Siderite Formation by Mechanochemical and High Pressure–High Temperature Processes for CO2 Capture Using Iron Ore as the Initial Sorbent
Abstract
:1. Introduction
2. Experimental
3. Results
3.1. Iron Ore Characterization
3.2. Thermodynamics Simulation of Iron Ore Carbonation
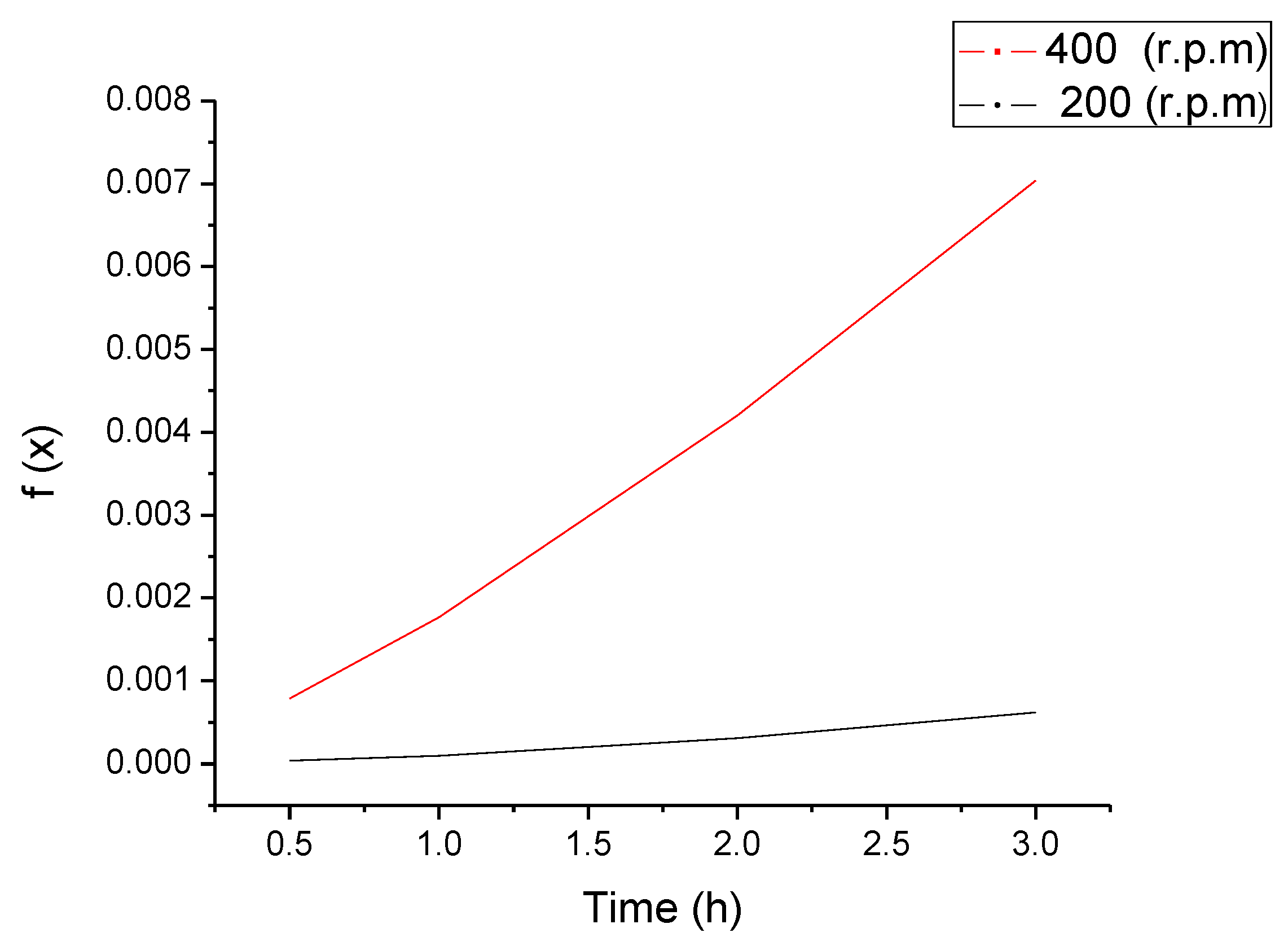
3.3. Iron Ore Carbonation in Mechanochemical Process
3.4. Iron Ore Carbonation in the HTHP Process
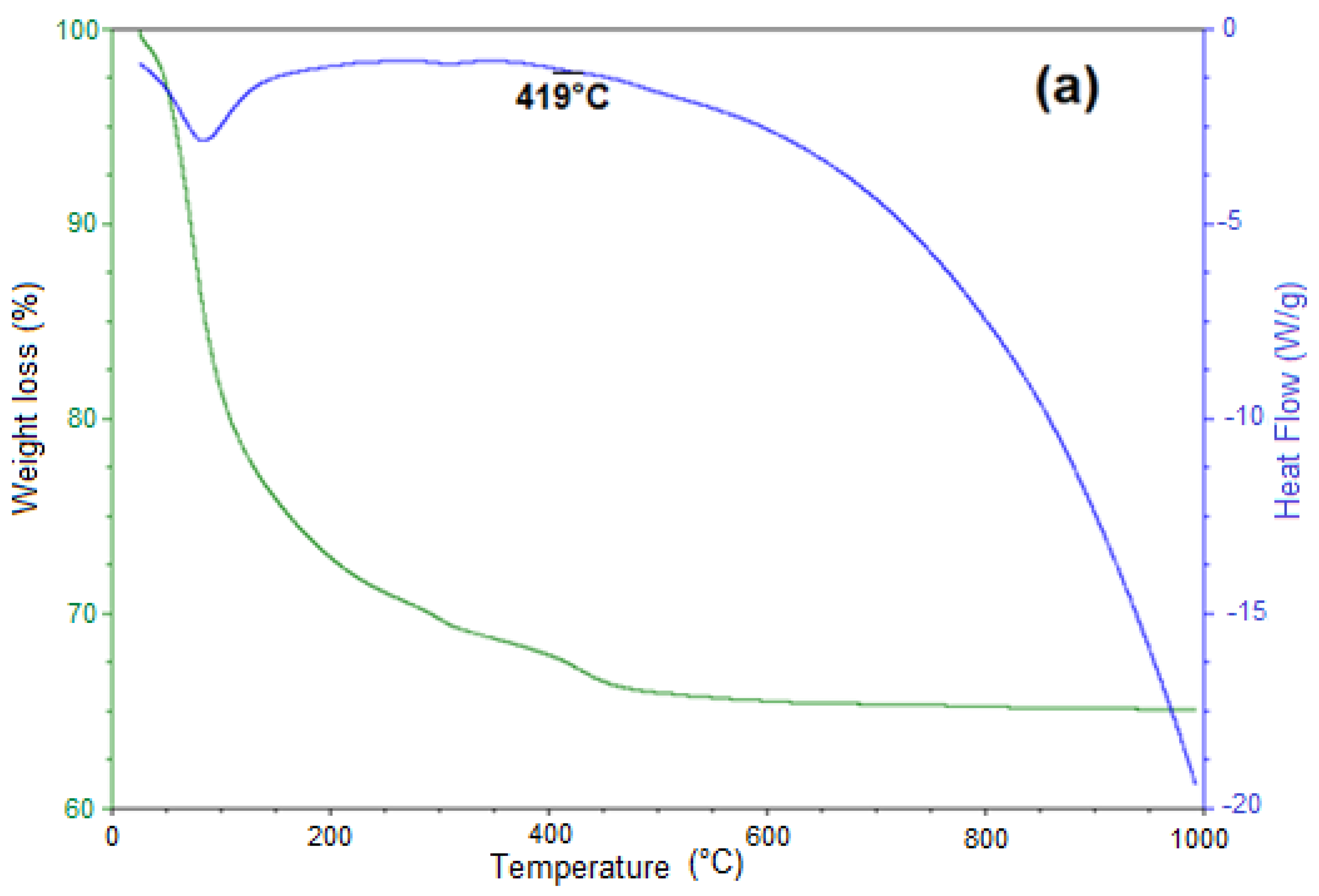
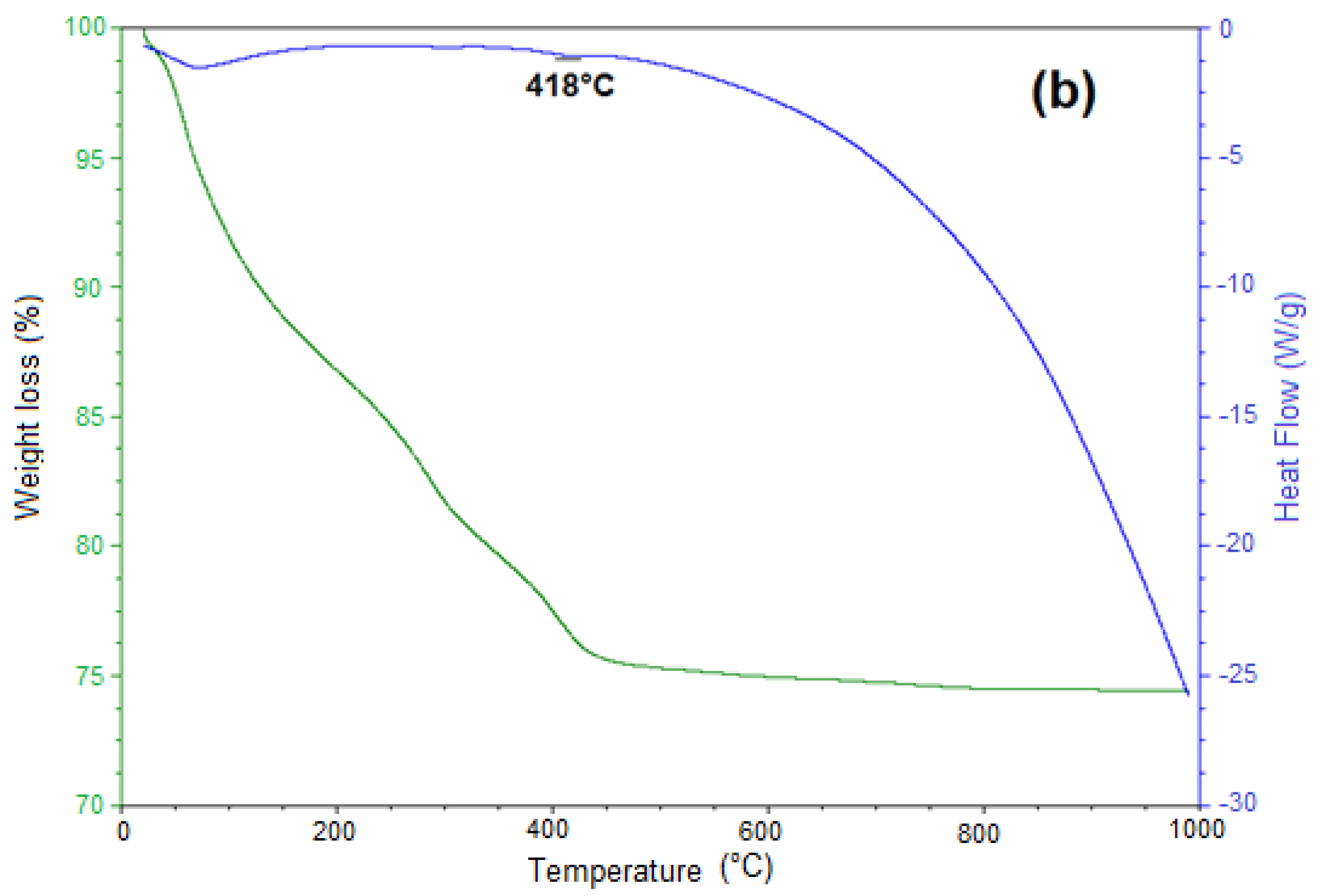
3.5. Thermal Decomposition of Siderite Studied by Thermogravimetric Analysis
3.6. Carbonation–Calcination Cycles
3.7. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanz-Pérez, E.; Murdock, C.; Didas, S.; Jones, C. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Wiers, B. Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal−Organic Framework mmen-Mg2(dobpdc). J. Am. Chem. Soc. 2012, 7056–7065. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A Review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Kim, Y.; Worrell, E. International comparison of CO2 emission trends in the iron and steel industry. Energy Policy 2002, 30, 827–838. