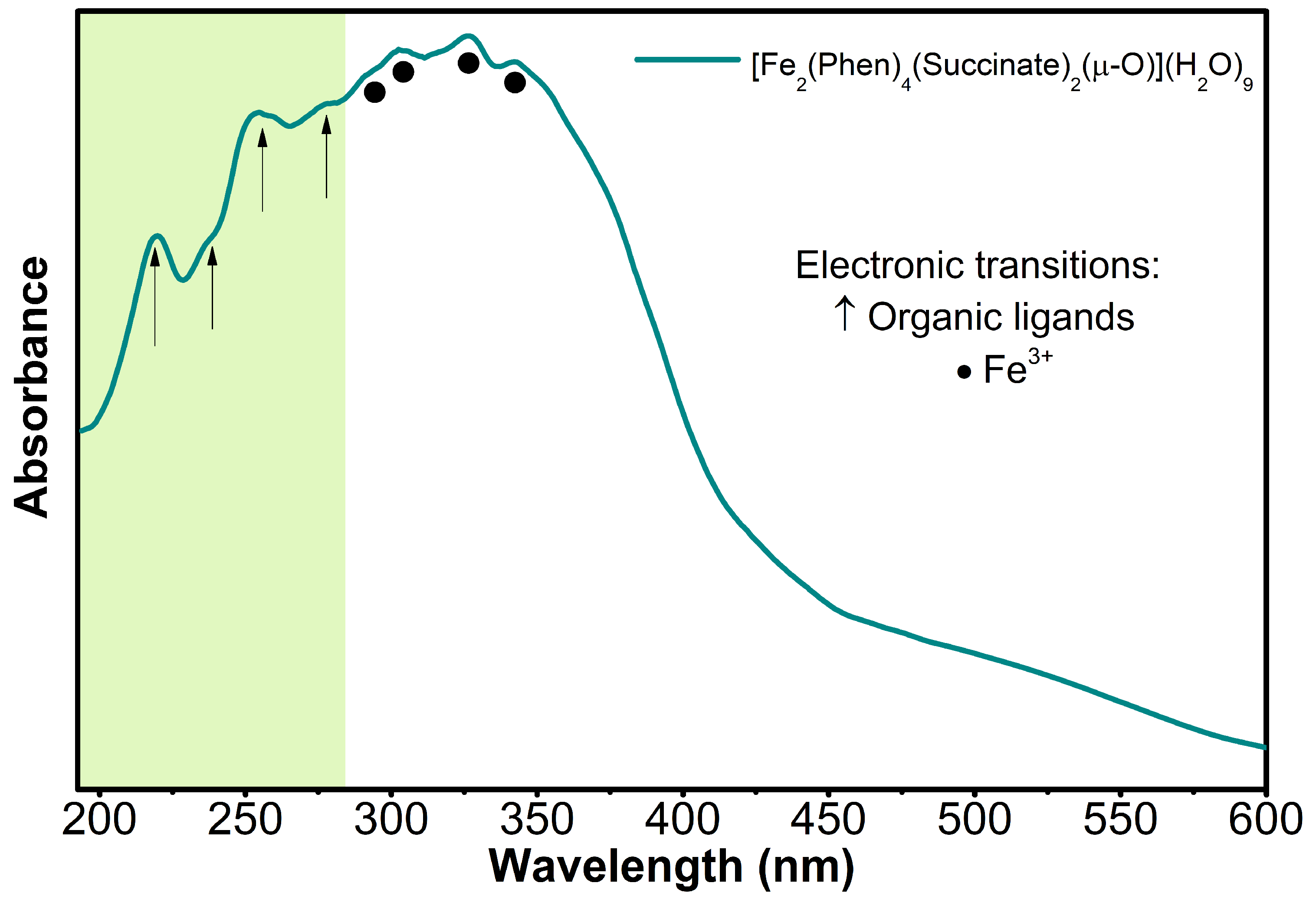
3.5. Electronic Properties
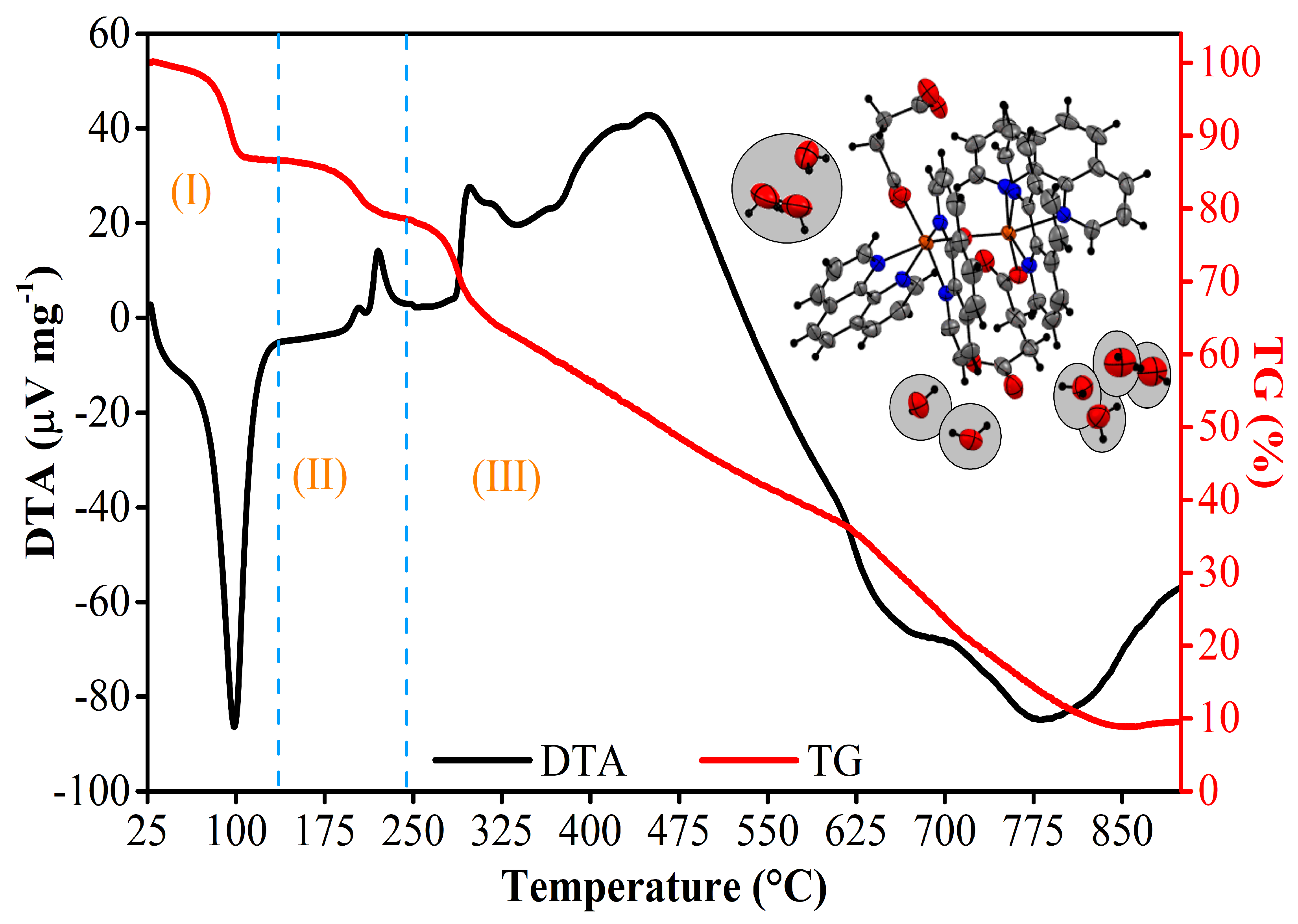
3.6. Thermal Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Xie, J.; Zhu, Z. 1,10-Phenanthroline: A Versatile Ligand to Promote Copper-Catalyzed Cascade Reactions. Appl. Organomet. Chem. 2020, 34, e5926. [Google Scholar] [CrossRef]
- Kunz, D.; Flaig, K.S. The Coordinative Flexibility of Rigid Phenanthroline-Analogous Di(NHC)-Ligands. Coord. Chem. Rev. 2018, 377, 73–85. [Google Scholar] [CrossRef]
- Dalal, A.; Nehra, K.; Hooda, A.; Singh, D.; Kumar, P.; Kumar, S.; Malik, R.S.; Rathi, B. Luminous Lanthanide Diketonates: Review on Synthesis and Optoelectronic Characterizations. Inorganica Chim. Acta 2023, 550, 121406. [Google Scholar] [CrossRef]
- Binnemans, K.; Lenaerts, P.; Driesen, K.; Görller-Walrand, C. A Luminescent Tris(2-Thenoyltrifluoroacetonato)Europium(Iii) Complex Covalently Linked to a 1,10-Phenanthroline-Functionalised Sol-Gel Glass. J. Mater. Chem. 2004, 14, 191. [Google Scholar] [CrossRef]
- von Eschwege, K.G.; Conradie, J. Review of DFT-Simulated and Experimental Electrochemistry Properties of the Polypyridyl Row-1 Mn, Fe & Co, and Group-8 Fe, Ru and Os MLCT Complexes. Electrochem. Commun. 2022, 136, 107225. [Google Scholar] [CrossRef]
- Kipgen, L.; Bernien, M.; Tuczek, F.; Kuch, W. Spin-Crossover Molecules on Surfaces: From Isolated Molecules to Ultrathin Films. Adv. Mater. 2021, 33, 2008141. [Google Scholar] [CrossRef]
- Butler, S.M.; Jolliffe, K.A. Molecular Recognition and Sensing of Dicarboxylates and Dicarboxylic Acids. Org. Biomol. Chem. 2020, 18, 8236–8254. [Google Scholar] [CrossRef]
- Neto, J.G.O.; da Silva Filho, J.G.; Cruz, N.S.; de Sousa, F.F.; Filho, P.F.F.; Santos, A.O. Growth, Structural, Vibrational, DFT and Thermal Studies of Bis(β-Alanine) Nickel(II) Dihydrate Crystals. J. Phys. Chem. Solids 2020, 141, 109435. [Google Scholar] [CrossRef]
- Ramos, M.C.; Neto, J.G.D.O.; Nogueira, C.E.S.; Reis, A.S.; Sousa, F.F.D.; Silva, L.M.; Santos, A.O. Structural, Vibrational, Thermal, and Cytotoxic Characterization of Aqua (1,10-Phenanthroline)(L-Serinato) Copper (II) Nitrate Complex Combined with DFT Calculations. Cryst. Res. Technol. 2023, 58, 2300240. [Google Scholar] [CrossRef]
