Optimization of Thiourea-Promoted Gold and Silver Leaching from Pyrite Cinders Using Response Surface Methodology (RSM)
Abstract
1. Introduction
2. Materials and Methods
2.1. Pretreatment and Characterization of Sample
2.2. Thiourea Leaching Experiment Procedure
2.3. Experimental Design
3. Results
3.1. Pretreatment and Characterization of Sample Results
- -
- One particle in free form—7.69%—particle size Au 0.8 × 0.8 µm;
- -
- One particle in conglomerate with hematite—7.69%—particle size Au 0.4 × 0.4 µm;
- -
- Three particles in conglomerate with goethite/hydrogoethite—23.08%—particle size from Au 0.3 to 1.2 µm;
- -
- Eight particles in aggregates with waste rock—61.54%—particle size varies from Au 0.3 to 1.4 µm.
- -
- Eight particles in free form—61.54%—particle size from Au 0.6 to 1.2 µm;
- -
- One particle in conglomerate with hematite—7.69%—particle size Au 1.2 × 1.5 µm;
- -
- Four particles in conglomerate with waste rock—30.77%—particle size varies from Au 0.5 to 1.3 µm.
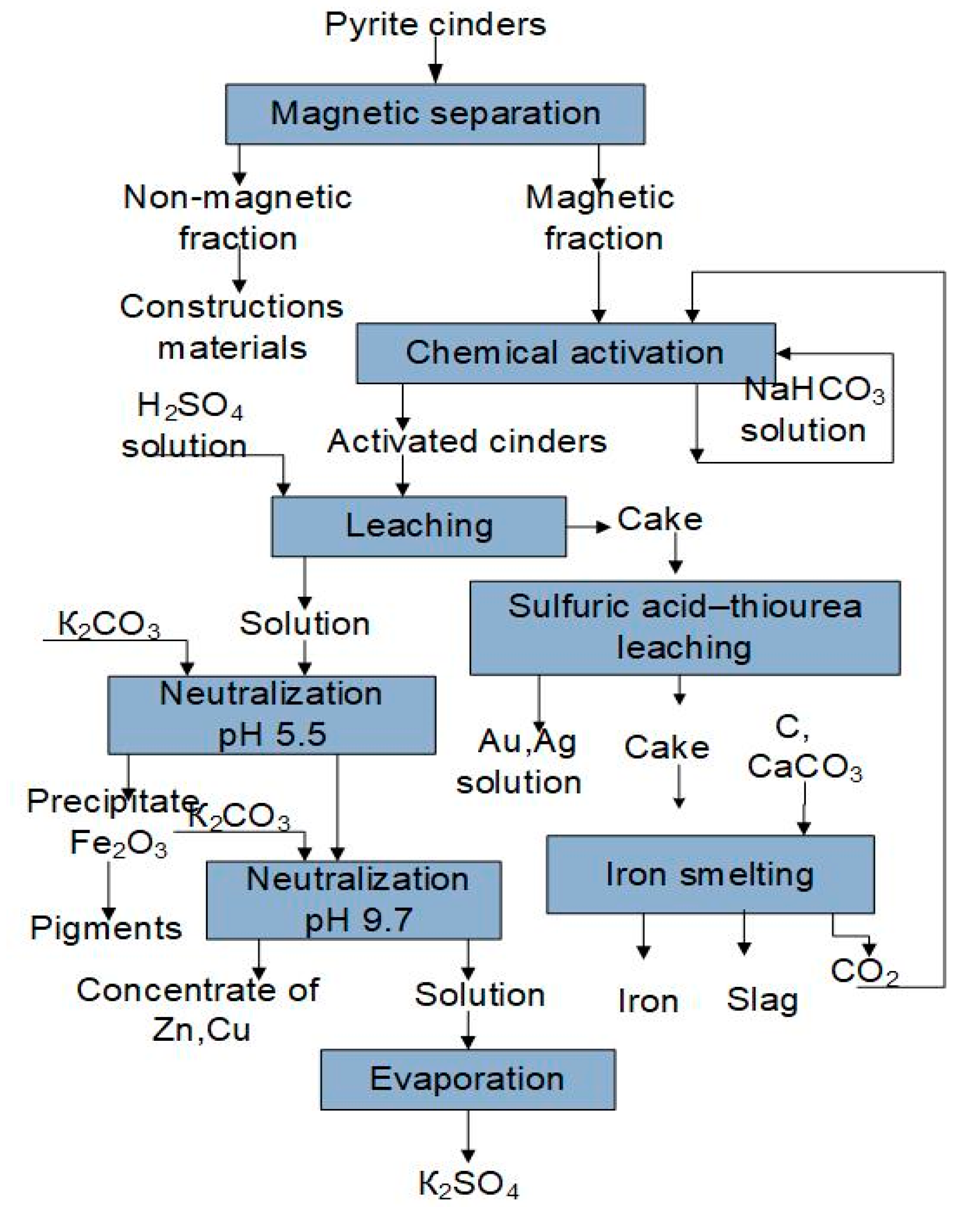
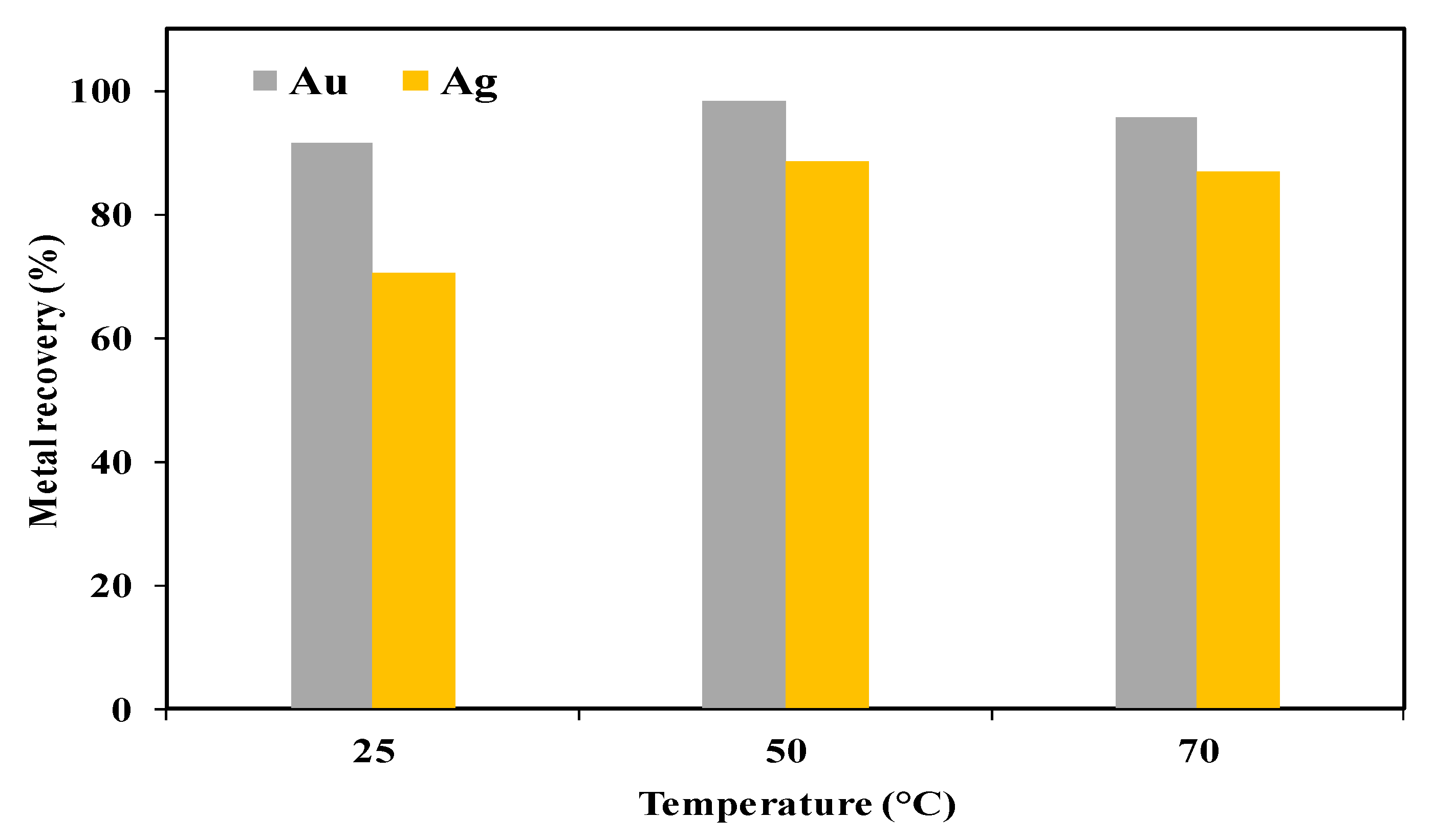
3.2. Selection of the Oxidizing Agent for Sulfuric Acid–Thiourea Leaching of the Pyrite Cinder Cake
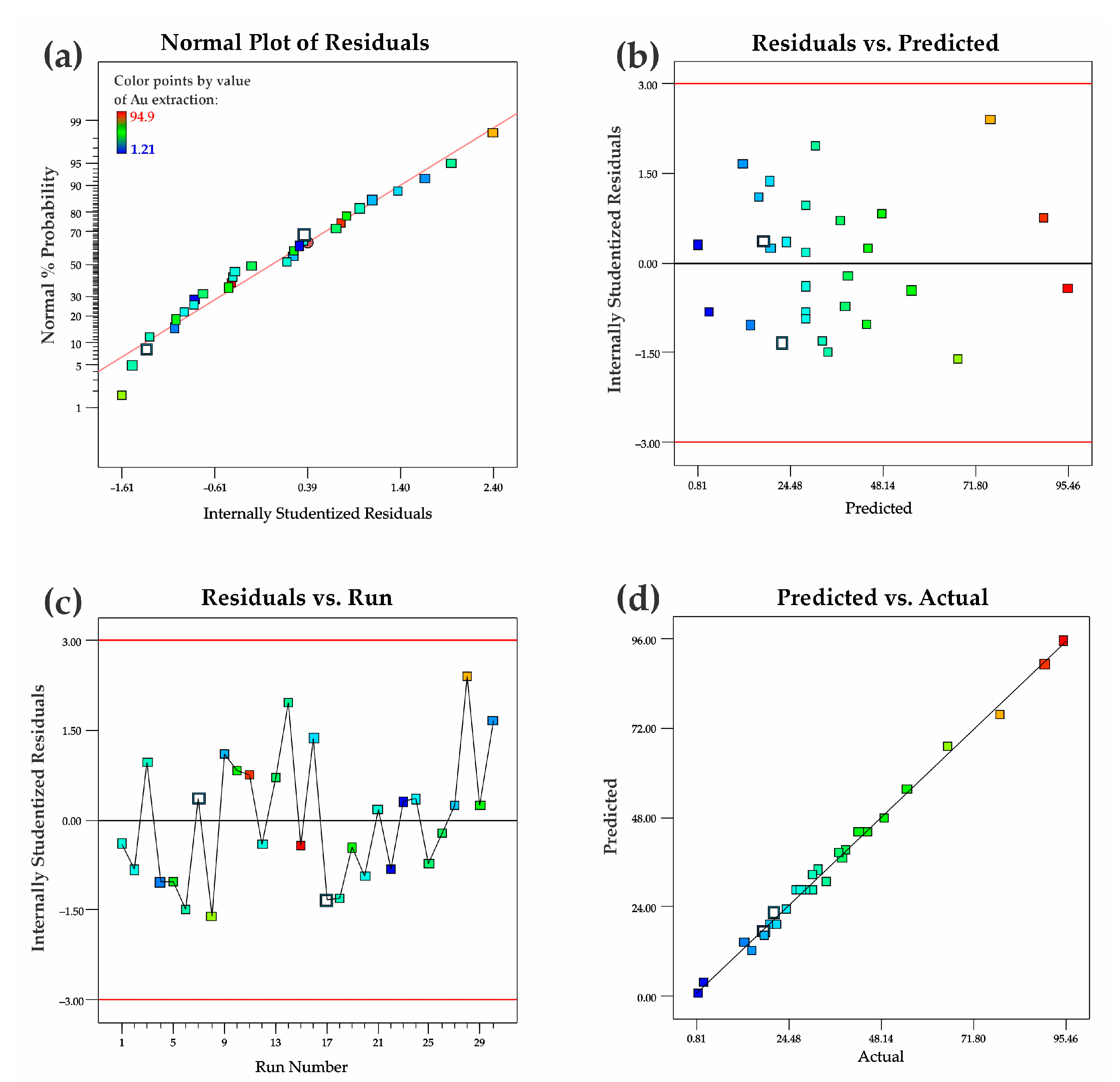
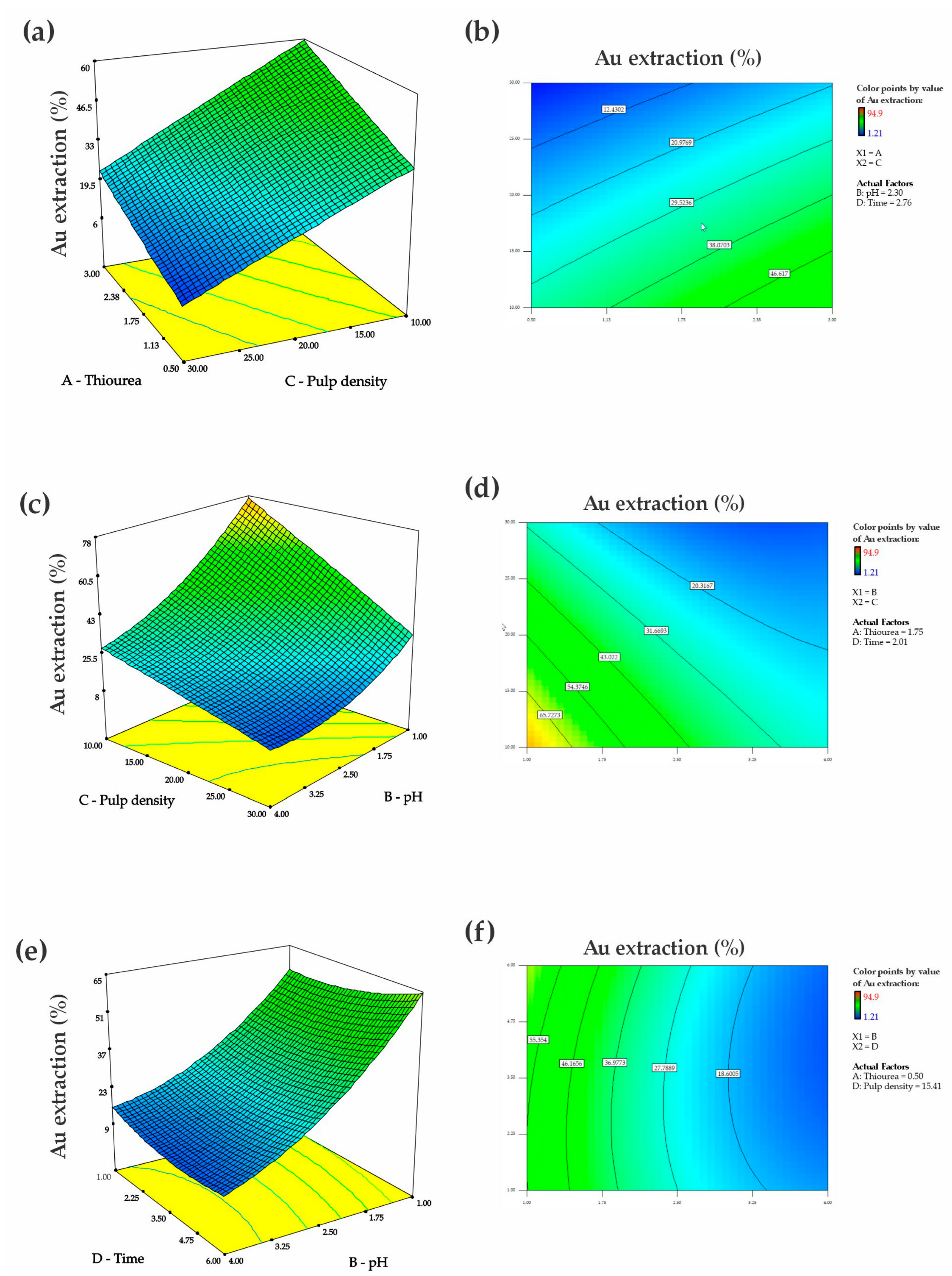
3.3. Statistical Analysis and Interpretation of Responses
3.4. Process Optimization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Müller, H. Sulfur dioxide, sulfuric acid and sulfur trioxide. In Ullmann Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 1994; Volume A25, pp. 569–703. [Google Scholar]