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, S. A comparative study of CO2 sorption properties for different oxides. Mater. Renew. Sustain. Energy 2014, 1, 2. [Google Scholar] [CrossRef]
- Han, K.; Ahn, C.; Lee, M.S. Performance of an ammonia-based CO2 capture pilot facility in iron and steel industry. Int. J. Greenh. Gas Control 2014, 27, 239–246. [Google Scholar] [CrossRef]
- Pannocchia, G.; Puccini, M.; Seggiani, M.; Vitolo, S. Experimental and modeling studies on high-temperature capture of CO2 Using Lithium Zirconate Based Sorbents. Ind. Eng. Chem. Res. 2007, 46, 6696–6706. [Google Scholar] [CrossRef]
- Kumar, A.; Ramaprabhu, S. Nano magnetite decorated multiwalled carbon nanotubes: A robust nanomaterial for enhanced carbon dioxide adsorption. Energy Environ. Sci. 2011, 4, 889–895. [Google Scholar]
- Ho, M.; Leamon, M.; Allinson, D.; Wiley, D. Economics of CO2 and mixed gas geosequestration of flue gas using gas separation membranes. J. Membr. Sci. 2006, 45, 2546–2552. [Google Scholar]
- Zhao, L.; Rienache, E.; Menzer, R.; Blum, L.; Stolen, D. A parametric study of CO2/N2 gas separation membrane processes for post-combustion capture. J. Membr. Sci. 2008, 325, 284–294. [Google Scholar] [CrossRef]
- Zaman, M.; Hyung Lee, J. Carbon capture from stationary power generation sources: A review of the current status of the technologies. Korean J. Chem. Eng. 2013, 30, 1497–1526. [Google Scholar] [CrossRef]
- Salvador, C.; Lu, D.; Anthony, E.; Abanades, J. Enhancement of CaO for CO2 capture in an FBC environment. Chem. Eng. 2003, 96, 187–195. [Google Scholar] [CrossRef]
- Kumar, S.; Drozd, V.; Durygin, A.; Saxena, S. Capturing CO2 Emissions in the iron industries using a magnetite–iron mixture. Energy Technol. 2016, 1–5. [Google Scholar] [CrossRef]
- Mora, E.; Sarmiento, A.; Lopez, E.; Drozd, V.; Durigyn, A.; Saxena, S.; Chen, J. Iron oxides as efficient sorbents for CO2 capture. J. Mater. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Gotor, F.; Macias, M.; Ortega, A.; Criado, J. Comparative study of the kinetics of the thermal decomposition of synthetic and natural siderite samples. Phys. Chem. Miner. 2000, 27, 475–503. [Google Scholar] [CrossRef]
- Dhupe, A.; Gokanrn, N. Studies in the Thermal Decomposition of Natural Siderites in the esence of Air. Int. J. Miner. Process. 1990, 28, 209–220. [Google Scholar] [CrossRef]
- Fosbol, P.; Thompsen, K.; Stenby, E. Review and recommended thermodynamic properties of FeCO3. Corros. Eng. Sci. Technol. 2013, 45, 115–135. [Google Scholar] [CrossRef]
- Hammersley, A. ESRF Internal Report, ESRF97HA02T, FIT2D: An Introduction and Overview; European Synchrotron Radiation Facility: Grenoble, France, 1997. [Google Scholar]
- Toby, B. EXPGUI, a graphical interface for GSAS. J. Appl. Cryst. 2001, 34, 210–221. [Google Scholar] [CrossRef]
- Larson, A.; Von Dreele, R. General Structure Analysis System (GSAS); Report LAUR 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2004.