- de Oliveira Neto, J.G.; Viana, J.R.; Butarelli, A.L.A.; dos Santos, A.P.A.; Lage, M.R.; dos Santos, A.O. Synthesis, Physicochemical Properties, and Antitumor Cytotoxic Activity of the Mg(II) Coordination Complex Containing 1,10-Phenanthroline and Sulfate Ligands. Inorganica Chim. Acta 2023, 556, 121658. [Google Scholar] [CrossRef]
- Engel, E.R.; Engel, E.R.; Scott, J.L. Advances in the Green Chemistry of Coordination Polymer Materials. Green Chem. 2020, 22, 3693–3715. [Google Scholar] [CrossRef]
- Fu, W.; Yi, J.; Cheng, M.; Liu, Y.; Zhang, G.; Li, L.; Du, L.; Li, B.; Wang, G.; Yang, X. When Bimetallic Oxides and Their Complexes Meet Fenton-like Process. J. Hazard. Mater. 2022, 424, 127419. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Nath, H.; Frontera, A.; Bhattacharyya, M.K. A Novel Oxalato Bridged Supramolecular Ternary Complex of Cu(II) Involving Energetically Significant π-Hole Interaction: Experimental and Theoretical Studies. Inorganica Chim. Acta 2019, 487, 354–361. [Google Scholar] [CrossRef]
- Emami, F.; Aliomrani, M.; Tangestaninejad, S.; Kazemian, H.; Moradi, M.; Rostami, M. Copper-Curcumin-Bipyridine Dicarboxylate Complexes as Anticancer Candidates. Chem. Biodivers. 2022, 19, e202200202. [Google Scholar] [CrossRef]
- Beheshti, A.; Lalegani, A.; Bruno, G.; Amiri Rudbari, H. Investigating the Effect of Flexible Ligands on the Crystal Engineering of the Iron(II) Coordination Compounds. J. Mol. Struct. 2013, 1051, 244–249. [Google Scholar] [CrossRef]
- Malinowski, J.; Zych, D.; Jacewicz, D.; Gawdzik, B.; Drzeżdżon, J. Application of Coordination Compounds with Transition Metal Ions in the Chemical Industry—A Review. Int. J. Mol. Sci. 2020, 21, 5443. [Google Scholar] [CrossRef]
- Matsumoto, Y. Energy Positions of Oxide Semiconductors and Photocatalysis with Iron Complex Oxides. J. Solid State Chem. 1996, 126, 227–234. [Google Scholar] [CrossRef]
- Que, L.; Tolman, W.B. Biologically Inspired Oxidation Catalysis. Nature 2008, 455, 333–340. [Google Scholar] [CrossRef]
- Hauser, A. Ligand Field Theoretical Considerations. Adv. Polym. Sci. 2004, 233, 49–58. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Real, J.A.; Tanaka, K. Spin Crossover and Photomagnetism in Dinuclear Iron(II) Compounds. Coord. Chem. Rev. 2007, 251, 1822–1833. [Google Scholar] [CrossRef]
- Olguín, J.; Brooker, S. Spin Crossover Active Iron(II) Complexes of Selected Pyrazole-Pyridine/Pyrazine Ligands. Coord. Chem. Rev. 2011, 255, 203–240. [Google Scholar] [CrossRef]