- Kirk-Othmer Encyclopedia of Chemical Technology; Wiley-Interscience: Hoboken, NJ, USA, 1997; Volume 23, pp. 258–278.
- Aksenchik, K.V. Technology of Sulfuric Acid; Chelyabinsk State University: Cherepovets, Russia, 2007; p. 209. [Google Scholar]
- Dementiev, V.; Khmelnitskaya, O.; Mullov, V.; Komlev, M.; Lanchakova, O. The development plant testing of valuable metals recovery technology from pyrite cinders. In Proceedings of the 26th International Mineral Processing Congress, IMPC 2012: Innovative Processing for Sustainable Growth, New Delhi, India, 24–28 September 2012; pp. 2412–2420. [Google Scholar]
- Surimbayev, B.; Akcil, A.; Bolotova, L.S.; Shalgymbayev, S.; Baykonurova, A. Processing of Refractory Gold-Bearing Sulfide Concentrates: A Review. Miner. Process. Extr. Metall. Rev. 2023, 45, 573–591. [Google Scholar] [CrossRef]
- Rintala, L.; Leikola, M.; Sauer, C.; Aromaa, J.; Roth Berghofer, T.; Forsén, O.; Lundström, M. Designing gold extraction processes: Performance study of a case-based reasoning system. Miner. Eng. 2017, 109, 42–53. [Google Scholar] [CrossRef]
- Li, J.; Zhu, T.; Zhang, F. Comparative study on the thiourea leaching of gold and silver from a sulfide concentrate and its calcine. Eng. Chem. Metall. 1992, 14, 311–318. (In Chinese) [Google Scholar]
- Wan, R.Y.; Luinstra, L.; Brierley, J.A. Gold recovery from refractory sulfidic-carbonaceous ore, Part (II): Thiourea leaching following biooxidation-heap pretreatment. In EPD Congress 1995; Warren, G.W., Ed.; The Minerals, Metals and Materials Society: Warrendale, PA, USA; pp. 165–173.
