Influence of Chromium Content in Alloys on Corrosion in Saline Water Saturated with Supercritical CO2
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Testing Materials and Solution Preparation
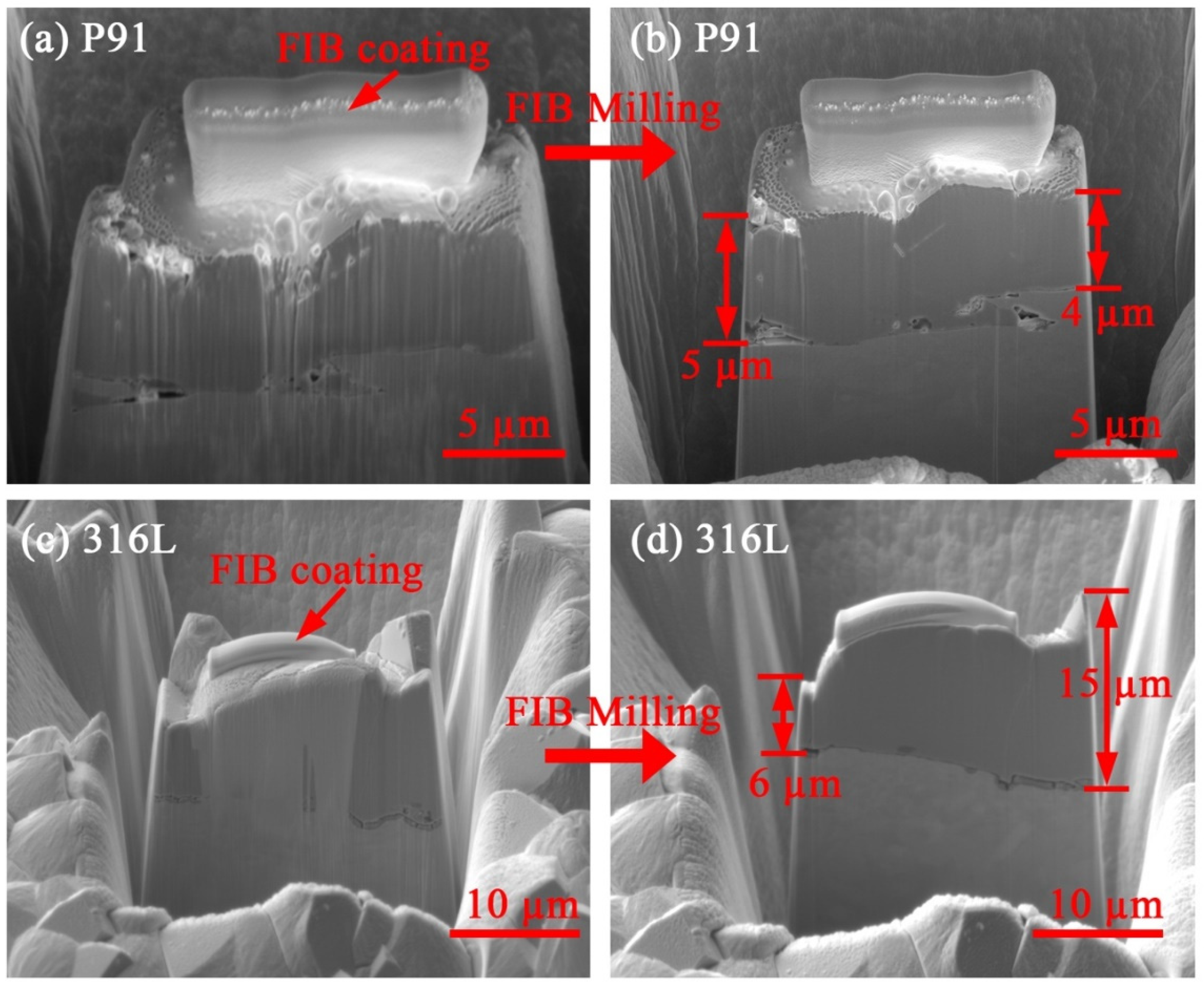
2.2. Autoclave Corrosion Experiments and Post-Experiment Characterization
3. Results
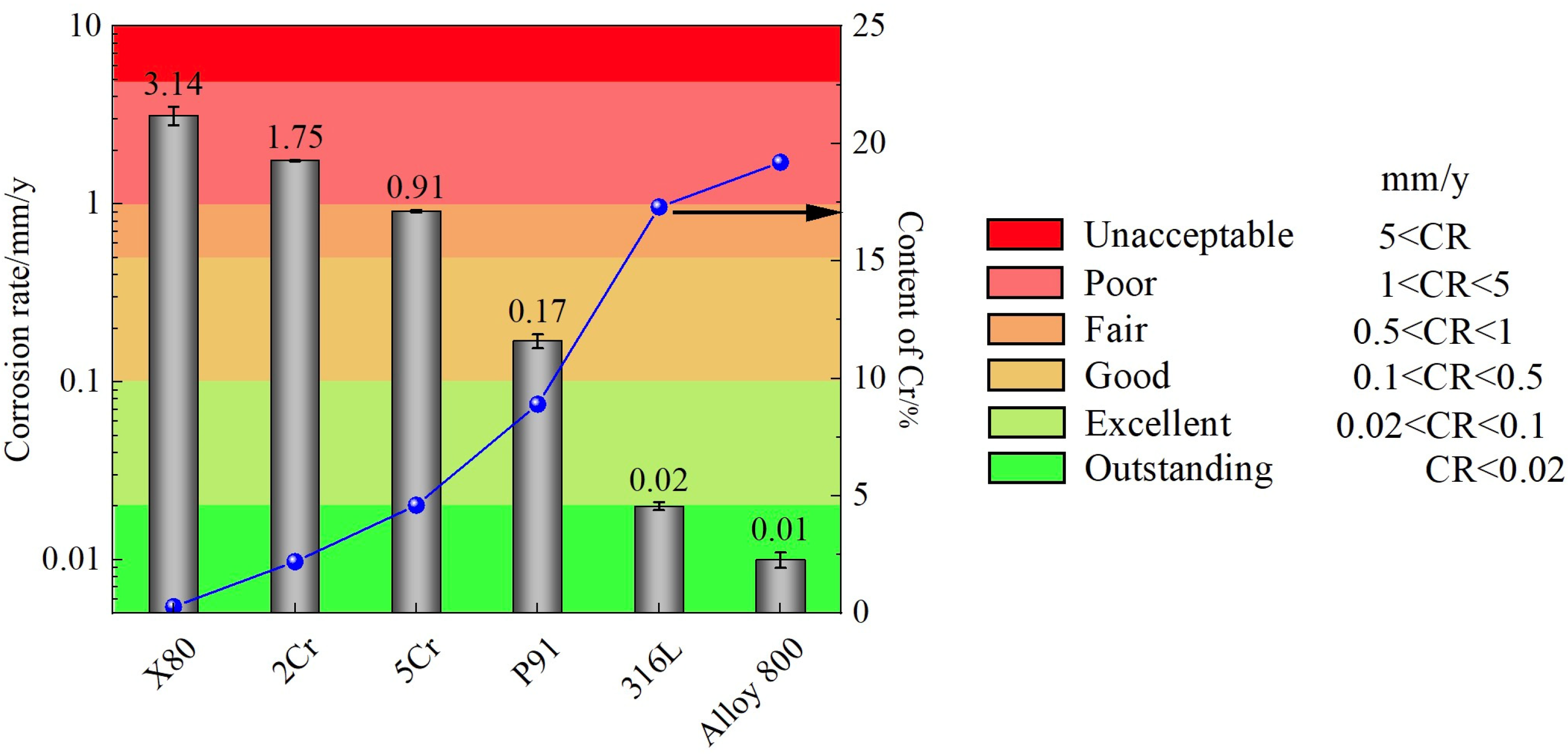
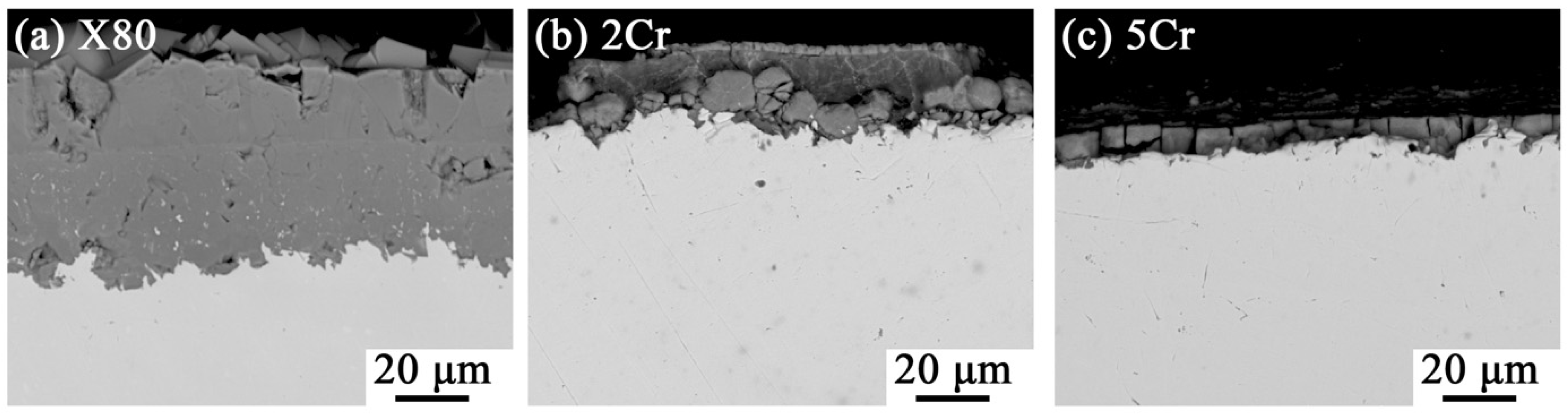