- Roth, H.C.; Schwaminger, S.P.; Schindler, M.; Wagner, F.E.; Berensmeier, S. Influencing Factors in the CO-Precipitation Process of Superparamagnetic Iron Oxide Nano Particles: A Model Based Study. J. Magn. Magn. Mater. 2015, 377, 81–89. [Google Scholar] [CrossRef]
- APEX4 Data Collection Software, Version 2021.4-0; Bruker AXS Inc.: Madison, WI, USA, 2021.
- SAINT Data Reduction Software; Version 8.36; Bruker AXS Inc.: Madison, WI, USA, 2019.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A Version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Tan, H.L.; Abdi, F.F.; Ng, Y.H. Heterogeneous Photocatalysts: An Overview of Classic and Modern Approaches for Optical, Electronic, and Charge Dynamics Evaluation. Chem. Soc. Rev. 2019, 48, 1255–1271. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Gromwood, D.J.; Spackman, P.R.; Jay-alitaka, D.M.A.S. Crystal Explorer, Version 17.5; The University of Western Australia (UWA): Perth, Australia, 2017.
- Romańczyk, P.P.; Kurek, S.S. Molecular Electrochemistry of Coordination Compounds—A Correlation Between Quantum Chemical Calculations and Experiment; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Ramezanpour, B.; Mirzaei, M.; Jodaian, V.; Niknam Shahrak, M.; Frontera, A.; Molins, E. Seven and Eight-Coordinate Fe(III) Complexes Containing Pre-Organized Ligand 1,10-Phenanthroline-2,9-Dicarboxylic Acid: Solvent Effects, Supramolecular Interactions and DFT Calculations. Inorganica Chim. Acta 2019, 484, 264–275. [Google Scholar] [CrossRef]
- Vlasenko, V.G.; Kubrin, S.P.; Garnovskii, D.A.; Guda, A.A.; Pankin, I.A.; Trigub, A.L. Spin-Crossover in the Iron(II) Complex Based on Dihydro-Bis(Pyrazolyl)Borate and 1,10-Phenanthroline-5,6-Dione. Chem. Phys. Lett. 2020, 739, 136970. [Google Scholar] [CrossRef]
- Dimiza, F.; Barmpa, A.; Chronakis, A.; Hatzidimitriou, A.G.; Sanakis, Y.; Papadopoulos, A.N.; Psomas, G. Iron(III) Complexes with Non-Steroidal Anti-Inflammatory Drugs: Structure, Antioxidant and Anticholinergic Activity, and Interaction with Biomolecules. Int. J. Mol. Sci. 2023, 24, 6391. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.M.; Alattar, R.A.; Abass, S.K.; Mihsen, H.H.; Abbas, Z.F.; Hussain, K.A. Synthesis, characterization and biological activity of mixed ligand (imine of benzidine and 1,10-phenanthroline) complexes with Fe(II), Co(II), Ni(II) and Cu(II) ions. Chem. Chem. Technol. 