- Swamy, K.M. Application of Ultrasound in Leaching. Miner. Process. Extr. Metall. 1995, 4, 179–192. [Google Scholar] [CrossRef]
- Haque, K.E. Gold leaching from refractory ores—Literature survey. Miner. Process. Extr. Metall. Rev. 1987, 2, 235–253. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Surkova, T.; Berkinbayeva, A.; Baltabekova, Z.; Smailov, K.; Abikak, Y.; Saulebekkyzy, S.; Tolegenova, N.; Omirbek, T.; Dosymbaeva, Z. Innovative Methods for Intensifying the Processing of Zinc Clinker: Synergy of Microwave Treatment and Ultrasonic Leaching. Metals 2025, 15, 246. [Google Scholar] [CrossRef]
- Kenzhaliev, B.K.; Dyusenova, S.B.; Abdulvaliev, R.A.; Gladyshev, S.V.; Omarova, S.A.; Manapova, A.I.; Imangalieva, L.M. Method for Obtaining Chromite Concentrate from Poor Chromite-Containing Ores. Republic of Kazakstan Patent No. 33583, 19 July 2019. [Google Scholar]
- Akhmadiyeva, N.K.; Abdulvaliyev, R.A.; Akcil, A.; Manapova, A.I. Preactivation of nepheline before the enrichment. Complex Use Miner. Resour. 2023, 327, 82–89. [Google Scholar]
- Kurganov, K.P. A Method for Extracting Gold and a Device for Enriching Gold-Containing Raw Materials. Russian Federation Patent No 2483807, 2 February 2013. [Google Scholar]
- Bocharov, V.A.; Ignatkina, V.A.; Chanturiya, E.L.; Yushina, T.I.; Khachatryan, L.S.; Dunayeva, V.N. On the choice of possible ways of complex use of technogenic pyrite tailings in connection with their processing. Min. Inf. Anal. Bull. 2005, 10, 92–99. [Google Scholar]
- Bykov, A.A.; Arzhannikov, G.I. Method of Mineral Raw Material Enrichment. Russian Federation Patent No 2149706, 25 May 2000. [Google Scholar]
- Gorlova, O.E.; Shadrunova, I.V.; Zhilina, V.A.; Chekushina, T.V. Increasing the completeness of gold extraction from the lying waste processing of gold-bearing ores. Proc. Tula State Univ. 2020, 1, 193–2101. [Google Scholar]
- Schelkonogov, M.A.; Litvinenko, L.G.; Litvinenko, V.G.; Morozov, A.A. Method of Complex Processing of Pyrite Cinders. Russian Federation Patent No 2623948, 26 June 2017. [Google Scholar]
- Vasilkova, N.A.; Zhuchkov, I.A.; Ignatieva, K.D.; Lodeishchikov, V.V.; Panchenko, A.F.; Stakheev, I.S.; Shubina, O.A. Tekhnika i Tekhnologiya Izvlecheniya Zolota iz Rud za Rubezhom [Machinery and Technology of Gold Extraction from Ores Abroad]; Metallurgy: Moscow, Russia, 1973; 288p. (In Russian) [Google Scholar]
- Otu, E.O.; Wilson, J. Supercritical Carbon Dioxide Elution of Gold—Cyanide Complex from Activated Carbon. Sep. Sci. Technol. 2000, 35, 1879–1886. [Google Scholar] [CrossRef]
- Bidari, E.; Valeh, A. Alkaline leaching pretreatment and cyanidation of arsenical gold ore from the Carlin-type Zarshuran deposit. Can. Metall. Q. 2018, 57, 83–293. [Google Scholar] [CrossRef]
- Akcil, A. A New Global Approach of Cyanide Management: International Cyanide Management Code for the Manufacture, Transport, and Use of Cyanide in the Production of Gold. Miner. Process. Extr. Metall. Rev. 2010, 31, 135–149. [Google Scholar] [CrossRef]
- Martínková, L.; Bojarová, P.; Sedova, A.; Křen, V. Recent trends in the treatment of cyanide-containing effluents: Comparison of different approaches. Crit. Rev. Environ. Sci. Technol. 2023, 53, 416–434. [Google Scholar] [CrossRef]
- Udupa, A.R.; Kawatra, S.K.; Prasad, M.S. Developments in gold leaching: A literature survey. Miner. Process. Extr. Metall. Rev. 1990, 2, 115–135. [Google Scholar] [CrossRef]
- Konyratbekova, S.S.; Baikonurova, A.; Akcil, A. Non-cyanide leaching processes in gold hydrometallurgy and iodine-iodide applications: A review. Miner. Process. Extr. Metall. Rev. 2015, 36, 198–212. [Google Scholar] [CrossRef]