3.1. Average Corrosion Rates of Alloys
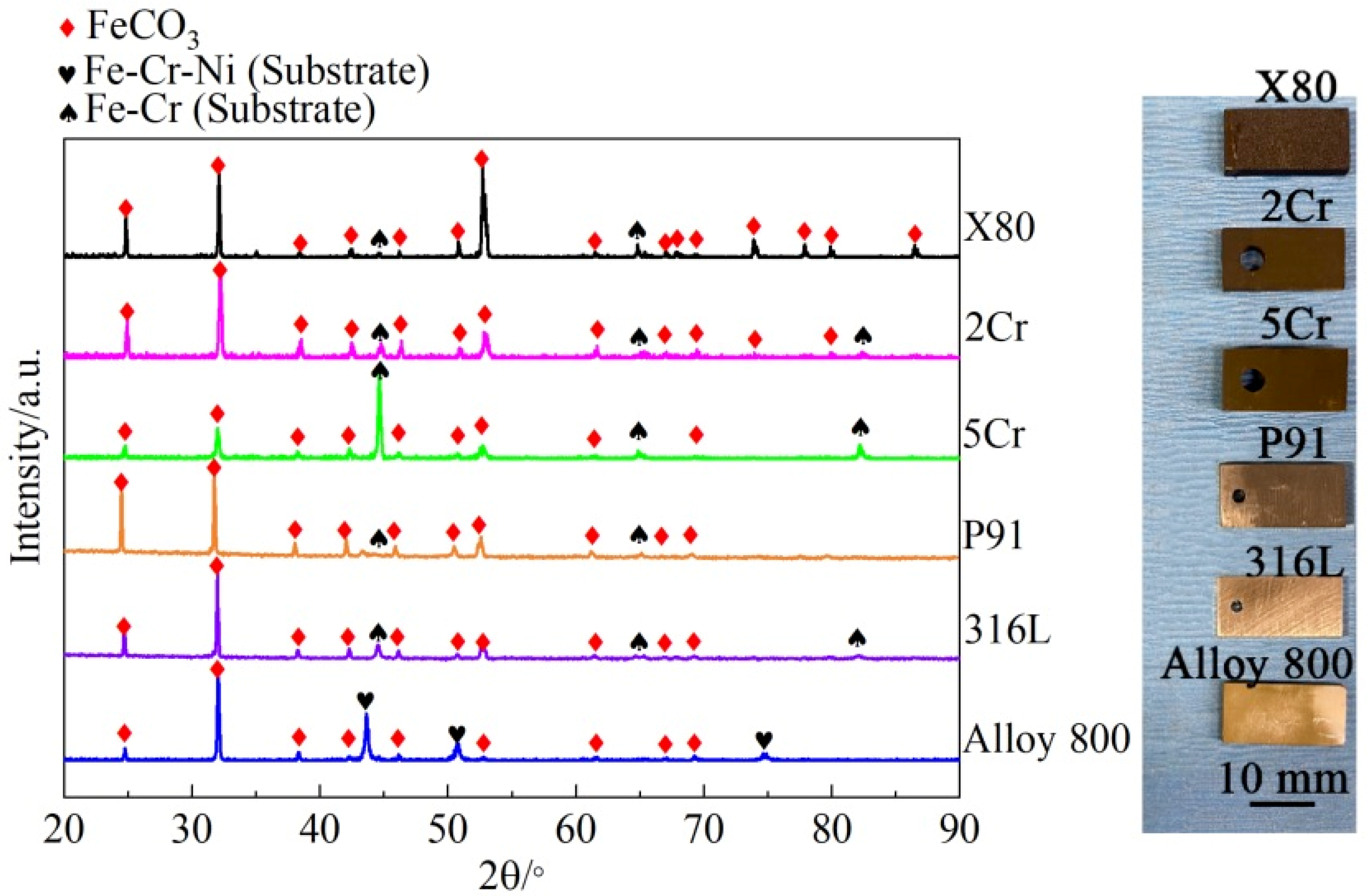
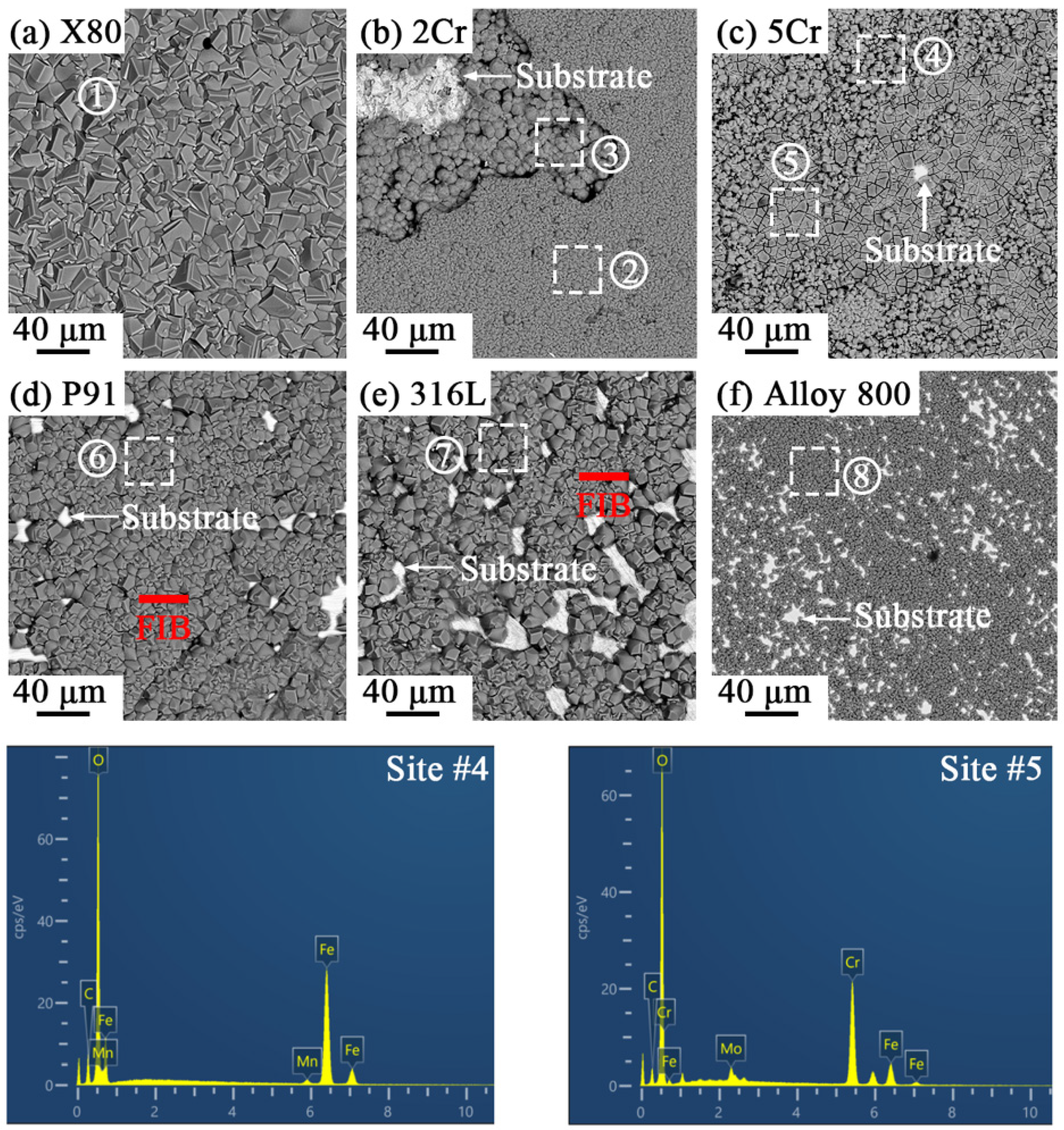
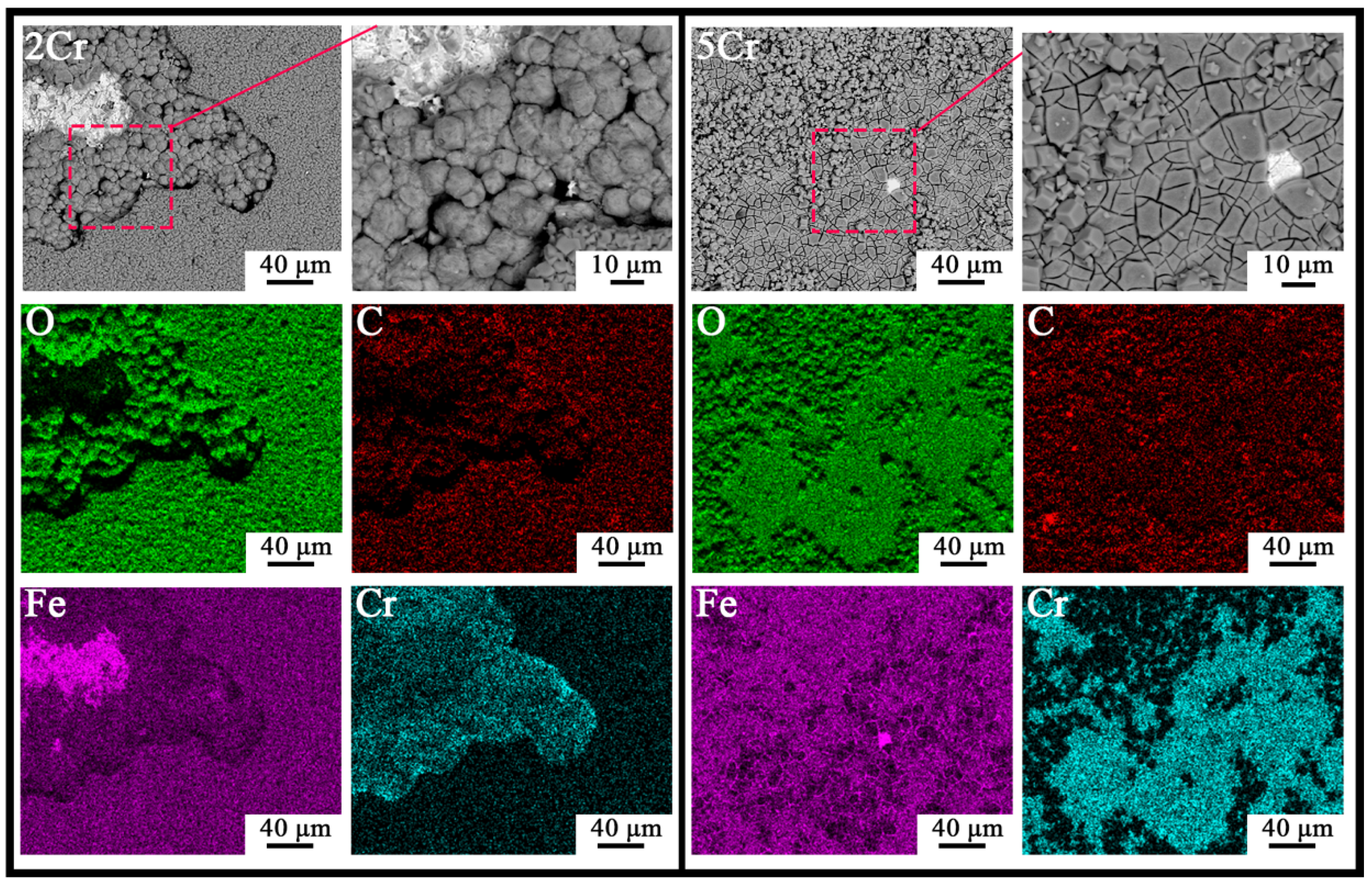
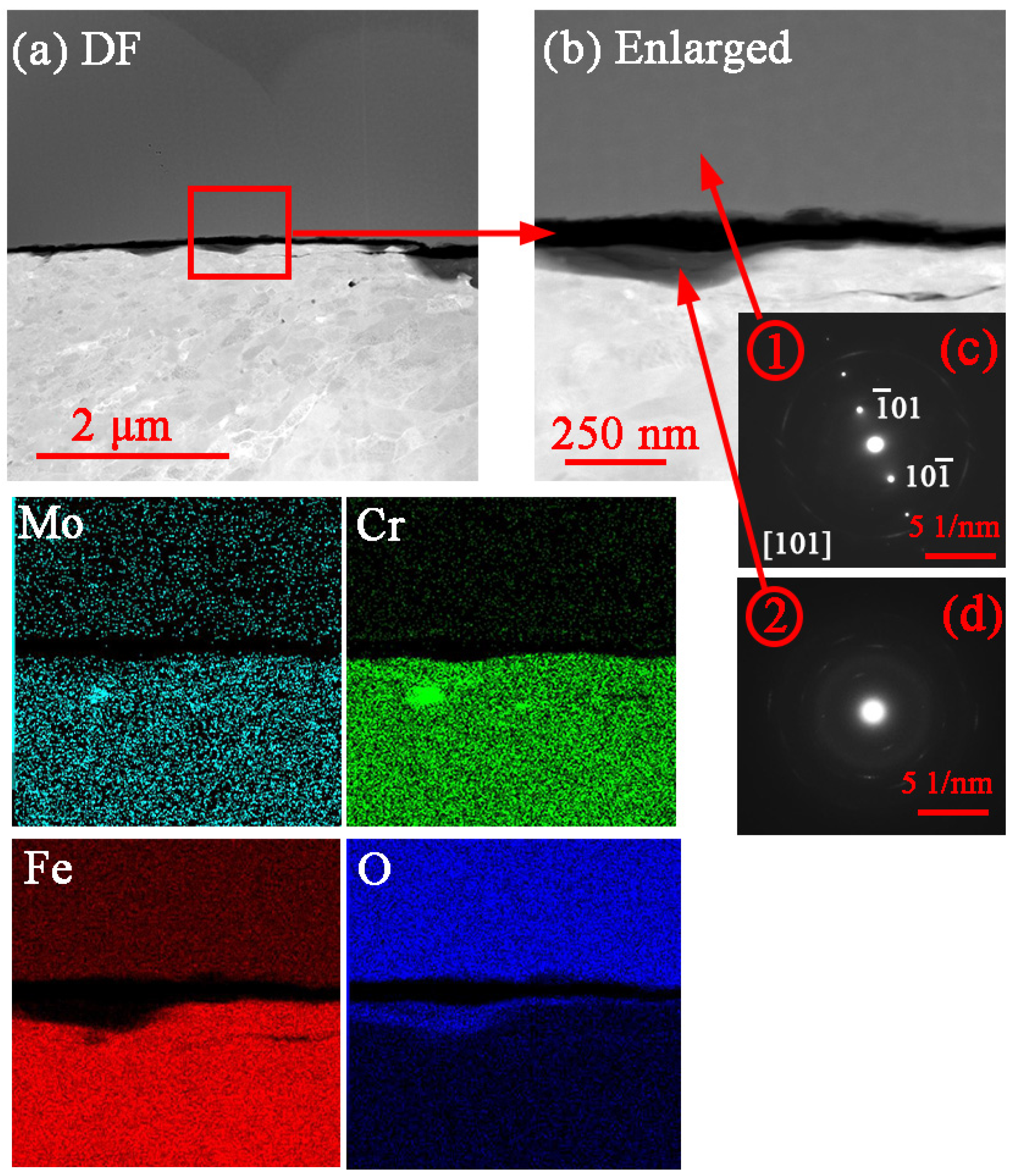
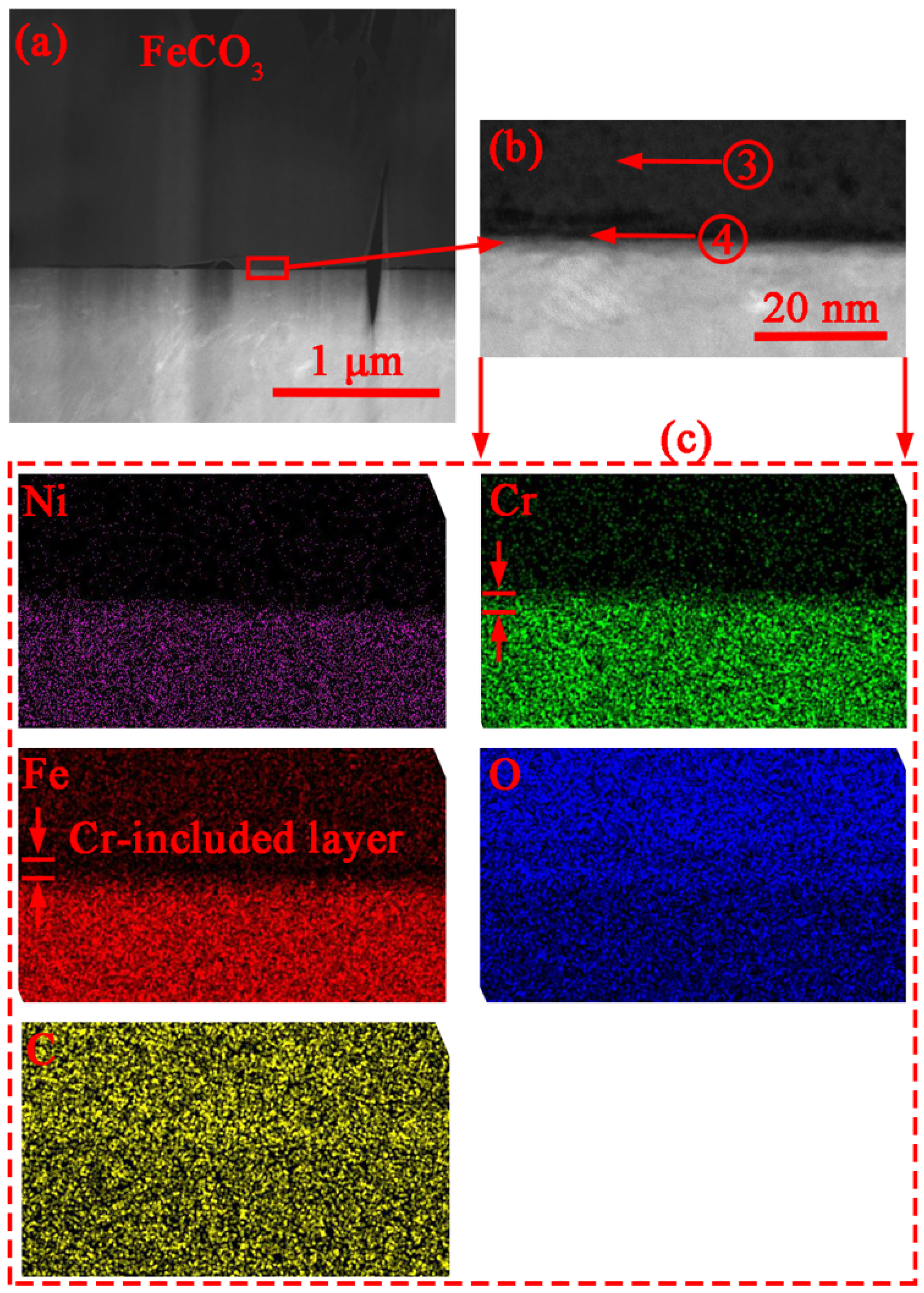
3.2. Corrosion Product Characterization
4. Discussion
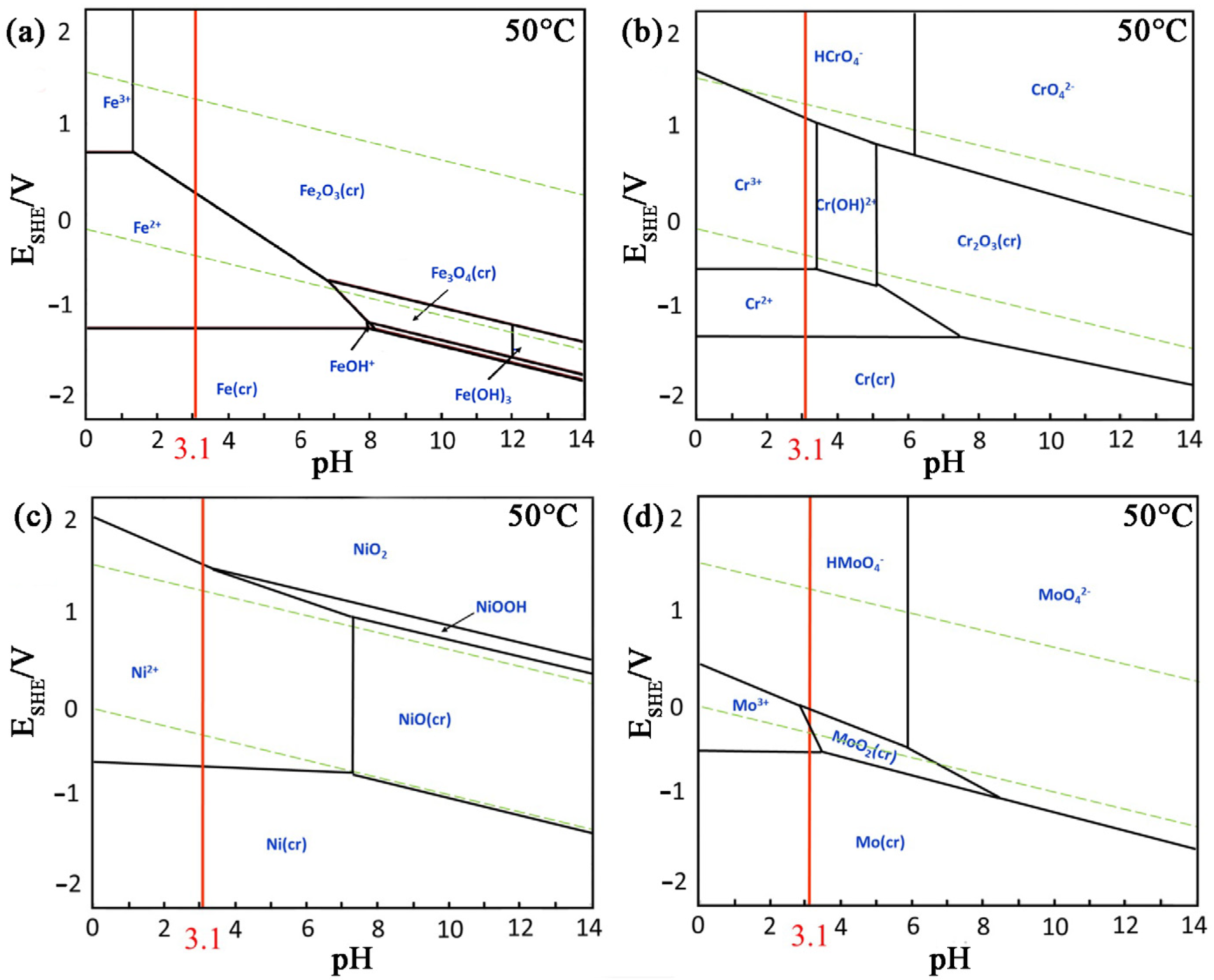
4.1. Thermodynamic Calculation of the Tested Alloy
4.2. Formation and Dissolution of Carbonate and Oxide
5. Conclusions
- (1)
- Corrosion rates consistently decrease with increasing Cr content in Fe-Cr and Fe-Cr-Ni alloys. There is a critical Cr content (~9 wt.%), above which the alloys exhibit a satisfactory corrosion performance for s-CO2 permanent storage or enhanced oil recovery in saline water reservoirs.
- (2)
- During exposure to the s-CO2 saline water environment, a non-protective FeCO3 layer forms on the constructional alloys, likely via a precipitation mechanism due to environmental acidity and the presence of high amounts of HCO3− and CO32−. For alloys with more than 2 wt.% Cr, an inner Cr-enriched layer forms between the outer FeCO3 layer and the substrate, acting as the main barrier controlling the corrosion process in the s-CO2 saline water. The properties of the inner Cr-containing layer significantly improve with increasing bulk Cr content above ~9 wt.%.
- (3)
- In the s-CO2 saline water environment, pitting is unlikely to occur on candidate construction alloys due to the solution’s acidity and the presence of bicarbonate ions, even though the Cl− ion concentration reaches up to 3.5 wt.%.