2022, 16, 15–24. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Kubasov, A.S.; Korolenko, S.E.; Privalov, V.I.; Malinina, E.A.; Kuznetsov, N.T. Iron(II), Cobalt(II), and Nickel(II) Complexes with 1,10-Phenanthroline and 2,2′-Bipyridyl and the Macropolyhedral Borane Cluster [Trans-B20H18]2- as Counterion. Polyhedron 2022, 217, 115740. [Google Scholar] [CrossRef]
- Oliveira Neto, J.G.D.; Viana, J.R.; Kamila, R.V.; Luiz, R.A.; Silva, F.L.; Lage, M.R.; Stoyanov, S.R.; Sousa, F.F.D.; Lang, R.; Adenilson, O. Tutton Salt (NH4)2Zn(SO4)2(H2O)6: Thermostructural, Spectroscopic, Hirshfeld Surface, and DFT Investigations. J. Mol. Model. 2024, 30, 339. [Google Scholar] [CrossRef]
- de Oliveira Neto, J.G.; Viana, J.R.; Abreu, K.R.; Butarelli, A.L.A.; dos Santos, A.P.A.; Lage, M.R.; de Sousa, F.F.; Souto, E.B.; dos Santos, A.O. Antitumor Neodymium(III) Complex with 1,10-Phenanthroline and Nitrate Ligands: A Comprehensive Experimental-Theoretical Study, in Silico Pharmacokinetic and Cytotoxic Properties. J. Mol. Struct. 2025, 1321, 139757. [Google Scholar] [CrossRef]
- Maradudin, A.A.; Vosko, S.H. Symmetry Properties of the Normal Vibrations of a Crystal. Rev. Mod. Phys. 1968, 40, 1–37. [Google Scholar] [CrossRef]
- Schöne, S.; Radoske, T.; März, J.; Stumpf, T.; Ikeda-Ohno, A. Synthesis and Characterization of Heterometallic Iron-Uranium Complexes with a Bidentate N-Donor Ligand (2,2′-Bipyridine or 1,10-Phenanthroline). Inorg. Chem. 2018, 57, 13318–13329. [Google Scholar] [CrossRef]
- Smith, G.L.; Reutovich, A.A.; Srivastava, A.K.; Reichard, R.E.; Welsh, C.H.; Melman, A.; Bou-Abdallah, F. Complexation of Ferrous Ions by Ferrozine, 2,2′-Bipyridine and 1,10-Phenanthroline: Implication for the Quantification of Iron in Biological Systems. J. Inorg. Biochem. 2021, 220, 111460. [Google Scholar] [CrossRef]
- Pereira, W.O.; Neto, J.G.O.; Viana, J.R.; Carvalho, G.G.C.; Santos, A.O. Synthesis, Electronic and Vibrational Properties, Solvation Effects, ADME and Cytotoxic Assay of Aqua -(1,10-phenanthroline)-tyrosinato-copper(II) Nitrate Dihydrate Complex: Experimental and Theoretical Studies. J. Mater. Sci. 2024, 1, 9502–9518. [Google Scholar] [CrossRef]