- Garcia, S.; Rosenbauer, R.; Palandri, J.; Maroto-Valer, M. Sequestration of non-pure carbon dioxide streams in iron oxyhydroxide-containing saline repositories. Int. J. Greenh. Gas Control 2012, 7, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Saxena, S.; Drozd, V.; Durygin, A. An experimental investigation of mesoporous MgO as a potential pre-combustion CO2 sorbent. Mater. Renew. Sustain. Energy 2015, 4. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Liu, C.; Shih, S. Kinetics of the reaction of iron blast furnace slag/hydrated lime sorbents with SO2 at low temperatures: Effects of the presence of CO2, O2, and NOX. Ind. Eng. Chem. 2009, 48, 8335–8340. [Google Scholar] [CrossRef]
- Betancur, J.; Barrero, D.; Greneche, F.; Goya, F. El efecto del contenido de agua en la magnetita y en las propiedades estructurales de la goethita. J. Alloy. Comp. 2004, 369, 247–251. [Google Scholar] [CrossRef]
- Gallagher, P.; Warne, S. Thermogravimetry and thermal decomposition of siderite. Thermochim. Acta 1980, 43, 253–267. [Google Scholar] [CrossRef]
- Patterson, J.; Levi, J. Relevance of carbonate minerals in the processing of Australian oil shales. Fuel 1991, 70, 1252–1259. [Google Scholar] [CrossRef]
- Hodkiewicks, J. Characterizing Carbon Materials with Raman Spectroscopy; Thermo Fisher Scientific: Madison, WI, USA, 2010. [Google Scholar]
- Reitch, S.; Thomsen, C. Raman spectroscopy of graphite. R. Soc. 2004, 362, 2271–2278. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, S.; McCreery, S. Raman Spectroscopy of Carbon Materials: Structural Basis of Observed Spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Kummar, S. The effect of elevated pressure, temperature and particles morphology on the carbon dioxide capture using zinc oxide. J. CO2 Util. 2014, 8, 60–66. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Abbasian, J. Regenerable MgO-based sorbents for high-temperature CO2 removal from syngas: 1. Sorbent development, evaluation, and reaction modeling. Fuel 2010, 89, 1287–1297. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Y.; Guo, Y.; Xu, Y.; Li, W.; Zhao, A.; Liu, W.; Lu, P. Stabilized CO2 capture performance of wet mechanically activated dolomite. Fuel 2018, 222, 334–342. [Google Scholar] [CrossRef]
- Ounoughene, G.; Buskens, E.; Santos, R.; Cizer, O.; Van Gerven, T. Solvochemical carbonation of lime using ethanol: Mechanism and enhancement for direct atmospheric CO2 capture. J. CO2 Util. 2018, 26, 143–151. [Google Scholar] [CrossRef]
- Benitez, M.; Valverde, J.; Perejon, A.; Sanchez, P.; Perez, L. Effect of milling mechanism on the CO2 capture performance of limestone in the Calcium Looping process. Chem. Eng. J. 2018, 346, 549–556. [Google Scholar] [CrossRef]
- Alkaç, D.; Atalay, U. Kinetics of thermal decomposition of Hekimhan–Deveci siderite ore samples. Int. J. Miner. Process. 2008, 87, 120–128. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Vasiliades, M.; Ioannou, I.; Esftathiou, A.; Godelistsas, A.; Kyratsy, T. Enhancing the rate of ex situ mineral carbonation in dunites via ball milling. Adv. Powder Technol. 2016, 27, 360–371. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Delimitis, A.; Ioannou, I.; Esftathiou, A.; Kyratsy, T. Effect of ball milling on the carbon sequestration efficiency of serpentinized peridotites. Miner. Eng. 2018, 120, 66–74. [Google Scholar] [CrossRef]