- (4)
- Alloys with a Cr content above 9% are suitable candidates for next-phase long-term corrosion kinetic evaluations. Given the complicate chemistry of s-CO2 storage environments, it is highly recommended to assess their performance in s-CO2-saturated solutions with typical ions (such as, Ca2⁺, Mg2⁺, or S2⁻ presented in the reservoirs) at higher temperatures, as the testing temperature (50 °C) in this study is the lower end of the 50–80 °C operation range under s-CO2 storage.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Material | T /°C | P /MPa | NaCl | Time/h | Rate /mm/y | Refs. |
|---|---|---|---|---|---|---|
| X70 | 80 | 9.5 | 3.5 wt.% | 0.5 | 119 | Gao 2015 [16] |
| X70 | 80 | 9.5 | 7 | 49.5 | ||
| X70 | 80 | 9.5 | 12 | 57.4 | ||
| X70 | 80 | 9.5 | 24 | 37 | ||
| X70 | 80 | 9.5 | 96 | 7 | ||
| X70 | 80 | 9.5 | 168 | 5 | ||
| X70 | 80 | 9.5 | 384 | 1.5 | ||
| X65 | 80 | 10 | 3.5 wt.% | 240 | 8.46 | Gao 2016 [27] |
| X65 1 | 80 | 10 | 240 | 15.48 | ||
| P110 2 | 80 | 10 | 3.5 wt.% | 240 | 10 | Gao 2016 [21] |
| 3Cr 2 | 80 | 10 | 240 | 2.2 | ||
| 316L 2 | 80 | 10 | 240 | 0.018 | ||
| P110 | 80 | 9.5 | 3.5 wt.% | 0.5 | 84.2 | Gao 2018 [20] |
| P110 | 80 | 9.5 | 12 | 48 | ||
| P110 | 80 | 9.5 | 50 | 14.4 | ||
| P110 | 80 | 9.5 | 96 | 13.0 | ||
| P110 | 80 | 9.5 | 168 | 6.12 | ||
| P110 | 80 | 9.5 | 384 | 1.8 | ||
| X65 | 60 | 10 | 35,249 ppm Cl− | 6 | 20 | Hua 2018 [30] |
| X65 | 60 | 10 | 24 | 14 | ||
| X65 | 60 | 10 | 48 | 7.5 | ||
| X65 | 60 | 10 | 96 | 3.8 | ||
| X65 | 60 | 10 | 1.0 wt.% | 6 | 10.8 | Hua 2020 [62] |
| 1Cr | 60 | 10 | 6 | 10.5 | ||
| 3Cr | 60 | 10 | 6 | 10.1 | ||
| 5Cr | 60 | 10 | 6 | 6.8 | ||
| X65 | 60 | 10 | 192 | 1.5 | ||
| 1Cr | 60 | 10 | 192 | 1.9 | ||
| 3Cr | 60 | 10 | 192 | 2.0 | ||
| 5Cr | 60 | 10 | 192 | 2.1 | ||
| N80 | 80 | 8 | 3.0 wt.% | 1 | 45 | Zhang 2021 [63] |
| N80 | 80 | 8 | 12 | 29 | ||
| N80 | 80 | 8 | 24 | 20 | ||
| N80 | 80 | 8 | 48 | 14 | ||
| N80 | 80 | 8 | 96 | 8 | ||
| N80 | 80 | 8 | 168 | 6 | ||
| X65 3 | 50 | 8 | 3.5 wt.% | 1.5 | 21 | Sun 2021 [12] |
| X65 3 | 50 | 8 | 24 | 4 | ||
| X65 3 | 50 | 8 | 72 | 2.4 | ||
| X65 | 50 | 8 | DI | 96 | 5.0 | Li 2024 [26] |
| X65 | 50 | 8 | 3.5 wt.% | 96 | 1.5 | |
| X65 | 75 | 8 | 96 | 1.0 | ||
| X65 | 100 | 8 | 96 | 0.9 |
References
- EEA. EN01 Energy Related Greenhouse Gas Emissions; European Environment Agency: Copenhagen, Denmark, 2011.
- Onyebuchi, V.; Kolios, A.; Hanak, D.; Biliyok, C.; Manovic, V. A systematic review of key challenges of CO2 transport via pipelines. Renew. Sustain. Energy Rev. 2018, 81, 2563–2583. [Google Scholar] [CrossRef]
- Gaurina-Međimurec, N.; Pašić, B. Design and mechanical integrity of CO2 injection wells. Rud. Geol. Naft. Zb. 2011, 23, 1–8. [Google Scholar]
- Melzer, L.S. Carbon Dioxide Enhanced Oil Recovery (CO2 EOR): Factors Involved in Adding Carbon Capture, Utilization and Storage (CCUS) to Enhanced Oil Recovery; Center for Climate and Energy Solutions: Washington, DC, USA, 2012. [Google Scholar]
- Kuuskraa, V.A.; Godec, M.L.; Dipietro, P. CO2 utilization from “Next Generation” CO2 enhanced oil recovery technology. Energy Procedia 2013, 37, 6854–6866. [Google Scholar] [CrossRef]
- Jung, W.; Nicot, J.P. Impurities in CO2-Rich mixtures impact CO2 pipeline design: Implications for calculating CO2 transport capacity. In Proceedings of the SPE International Conference on CO2 Capture, Storage, and Utilization, New Orleans, LA, USA, 10–12 November 2010; p. SPE-139712-MS. [Google Scholar]
- Wetenhall, B.; Race, J.; Downie, M. The effect of impurities on a simplified CCS network. In Proceedings of the PSIG Annual Meeting, Prague, Czech Republic, 16–19 April 2013; p. PSIG-1306. [Google Scholar]
- Global-CCS-Institute. The Global Status of CCS; Global CCS Institute: Melbourne, Australia, 2017. [Google Scholar]
- Lucci, A.; Demofonti, G.; Tudori, P.; Spinelli, C.M. CCTS (Carbon Capture Transportation & Storage) transportation issues. In Proceedings of the Twenty-first International Offshore and Polar Engineering Conference, Maui, HI, USA, 19–24 June 2011; p. ISOPE-I-11-162. [Google Scholar]
- Sun, H.; Wang, H.; Zeng, Y.; Liu, J. Corrosion challenges in supercritical CO2 transportation, storage, and utilization—A review. Renew. Sustain. Energy Rev. 2023, 179, 113292. [Google Scholar] [CrossRef]
- Cui, G.; Yang, Z.; Liu, J.; Li, Z. A comprehensive review of metal corrosion in a supercritical CO2 environment. Int. J. Greenh. Gas Control 2019, 90, 102814. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Sun, J.; Lin, X.; Wang, Y. Probing the initial corrosion behavior of X65 steel in CCUS-EOR environments with impure supercritical CO2 fluids. Corros. Sci. 2021, 189, 109585. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, M.; Choi, Y.-S. State-of-the-art overview of pipeline steel corrosion in impure dense CO2 for CCS transportation: Mechanisms and models. Corros. Eng. Sci. Technol. 2017, 52, 485–509. [Google Scholar] [CrossRef]
- Sim, S.; Cole, I.; Choi, Y.-S.; Birbilis, N. A review of the protection strategies against internal corrosion for the safe transport of supercritical CO2 via steel pipelines for CCS purposes. Int. J. Greenh. Gas Control 2014, 29, 185–199. [Google Scholar] [CrossRef]
- Barker, R.; Hua, Y.; Neville, A. Neville, Internal corrosion of carbon steel pipelines for dense-phase CO2 transport in carbon capture and storage (CCS)—A review. Int. Mater. Rev. 2016, 62, 1–31. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Liu, C.; Gao, K. Formation mechanism and protective property of corrosion product scale on X70 steel under supercritical CO2 environment. Corros. Sci. 2015, 100, 404–420. [Google Scholar] [CrossRef]
- Yevtushenko, O.; ßler, R.B. Water Impact on Corrosion Resistance of Pipeline Steels in Circulating Supercritical CO2 with SO2- and NO2-Impurities; NACE Corrosion: Houston, TX, USA, 2014; NACE-2014-3838. [Google Scholar]