- Rodrigues, J.A.O.; Torres, A.U.; de Sousa, N.A.B.; de Sousa, T.J.D.; Neto, J.G.O.; Reis, A.S.; Lage, M.R.; dos Santos, A.O.; dos Santos, C.C.; de Menezes, A.S.; et al. Synthesis, Characterization, DFT Study, and Antibacterial Activity of a Coordination Complex of Cu(II) with 1,10-Phenanthroline and L-Methionine Ligands. J. Mol. Struct. 2023, 1293, 136197. [Google Scholar] [CrossRef]
- de Oliveira Neto, J.G.; Filho, J.G.S.; Bittar, E.M.; Silva, L.M.; de Sousa, F.F.; Domingos, H.V.; Costa-Lotufo, L.V.; Reis, A.S.; dos Santos, A.O. Structural, Thermal, Electronic, Vibrational, Magnetic, and Cytotoxic Properties of Chloro(Glycinato-N,O)(1,10-Phenanthroline-N,N′)-copper(II) Trihydrate Coordination Complex. J. Inorg. Biochem. 2022, 226, 111658. [Google Scholar] [CrossRef] [PubMed]
- Nurani, D.A.; Butar, B.C.B.; Krisnandi, Y.K. Synthesis and Characterization of Metal Organic Framework Using Succinic Acid Ligand with Cobalt and Iron Metals as Methylene Blue Dye Adsorbent. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 4th International Symposium on Current Progress in Functional Materials, Bali, Indonesia, 6–7 November 2019; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 902. [Google Scholar] [CrossRef]
- Tigineh, G.T.; Sitotaw, G.; Workie, A.; Abebe, A. Synthesis, Characterization and in Vitro Antibacterial Studies on Mixed Ligand Complexes of Iron(III) Based on 1,10-Phenanthroline. J. Korean Chem. Soc. 2021, 65, 203–208. [Google Scholar] [CrossRef]
- Ouyang, Z.J.; Mo, X.Y.; Ye, J.Q.; Yu, X.X.; Huang, S.Y.; Liu, X.L.; Chen, W.B.; Gao, S.; Dong, W. High Temperature Anionic Fe(Iii) Spin Crossover Behavior in a Mixed-Valence Fe(Ii)/Fe(Iii) Complex. Dalt. Trans. 2021, 50, 5960–5967. [Google Scholar] [CrossRef]
- Azad, U.P.; Turllapati, S.; Rastogi, P.K.; Ganesan, V. Tris(1,10-Phenanthroline)Iron(II)-Bentonite Film as Efficient Electrochemical Sensing Platform for Nitrite Determination. Electrochim. Acta 2014, 127, 193–199. [Google Scholar] [CrossRef]
- Šima, J.; Makáňová, J. Photochemistry of Iron (III) Complexes. Coord. Chem. Rev. 1997, 160, 161–189. [Google Scholar] [CrossRef]
- Singh, J.; Srivastav, A.N.; Sinh, N.; Singh, A. Stability Constants of Metal Complexes in Solution. In Stability and Applications of Coordination Compounds; Srivastav, A.N., Ed.; IntechOpen: London, UK, 2020; Volume 1, pp. 1–18. [Google Scholar] [CrossRef]