- Arslan, F.; Sayiner, B. Extraction of Gold and Silver from Turkish Gold Ore by Ammoniacal Thiosulphate Leaching. Miner. Process. Extr. Metall. Rev. 2008, 29, 68–82. [Google Scholar] [CrossRef]
- Abbruzzese, C.; Fornari, P.; Massidda, R.; Vegliò, F.; Ubaldini, S. Thiosulfate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265. [Google Scholar] [CrossRef]
- Zhang, X.; Senanayake, G. A Review of Ammoniacal Thiosulfate Leaching of Gold: An Update Useful for Further Research in Non-cyanide Gold Lixiviants. Miner. Process. Extr. Metall. Rev. 2016, 37, 385–411. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Surkova, T.; Yessimova, D.; Abikak, Y.; Mukhanova, A.; Fischer, D. On the question of the complex processing of pyrite cinders. Inorganics 2023, 11, 171. [Google Scholar] [CrossRef]
- Groenewald, T. Determination of gold in solutions of thiourea. Anal. Chem. 1971, 43, 1689–1691. [Google Scholar] [CrossRef]
- Li, J.; Miller, J.D. A Review of Gold Leaching in Acid Thiourea Solutions. Miner. Process. Extr. Metall. Rev. 2006, 27, 177–214. [Google Scholar] [CrossRef]
- Celik, H. Extraction of gold and silver from a Turkish gold ore through thiourea leaching. Min. Metall. Explor. 2004, 21, 144–148. [Google Scholar] [CrossRef]
- Örgül, S.; Atalay, Ü. Gold Extraction from kaymaz gold ore by thiourea leaching. Dev. Miner. Process 2000, 13, C6–C22. [Google Scholar]
- Zaikin, S.Y.; Shvyryaev, A.Y.; Travkina, V.A. Method of Leaching and Extraction of Gold and Silver from Pyrite Cinders. Russian Federation Patent No 2721731, 21 January 2021. [Google Scholar]
- Abikak, Y.B.; Kenzhaliev, B.K. Development of an integrated technology intended to process pyrite slag using chemical pre-activation. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2022, 3, 32–51. [Google Scholar] [CrossRef]
- Ait-Amir, B.; Pougnet, P.; El Hami, A. Meta-Model Development. In Embedded Mechatronic Systems 2; ISTE: Washington, DC, USA, 2015; pp. 151–179. [Google Scholar]
- Ren, G.; Heo, S.; Kim, T.; Cheong, C. Response surface method-based optimization of the shroud of an axial cooling fan for high performance and low noise. J. Mech. Sci. Technol. 2013, 27, 33–42. [Google Scholar] [CrossRef]
- Dembele, S.; Akcil, A.; Panda, S. Investigation of the characteristics of stibnite (Sb2S3) flotation tailings and extraction of critical metals (Sb and As): Optimization and scale-up. Miner. Eng. 2024, 216, 108883. [Google Scholar] [CrossRef]
- Rana, M.M.; Khan, I.H.; Nshizirungu, T.; Jo, Y.-T.; Park, J.H. Green and sustainable route for the efficient leaching and recovery of valuable metals from spent Ni- Cd batteries. Chem. Eng. J. 2023, 455, 140626. [Google Scholar] [CrossRef]
- Jing-Ying, L.; Xiu-Li, X.; Wen-Quan, L. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Manag. 2012, 32, 1209–1212. [Google Scholar] [CrossRef]
- Heydarian, A.; Mousavi, S.M.; Vakilchap, F.; Baniasadi, M. Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J. Power Sources 2018, 378, 19–30. [Google Scholar] [CrossRef]
- Rezaei, H.; Shafaei, S.Z.; Abdollahi, H.; Shahidi, A.; Ghassa, S. A sustainable method for germanium, vanadium and lithium extraction from coal fly ash: Sodium salts roasting and organic acids leaching. Fuel 2022, 312, 122844. [Google Scholar] [CrossRef]
- Birloaga, I.; Michelis, I.D.; Ferella, F.; Buzatu, M.; Francesco, V. Study on the influence of various factors in the hydrometallurgical processing of printed waste circuit boards for copper and gold recovery. Waste Manag. 2013, 33, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, G.; Ghali, E. Leaching of gold from a chalcopyrite concentrate by thiourea. Hydrometallurgy 1988, 20, 179–202. [Google Scholar] [CrossRef]