- Pfennig, A.; Kranzmann, A. Effect of CO2 and pressure on the stability of steels with different amounts of chromium in saline water. Corros. Sci. 2012, 65, 441–452. [Google Scholar] [CrossRef]
- Huang, S.; Worthington, D.L.; Asta, M.; Ozolins, V.; Ghosh, G.; Liaw, P.K. Liaw, Calculation of impurity diffusivities in α-Fe using first-principles methods. Acta Mater. 2010, 58, 1982–1993. [Google Scholar] [CrossRef]
- Wei, L.; Gao, K.; Li, Q. Corrosion of low alloy steel containing 0.5% chromium in supercritical CO2-saturated brine and water-saturated supercritical CO2 environments. Appl. Surf. Sci. 2018, 440, 524–534. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Corrosion of low alloy steel and stainless steel in supercritical CO2/H2O/H2S systems. Corros. Sci. 2016, 111, 637–648. [Google Scholar] [CrossRef]
- Collier, J.; Papavinasam, S.; Li, J.; Shi, C.; Liu, P.; Gravel, J.P. Effect of impurities on the corrosion performance of steels in supercritical carbon dioxide: Optimization of experimental procedure. In Proceedings of the Corrosion Conference and Expo 2013, Orlando, FL, USA, 17–21 March 2013; p. NACE-2013-2357. [Google Scholar]
- Li, K.; Zeng, Y.; Luo, J. Condensed phase corrosion of P91 and DSS 2205 steels at advanced oxygen-fired pressurized fluidized bed combustion plants. Mater. Corros. 2020, 72, 757–771. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, X.; Qu, S.; Li, X.; Gao, K. The relationship between fracture toughness of CO2 corrosion scale and corrosion rate of X65 pipeline steel under supercritical CO2 condition. Int. J. Greenh. Gas Control 2011, 5, 1643–1650. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, X.; Qu, S.; Li, X.; Gao, K. Discussion of the CO2 corrosion mechanism between low partial pressure and supercritical condition. Corros. Sci. 2012, 59, 186–197. [Google Scholar] [CrossRef]
- Li, M.; Gross, A.; Taylor, B.; Zhang, H.; Liu, J. Effects of Cl-ion and temperature variations on steel corrosion in supercritical CO2 saturated aqueous environments. Process. Saf. Environ. Prot. 2024, 187, 1446–1453. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Effect of small amount of H2S on the corrosion behavior of carbon steel in the dynamic supercritical CO2 environments. Corros. Sci. 2016, 103, 132–144. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y. Advancing the mechanistic understanding of corrosion in supercritical CO2 with H2O and O2 impurities. Corros. Sci. 2023, 213, 110981. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Hua, Y.; Shamsa, A.; Barker, R.; Neville, A. Protectiveness, morphology and composition of corrosion products formed on carbon steel in the presence of Cl−, Ca2+ and Mg2+ in high pressure CO2 environments. Appl. Surf. Sci. 2018, 455, 667–682. [Google Scholar] [CrossRef]
- G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2003.
- G31-21; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2021.
- Denny, A.J. Principles and Prevention of Corrosion; Prentice Hall: Hoboken, NJ, USA, 1996. [Google Scholar]
- Guo, S.; Xu, L.; Zhang, L.; Chang, W.; Lu, M. Corrosion of alloy steels containing 2% chromium in CO2 environments. Corros. Sci. 2012, 63, 246–258. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.; Coy, A.; Viejo, F.; Arrabal, R.; Matykina, E. Pitting corrosion behaviour of austenitic stainless steels—Combining effects of Mn and Mo additions. Corros. Sci. 2008, 50, 1796–1806. [Google Scholar] [CrossRef]
- Muwila, A. The Effect of Manganese, Nitrogen and Molybdenum on the Corrosion Resistance of a Low Nickel (<2 wt%) Austenitic Stainless Steel. Ph.D. Thesis, Wits University, Johannesburg, South Africa, 2006. [Google Scholar]
- Morland, B.H.; Svenningsen, G. Pitfalls and Artefacts in Corrosion Experiments with Dense Phase CO2; NACE Corrosion: Houston, TX, USA, 2021; p. D121S051R004. [Google Scholar]
- Xiang, Y.; Xie, W.; Ni, S.; He, X. Comparative study of A106 steel corrosion in fresh and dirty MEA solutions during the CO2 capture process: Effect of NO3−. Corros. Sci. 2020, 167, 108521. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Liu, W.; Lu, M. The formation mechanism of corrosion scale and electrochemical characteristic of low alloy steel in carbon dioxide-saturated solution. Corros. Sci. 2012, 57, 131–138. [Google Scholar] [CrossRef]
- Bardal, E. Corrosion and Protection; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Liu, M.; Zeng, Y.; Luo, J.-L. Roles of major alloying elements in steels and alloys on corrosion under biomass hydrothermal liquefaction (HTL) conversion. Corros. Sci. 2023, 218, 111148. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, Y.; Luo, J.-L. Impacts of catalyst, inorganic and organic corrodants on corrosion under batch-mode catalytic biomass hydrothermal liquefaction conversion. Corros. Sci. 2022, 204, 110409. [Google Scholar] [CrossRef]
- Ezuber, H.M. Influence of temperature and thiosulfate on the corrosion behavior of steel in chloride solutions saturated in CO2. Mater. Des. 2009, 30, 3420–3427. [Google Scholar] [CrossRef]
- Dugstad, A. Fundamental Aspects of CO2 Metal Loss Corrosion, Part I: Mechanism. In Proceedings of the Corrosion Conference and Expo 2015, Dallas, TX, USA, 15–19 March 2015. [Google Scholar]
- Garside, J. Advances in the Characterization of Crystal Growth; AIChE Symposium Series; American Institute of Chemical Engineers (AIChE): New York, NY, USA, 1984; pp. 23–38. [Google Scholar]
- Ueda, M.; Takabe, H. Effect of Environmental Factor and Microstructure on Morphology of Corrosion Products in CO2 Environments; NACE Corrosion: Houston, TX, USA, 1999; p. NACE-99013. [Google Scholar]