- Starikov, A.G.; Starikova, A.A.; Chegerev, M.G.; Minkin, V.I. Computational Modeling of Spin-Crossover in Mixed-Ligand Binuclear Iron and Cobalt Complexes with 5,6-Bis(Salicylideneimino)-1,10-Phenanthroline. Russ. J. Coord. Chem. Khimiya 2019, 45, 105–111. [Google Scholar] [CrossRef]
- Droghetti, A.; Alfè, D.; Sanvito, S. Assessment of Density Functional Theory for Iron(II) Molecules across the Spin-Crossover Transition. J. Chem. Phys. 2012, 137, 124303. [Google Scholar] [CrossRef]
- Kepp, K.P. The Electronic Determinants of Spin Crossover Described by Density Functional Theory. In Challenges and Advances in Computational Chemistry and Physics; Springer: Berlin/Heidelberg, Germany, 2019; Volume 29, pp. 1–33. [Google Scholar] [CrossRef]
- Krishnan, S.; Raj, C.J.; Robert, R.; Ramanand, A.; Jerome Das, S. Growth and Characterization of Succinic Acid Single Crystals. Cryst. Res. Technol. 2007, 42, 1087–1090. [Google Scholar] [CrossRef]
- Braun, D.E.; Schneeberger, A.; Griesser, U.J. Understanding the Role of Water in 1,10-Phenanthroline Monohydrate. CrystEngComm 2017, 19, 6133–6145. [Google Scholar] [CrossRef]


| CCDC Deposition Number | 2300427 |
|---|---|
| Chemical Formula * | C56H58Fe2N8O18·9(H2O) |
| Mr (g∙mol−1) | 1242.80 |
| a (Å) | 12.7772 (10) |
| b (Å) | 23.0786 (15) |
| c (Å) | 18.9982 (13) |
| α = γ (°) | 90 |
| β (°) | 93.047 (3) |
| V (Å3) | 5594.3 (7) |
| Z | 4 |
| Radiation Type | MoKα |
| µ (mm−1) | 0.601 |
| Crystal Size (mm3) | 0.36 × 0.33 × 0.24 |
| Data Collection | |
| Diffractometer | Bruker D8 Venture |
| Absorption Correction | Integration (SADAB) |
| Tmin, Tmax | 0.981, 0.993 |
| N° of Measured, Independent and Observed [I > 2σ(I)] reflections | 97,690, 10,259, 3420 |
| Rint, Rsigma | 0.0425, 0.0265 |
| (sin θ/λ)max (Å−1) | 0.588 |
| Refinement | |
| R[F2 > 2σ (F2)], wR(F2), S [all data] | 0.0617, 0.1219, 1.075 |
| N° of Reflections | 10,259 |
| N° of Parameters | 399 |
| H-atom treatment | Riding Model |
| Δρmax, Δρmin (e Å−3) | 0.43, −0.47 |
| Bonds | Distances (Å) | Angles (°) | ||
|---|---|---|---|---|
| D–H⋯A | D–H | H⋯A | D⋯A | D–H⋯A |
| C4–O3⋅⋅⋅H2WB (i) | 0.58 | 1.35 | 1.94 | 135.0 |
| C4–O4⋅⋅⋅H1WB (ii) | 0.58 | 1.36 | 1.94 | 166.5 |
| C4–O4⋅⋅⋅H1WA (iii) | 0.62 | 1.44 | 2.06 | 165.4 |
| C13A–H13A⋅⋅⋅H3WA (iv) | 0.63 | 1.46 | 2.09 | 84.6 |
| C4–O3⋅⋅⋅H3WB (v) | 0.63 | 1.48 | 2.11 | 114.6 |
| C11B–H11B⋅⋅⋅H2A (x) | 0.71 | 1.66 | 2.37 | 72.0 |