- Guzman, L.; Segarra, M.; Jimenos, J.M.; Fernandez, M.A.; Espiell, F. Cyanidation of gold using hydrogen peroxide. Hydrometallurgy 1999, 52, 21–35. [Google Scholar] [CrossRef]
- Kotlyar, Y.A.; Meretukov, M.A.; Strizhko, L.S. Metallurgy of Noble Metals; A Manual in 2 Volumes. Tutorial. T. 1.; Ore and Metals: Moscow, Russia, 2005; p. 431. [Google Scholar]
- Ippolito, N.M.; Birloaga, I.; Ferella, F.; Centofanti, M.; Vegliò, F. Preliminary study on gold recovery from high grade e-waste by thiourea leaching and electrowinning. Minerals 2021, 11, 235. [Google Scholar] [CrossRef]
- Qin, H.; Guo, X.; Tian, Q.; Yu, D.; Zhang, L. Recovery of gold from sulfide refractory gold ore: Oxidation roasting pretreatment and gold extraction. Miner. Eng. 2021, 164, 106822. [Google Scholar] [CrossRef]
- Beck, N.; Yu, R.; Zelinsky, A.G.; Weiss, A.A. Laws of anodic behavior of gold in sour thiocarbamide solutions according to cyclic voltammetry and quartz microgravimetry. Electrochemistry 2006, 3, 279–285. [Google Scholar]
- Moutiy, E.H.; Tran, L.H.; Mueller, K.K.; Coudert, L.; Blais, J.F. Optimized indium solubilization from LCD panels using H2SO4 leaching. Was. Manag. 2020, 114, 53–61. [Google Scholar]
- Murugesan, M.; Kannan, K.T.; Selvaganapathy, T. Bioleaching recovery of copper from printed circuit boards and optimization of various parameters using response surface methodology (RSM). Mater. Today Proc. 2020, 26, 2720–2728. [Google Scholar] [CrossRef]
- Zakrzewska-Koltuniewicz, G.; Herdzik-Koniecko, I.; Cojocaru, C.; Chajduk, E. Experimental design and optimization of leaching process for recovery of valuable chemical elements (U, La, V, Mo, Yb and Th) from low-grade uranium ore. J. Hazard. Mater. 2014, 275, 136–145. [Google Scholar] [CrossRef]
- Lee, H.; Molstad, E.; Mishra, B. Recovery of gold and silver from secondary sources of electronic waste processing by thiourea leaching. JOM 2018, 70, 1616–1621. [Google Scholar] [CrossRef]




| Process Parameter | Units | Symbol | Low Level | Middle Level | High Level |
|---|---|---|---|---|---|
| Thiourea | g/L | A | 5 | 17.5 | 30 |
| pH | - | B | 1 | 2.5 | 4 |
| Pulp density | % | C | 10 | 20 | 30 |
| Time | h | D | 1 | 3.5 | 6 |
| Name | Content, % | |
|---|---|---|
| Before Activation | After Activation | |
| Maghemite Fe2O3 | 23.4 | 29.1 |
| Hematite Fe2O3 | 17.5 | 21.8 |
| Quartz SiO2 | 16.7 | 14.9 |
| Albite Na(AlSi3O8) | 9.9 | 8.5 |
| Trisodium phosphate zinc oxide hydrate Na3Zn4O(PO4)3(H2O)6 | 9.2 | - |
| Sodium aluminum silicate NaAl3Si3O11 | 6.5 | 5.8 |
| Barium ferrite BaFe2O4 | 4.5 | 5.9 |
| Natrojarosite (Na0.67(H3O)0.33)Fe3(SO4)2(OH)6 | 4.1 | 4.3 |
| Pyrite FeS2 | 3.0 | - |
| Sodium thiophosphate Na2P2S6 | 2.9 | 5.7 |
| Dolomite CaMg(CO3)2 | 2.4 | - |
| Magnesium aluminum silicate (MgAl2Si3O10)0.6 | - | 2.4 |
| Calcium silicate CaSiO3 | - | 1.7 |
| Run | A: Thiourea (g/L) | B: pH | C: Pulp Density (%) | D: Time (h) | Au (%) | Ag (%) |
|---|---|---|---|---|---|---|
| 1 | 17.50 | 2.5 | 20 | 3.5 | 27.59 | 25.46 |
| 2 | 17.50 | 2.5 | 20 | 3.5 | 26.67 | 24.77 |
| 3 | 17.50 | 2.5 | 20 | 3.5 | 30.44 | 27.77 |
| 4 | 5.00 | 4 | 10 | 6 | 13.00 | 10.10 |
| 5 | 17.50 | 2.5 | 10 | 3.5 | 42.33 | 22.50 |
| 6 | 5.00 | 1 | 30 | 6 | 32.04 | 17.24 |
| 7 | 30.00 | 4 | 30 | 6 | 18.04 | 15.70 |
| 8 | 5.00 | 1 | 10 | 1 | 65.11 | 10.56 |
| 9 | 17.50 | 4 | 20 | 3.5 | 18.22 | 26.00 |
| 10 | 30.00 | 1 | 30 | 6 | 48.85 | 68.67 |
| 11 | 30.00 | 1 | 10 | 1 | 90.11 | 62.55 |
| 12 | 17.50 | 2.5 | 20 | 3.5 | 27.55 | 25.90 |
| 13 | 30.00 | 4 | 10 | 6 | 38.11 | 20.13 |
| 14 | 17.50 | 2.5 | 20 | 1 | 34.00 | 22.22 |
| 15 | 30.00 | 1 | 10 | 6 | 94.90 | 73.03 |