- Dugstad, A.; Lunde, L.; Videm, K. Parametric Study of CO2 Corrosion of Carbon Steel; NACE International: Houston, TX, USA, 1994. [Google Scholar]
- Pessu, F.; Barker, R.; Neville, A. The Influence of pH on Localized Corrosion Behavior of X65 Carbon Steel in CO2-Saturated Brines. Corrosion 2015, 71, 1452–1466. [Google Scholar] [CrossRef]
- Van Hunnik, E.; Hendriksen, E.; Pots, B.F. The Formation of Protective FeCO3 Corrosion Product Layers in CO2 Corrosion; NACE Corrosion: Houston, TX, USA, 1996; p. NACE-96006. [Google Scholar]
- Nazari, M.H.; Allahkaram, S.; Kermani, M. The effects of temperature and pH on the characteristics of corrosion product in CO2 corrosion of grade X70 steel. Mater. Des. 2010, 31, 3559–3563. [Google Scholar] [CrossRef]
- Ahmadi, P.; Chapoy, A. CO2 solubility in formation water under sequestration conditions. Fluid Phase Equilibria 2018, 463, 80–90. [Google Scholar] [CrossRef]
- Jolivet, J.; Chaneac, C.; Tronc, E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem. Commun. 2004, 35, 481–483. [Google Scholar]
- Rai, D.; Sass, B.M.; Moore, D.A. Moore, Chromium (III) hydrolysis constants and solubility of chromium (III) hydroxide. Inorg. Chem. 1987, 26, 345–349. [Google Scholar] [CrossRef]
- Pedeferri, P.; Ormellese, M. Corrosion Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Liu, M.; Zeng, Y.; Luo, J.-L. Influence of Major Operating Parameters (Temperature, Pressure, and Flow Rate) on the Corrosion of Candidate Alloys for the Construction of Hydrothermal Liquefaction Biorefining Reactors. Energy Fuels 2022, 36, 3134–3153. [Google Scholar] [CrossRef]
- Xiao, Q.; Jang, C.; Kim, C.; Kim, H.; Chen, J.; Lee, H.B. Corrosion behavior of stainless steels in simulated PWR primary water: The effect of composition and matrix phases. Corros. Sci. 2020, 177, 108991. [Google Scholar] [CrossRef]
- Zeng, Y.; Guzonas, D. Corrosion Assessment of Candidate Materials for Fuel Cladding in Canadian SCWR. JOM 2016, 68, 475–479. [Google Scholar] [CrossRef]
- Presuel-Moreno, F.; Scully, J.; Sharp, S. Literature Review of Commercially Available Alloys That Have Potential as Low-Cost Corrosion Resistant Concrete Reinforcement. Corrosion 2010, 66, 086001-1. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Al-Thani, N. A review of passivity breakdown on metal surfaces: Influence of chloride- and sulfide-ion concentrations, temperature, and pH. Emergent Mater. 2021, 4, 1187–1203. [Google Scholar] [CrossRef]
- Takasaki, S.; Yamada, Y. Effects of temperature and aggressive anions on corrosion of carbon steel in potable water. Corros. Sci. 2007, 49, 240–247. [Google Scholar] [CrossRef]
- Wasik, D.O.; Polat, H.M.; Ramdin, M.; Moultos, O.A.; Calero, S.; Vlugt, T.J.H. Vlugt, Solubility of CO2 in Aqueous Formic Acid Solutions and the Effect of NaCl Addition: A Molecular Simulation Study. J. Phys. Chem. C 2022, 126, 19424–19434. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Mohammed, S.; Barker, R.; Neville, A. Comparisons of corrosion behaviour for X65 and low Cr steels in high pressure CO2-saturated brine. J. Mater. Sci. Technol. 2020, 41, 21–32. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Hou, B.; Zhang, Q.; Zhang, G. A numerical model based on finite element method for predicting the corrosion of carbon steel under supercritical CO2 conditions. Process. Saf. Environ. Prot. 2021, 149, 866–884. [Google Scholar] [CrossRef]

| Alloys | Chemical Composition (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cr | Ni | Fe | Si | Mn | C | Al | Others | |
| X80 | 0.28 | - | Bal. | 0.17 | 1.83 | 0.04 | - | 0.3Mo |
| 2Cr | 2.2 | 0.1 | Bal. | 0.21 | 0.4 | 0.1 | 0.02 | 1.0Mo |
| 5Cr | 4.6 | 0.1 | Bal. | 0.33 | 0.3 | 0.1 | 0.05 | 0.5Mo |
| P91 | 8.9 | 0.1 | Bal. | 0.3 | 0.4 | 0.1 | 0.03 | 0.9Mo |
| 316L | 17.3 | 8.3 | Bal. | 0.4 | 1.0 | 0.02 | - | 0.2Mo |
| Alloy 800 | 19.2 | 31.2 | Bal. | 0.8 | 0.8 | 0.06 | 0.2 | - |
| Alloys | Site # | Average Composition (at. %) | Products | ||||
|---|---|---|---|---|---|---|---|
| O | Fe | Cr | Ni | C | |||
| X80 | 1 | 59.6 ± 2.6 | 17.6 ± 4.4 | - | - | 22.4 ± 1.8 | FeCO3 |
| 2Cr | 2 | 57.9 ± 3.5 | 19.5 ± 4.9 | - | - | 22.0 ± 2.2 | FeCO3 |
| 3 | 60.9 ± 1.9 | 14.5 ± 2.9 | 2.7 ± 0.4 | - | 21.2 ± 0.6 | - | |
| 5Cr | 4 | 61.5 ± 1.0 | 12.1 ± 1.7 | - | - | 25.6 ± 1.7 | FeCO3 |
| 5 | 60.9 ± 1.0 | 9.5 ± 0.7 | 10.8 ± 0.7 | - | 18.8 ± 0.5 | - | |
| P91 | 6 | 58.7 ± 1.3 | 19.3 ± 3.0 | - | - | 22.0 ± 1.7 | FeCO3 |
| 316L | 7 | 63.5 ± 2.4 | 19.0 ± 4.8 | - | - | 17.5 ± 2.4 | FeCO3 |
| Alloy 800 | 8 | 61.6 ± 0.8 | 18.6 ± 3.7 | - | - | 19.8 ± 2.9 | FeCO3 |
| Region # | Chemical Composition (at. %) | |||
|---|---|---|---|---|
| O | Fe | Cr | C | |
| 1 | 22 | 18 | - | 60 |
| 2 | 21 | 13 | 8 | 58 |
| 3 | 22 | 23 | - | 55 |
| 4 | 20 | 15 | 10 | 55 |
| Reaction | Gibbs Energy at 50 °C (kJ/mol) |
|---|---|
| 2Fe + 3H2O = Fe2O3 + 3H2 | −34.911 |
| 2Cr + 3H2O = Cr2O3 + 3H2 | −347.030 |
| Ni + H2O = NiO + H2 | 23.862 |
| Mo + 2H2O = MoO2 + 2H2 | −61.229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Liu, M.; Zeng, Y.; Liu, J. Influence of Chromium Content in Alloys on Corrosion in Saline Water Saturated with Supercritical CO2. Processes 2025, 13, 1334. https://doi.org/10.3390/pr13051334
Sun H, Liu M, Zeng Y, Liu J. Influence of Chromium Content in Alloys on Corrosion in Saline Water Saturated with Supercritical CO2. Processes. 2025; 13(5):1334. https://doi.org/10.3390/pr13051334
Chicago/Turabian StyleSun, Haofei, Minkang Liu, Yimin Zeng, and Jing Liu. 2025. "Influence of Chromium Content in Alloys on Corrosion in Saline Water Saturated with Supercritical CO2" Processes 13, no. 5: 1334. https://doi.org/10.3390/pr13051334
APA StyleSun, H., Liu, M., Zeng, Y., & Liu, J. (2025). Influence of Chromium Content in Alloys on Corrosion in Saline Water Saturated with Supercritical CO2. Processes, 13(5), 1334. https://doi.org/10.3390/pr13051334