- Criado, J.; Gonzalez, M.; Macias, M. Influence of grinding on both the stability and thermal decomposition mechanical of siderite. Thermochim. Acta 1988, 135, 219–223. [Google Scholar] [CrossRef]
- Bradley, W.; Burst, J.; Graf, D. Crystal chemistry and differential thermal effect of dolomite. Am. Mineral. 1953, 38, 207–211. [Google Scholar]
- Burgio, N.; Lasonna, A.; Magini, M.; Martelli, S.; Padella, F. Mechanical Alloying of the Fe-Zr System. Correlation between Input Energy and End Products. Il Nuovo Cim. D 1991, 13, 459–476. [Google Scholar] [CrossRef]









| Fe (Total) | 46.98 |
|---|---|
| SiO2 | 9.58 |
| CaO | 4.38 |
| Al2O3 | 5.43 |
| MgO | 0.43 |
| MnO | 0.23 |
| P2O5 | 2.72 |
| Na2O | 0.59 |
| K2O | 0.04 |
| S | 0.89 |
| Zn | 0.08 |
| Pressure (bar) | Revolution Speed (rev/min) | Time Reaction (h) | CO2 Capture Capacity (mmol CO2/g sorbent) |
|---|---|---|---|
| 10 | 400 | 3 | 1.075 |
| 10 | 400 | 6 | 1.9523 |
| 20 | 400 | 3 | 1.7545 |
| 20 | 400 | 6 | 2.9204 |
| 20 | 200 | 3 | 0.4318 |
| 30 | 200 | 3 | 0.6864 |
| 30 | 200 | 6 | 0.8545 |
| Pressure (bar) | Temperature (°C) | Reaction Time (h) | CO2 Capture Capacity (mmol CO2/g Sorbent) |
|---|---|---|---|
| 30 | 100 | 4 | 4.7927 |
| 30 | 200 | 1 | 1.6629 |
| 30 | 200 | 4 | 1.9945 |
| 50 | 100 | 1 | 2.9118 |
| 50 | 100 | 4 | 5.4392 |
| 50 | 150 | 4 | 5.7069 |
| 50 | 200 | 4 | 2.6713 |
| N° Cycle, Carb-Calc | Added Extra Substances | CO2 Capture Capacity (mmol CO2/g Sorbent) |
|---|---|---|
| 1 | Water | 3.7341 |
| 2 | Water | 4.1354 |
| 3 | Water | 6.2158 |
| 4 | Water | 6.9611 |
| N° Cycle, Carb-Calc | Added Extra Reactants | CO2 Capture Capacity (mmol CO2/g Sorbent) |
|---|---|---|
| 1 | Water, iron | 5.4392 |
| 2 | Water, iron | 4.8687 |
| 3 | Water, iron | 2.7125 |
| Reaction | Material | Amount (ton) |
|---|---|---|
| → | Fe2O3 | 1.21 |
| Fe | 0.42 | |
| → + | FeOOH | 1.35 |
| Fe | 0.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora Mendoza, E.Y.; Sarmiento Santos, A.; Vera López, E.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Siderite Formation by Mechanochemical and High Pressure–High Temperature Processes for CO2 Capture Using Iron Ore as the Initial Sorbent. Processes 2019, 7, 735. https://doi.org/10.3390/pr7100735
Mora Mendoza EY, Sarmiento Santos A, Vera López E, Drozd V, Durygin A, Chen J, Saxena SK. Siderite Formation by Mechanochemical and High Pressure–High Temperature Processes for CO2 Capture Using Iron Ore as the Initial Sorbent. Processes. 2019; 7(10):735. https://doi.org/10.3390/pr7100735
Chicago/Turabian StyleMora Mendoza, Eduin Yesid, Armando Sarmiento Santos, Enrique Vera López, Vadym Drozd, Andriy Durygin, Jiuhua Chen, and Surendra K. Saxena. 2019. "Siderite Formation by Mechanochemical and High Pressure–High Temperature Processes for CO2 Capture Using Iron Ore as the Initial Sorbent" Processes 7, no. 10: 735. https://doi.org/10.3390/pr7100735
APA StyleMora Mendoza, E. Y., Sarmiento Santos, A., Vera López, E., Drozd, V., Durygin, A., Chen, J., & Saxena, S. K. (2019). Siderite Formation by Mechanochemical and High Pressure–High Temperature Processes for CO2 Capture Using Iron Ore as the Initial Sorbent. Processes, 7(10), 735. https://doi.org/10.3390/pr7100735