| 16 | 5.00 | 2.5 | 20 | 3.5 | 21.33 | 6.20 |
| 17 | 30.00 | 4 | 30 | 1 | 20.67 | 20.45 |
| 18 | 17.50 | 2.5 | 20 | 6 | 30.56 | 23.82 |
| 19 | 17.50 | 1 | 20 | 3.5 | 54.78 | 53.40 |
| 20 | 17.50 | 2.5 | 20 | 3.5 | 26.44 | 26.77 |
| 21 | 17.50 | 2.5 | 20 | 3.5 | 28.78 | 26.97 |
| 22 | 5.00 | 4 | 30 | 1 | 2.55 | 13.50 |
| 23 | 5.00 | 4 | 30 | 6 | 1.21 | 6.47 |
| 24 | 5.00 | 1 | 30 | 1 | 23.89 | 5.33 |
| 25 | 30.00 | 2.5 | 20 | 3.5 | 37.33 | 38.80 |
| 26 | 30.00 | 1 | 30 | 1 | 39.00 | 59.48 |
| 27 | 5.00 | 4 | 10 | 1 | 19.67 | 16.47 |
| 28 | 5.00 | 1 | 10 | 6 | 78.67 | 19.51 |
| 29 | 30.00 | 4 | 10 | 1 | 44.56 | 24.00 |
| 30 | 17.50 | 2.5 | 30 | 3.5 | 14.93 | 21.06 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 14,849.14 | 9 | 1649.90 | 358.13 | <0.0001 | Significant |
| A: Thiourea | 1683.88 | 1 | 1683.88 | 365.50 | <0.0001 | |
| B: pH | 6856.97 | 1 | 6856.97 | 1488.37 | <0.0001 | |
| C: Pulp density | 4521.45 | 1 | 4521.45 | 981.42 | <0.0001 | |
| D: Time | 13.89 | 1 | 13.89 | 3.02 | 0.0978 | |
| AC | 37.12 | 1 | 37.12 | 8.06 | <0.0001 | |
| BC | 786.06 | 1 | 786.06 | 170.62 | <0.0001 | |
| BD | 178.43 | 1 | 178.43 | 38.73 | <0.0001 | |
| B2 | 200.17 | 1 | 200.17 | 43.45 | <0.0001 | |
| D2 | 39.84 | 1 | 39.84 | 8.65 | 0.0081 | |
| Residual | 92.14 | 20 | 4.61 | |||
| Lack of fit | 81.04 | 15 | 5.40 | 2.43 | 0.1661 | Not significant |
| Pure error | 11.10 | 5 | 2.22 | |||
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 9635.57 | 10 | 963.56 | 808.65 | <0.0001 | Significant |
| A: Thiourea | 4275.86 | 1 | 4275.86 | 3588.44 | <0.0001 | |
| B: pH | 2614.76 | 1 | 2614.76 | 2194.39 | <0.0001 | |
| C: Pulp density | 53.24 | 1 | 53.24 | 44.68 | <0.0001 | |
| D: Time | 22.51 | 1 | 22.51 | 18.89 | 0.0003 | |
| AB | 1965.32 | 1 | 1965.32 | 1649.36 | <0.0001 | |
| BD | 244.45 | 1 | 244.45 | 205.15 | <0.0001 | |
| A2 | 39.57 | 1 | 39.57 | 33.21 | <0.0001 | |
| B2 | 457.75 | 1 | 457.75 | 384.16 | <0.0001 | |
| C2 | 55.53 | 1 | 55.53 | 46.60 | <0.0001 | |
| D2 | 29.73 | 1 | 29.73 | 24.95 | <0.0001 | |
| Residual | 22.64 | 19 | 1.19 | |||
| Lack of fit | 16.60 | 14 | 1.19 | 0.98 | 0.5565 | Not significant |
| Pure error | 6.04 | 5 | 1.21 | |||
| Thiourea (g/L) | Pulp Density (%) | pH | Time (h) | Predicted Results | Experimental Results | |||
|---|---|---|---|---|---|---|---|---|
| Au (%) | Ag (%) | Desirability | Au (%) | Ag (%) | ||||
| 30 | 10 | 1 | 4 | 90.46 | 72.00 | 0.969 | 91.57 | 70.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abikak, Y.; Kenzhaliev, B.; Akcil, A.; Dembele, S.; Koizhanova, A.; Bakhytuly, N.; Kassymova, G. Optimization of Thiourea-Promoted Gold and Silver Leaching from Pyrite Cinders Using Response Surface Methodology (RSM). Processes 2025, 13, 1277. https://doi.org/10.3390/pr13051277
Abikak Y, Kenzhaliev B, Akcil A, Dembele S, Koizhanova A, Bakhytuly N, Kassymova G. Optimization of Thiourea-Promoted Gold and Silver Leaching from Pyrite Cinders Using Response Surface Methodology (RSM). Processes. 2025; 13(5):1277. https://doi.org/10.3390/pr13051277
Chicago/Turabian StyleAbikak, Yerkezhan, Bagdaulet Kenzhaliev, Ata Akcil, Seydou Dembele, Aigul Koizhanova, Nauryzbek Bakhytuly, and Gulzhaina Kassymova. 2025. "Optimization of Thiourea-Promoted Gold and Silver Leaching from Pyrite Cinders Using Response Surface Methodology (RSM)" Processes 13, no. 5: 1277. https://doi.org/10.3390/pr13051277
APA StyleAbikak, Y., Kenzhaliev, B., Akcil, A., Dembele, S., Koizhanova, A., Bakhytuly, N., & Kassymova, G. (2025). Optimization of Thiourea-Promoted Gold and Silver Leaching from Pyrite Cinders Using Response Surface Methodology (RSM). Processes, 13(5), 1277. https://doi.org/10.3390/pr13051277