| C2–H2A⋅⋅⋅H1WA (iii) | 0.72 | 1.68 | 2.39 | 161.7 |
| C12A–O2⋅⋅⋅H12A (vii) | 0.73 | 1.71 | 2.44 | 145.2 |
| C2B–H2BA⋅⋅⋅O2W (viii) | 0.74 | 1.73 | 2.47 | 124.3 |
| C3A–H3A⋅⋅⋅O4W (ix) | 0.76 | 1.76 | 2.52 | 55.6 |
| C1–O2⋅⋅⋅H5WB (iv) | 0.78 | 1.82 | 2.60 | 168.8 |
| C13A–H13A⋅⋅⋅O3W (iv) | 0.81 | 1.88 | 2.69 | 82.5 |
| C8B–H8B⋅⋅⋅O5W (vi) | 0.81 | 1.89 | 2.69 | 74.9 |
| General | DTA | TG | |||
|---|---|---|---|---|---|
| Frag. | Stage | Temp (°C) | Weight Loss | Molar Mass (g∙mol−1) | |
| Exp. | Calc. * | ||||
| 9 H2O | I (25–136 °C) | 80 (↓) | 13.3% 0.49 mg | 162.14 | 170.28 |
| C12H6N2O2 + C4H6O4 | II (136–242 °C) | 205 (↑) 222 (↑) | 8.15% 0.30 mg | 101.29 | 181.70 |
| C12H6N2O2 + C4H6O4 + FeO | III (242–855 °C) | 298 (↑) 341 (↓) 455 (↑) 671 (↓) 783 (↓) | 69.86% 2.58 mg | 868.22 | 890.82 |
| Total Molecular Mass (g∙mol−1) | 1132.69 | 1242.80 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavarize, D.G.; Oliveira Neto, J.G.d.; Abreu, K.R.; Ayala, A.P.; Sousa, F.F.d.; Santos, A.O.d. A Novel Fe(III)-Complex with 1,10-Phenanthroline and Succinate Ligands: Structure, Intermolecular Interactions, and Spectroscopic and Thermal Properties for Engineering Applications. Processes 2025, 13, 1267. https://doi.org/10.3390/pr13051267
Zavarize DG, Oliveira Neto JGd, Abreu KR, Ayala AP, Sousa FFd, Santos AOd. A Novel Fe(III)-Complex with 1,10-Phenanthroline and Succinate Ligands: Structure, Intermolecular Interactions, and Spectroscopic and Thermal Properties for Engineering Applications. Processes. 2025; 13(5):1267. https://doi.org/10.3390/pr13051267
Chicago/Turabian StyleZavarize, Danilo Gualberto, João G. de Oliveira Neto, Kamila Rodrigues Abreu, Alejandro Pedro Ayala, Francisco Ferreira de Sousa, and Adenilson Oliveira dos Santos. 2025. "A Novel Fe(III)-Complex with 1,10-Phenanthroline and Succinate Ligands: Structure, Intermolecular Interactions, and Spectroscopic and Thermal Properties for Engineering Applications" Processes 13, no. 5: 1267. https://doi.org/10.3390/pr13051267
APA StyleZavarize, D. G., Oliveira Neto, J. G. d., Abreu, K. R., Ayala, A. P., Sousa, F. F. d., & Santos, A. O. d. (2025). A Novel Fe(III)-Complex with 1,10-Phenanthroline and Succinate Ligands: Structure, Intermolecular Interactions, and Spectroscopic and Thermal Properties for Engineering Applications. Processes, 13(5), 1267. https://doi.org/10.3390/pr13051267









