Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Source-Separated Organics
Abstract
1. Introduction
2. Methods and Materials
2.1. Substrate and Inoculum
2.2. Hydrothermal Pretreatment
2.3. Acidification Experiment
2.4. Solubilization Study
- : Concentration of soluble COD of TWAS after HTP
- : Concentrations of soluble COD of the raw sample
- PCODRaw: Particulate COD of the raw substrate before adding to the reactor
- : Concentrations of total COD of the raw sample
- : Volatile suspended solids (VSS) concentration of the raw sample
- : Volatile suspended solids (VSS) concentration of the pretreated sample
- : Mass of soluble COD produced
- : Particulate COD of raw or pretreated samples that can be calculated as or
- : Mass of soluble COD after acidification
- : Mass of soluble COD before acidification
- : Soluble COD before acidification
- SCODSub: Soluble COD of the substrate (raw or pretreated)
- VSub: Volume of substrate added to the acidification reactor
- SCODSeed: Soluble COD of the inoculum
- VSeed: Volume of inoculum added to each acidification reactor
- PCODHTP: Particulate COD of the pretreated substrate before adding to the reactor
- TCODHTP: Total COD concentration of the pretreated sample
- SCODF: Soluble COD after acidification test.
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Hydrothermal Pretreatment on Source-Separated Organics
3.1.1. COD Solubilization of Source-Separated Organics
3.1.2. Carbohydrates and Proteins
3.1.3. Solid Reduction
3.1.4. Viscosity
3.2. Acidification of Organics
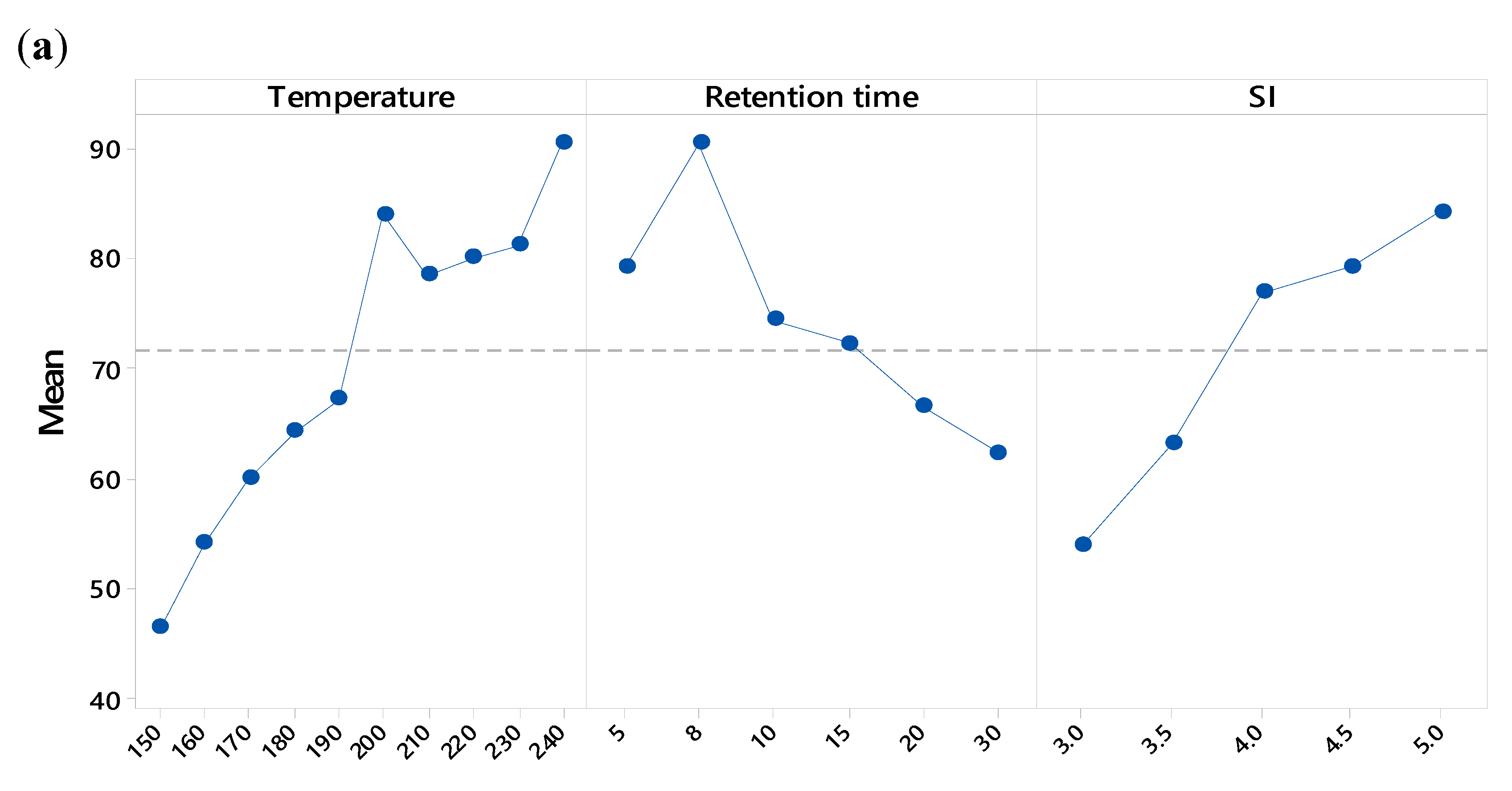
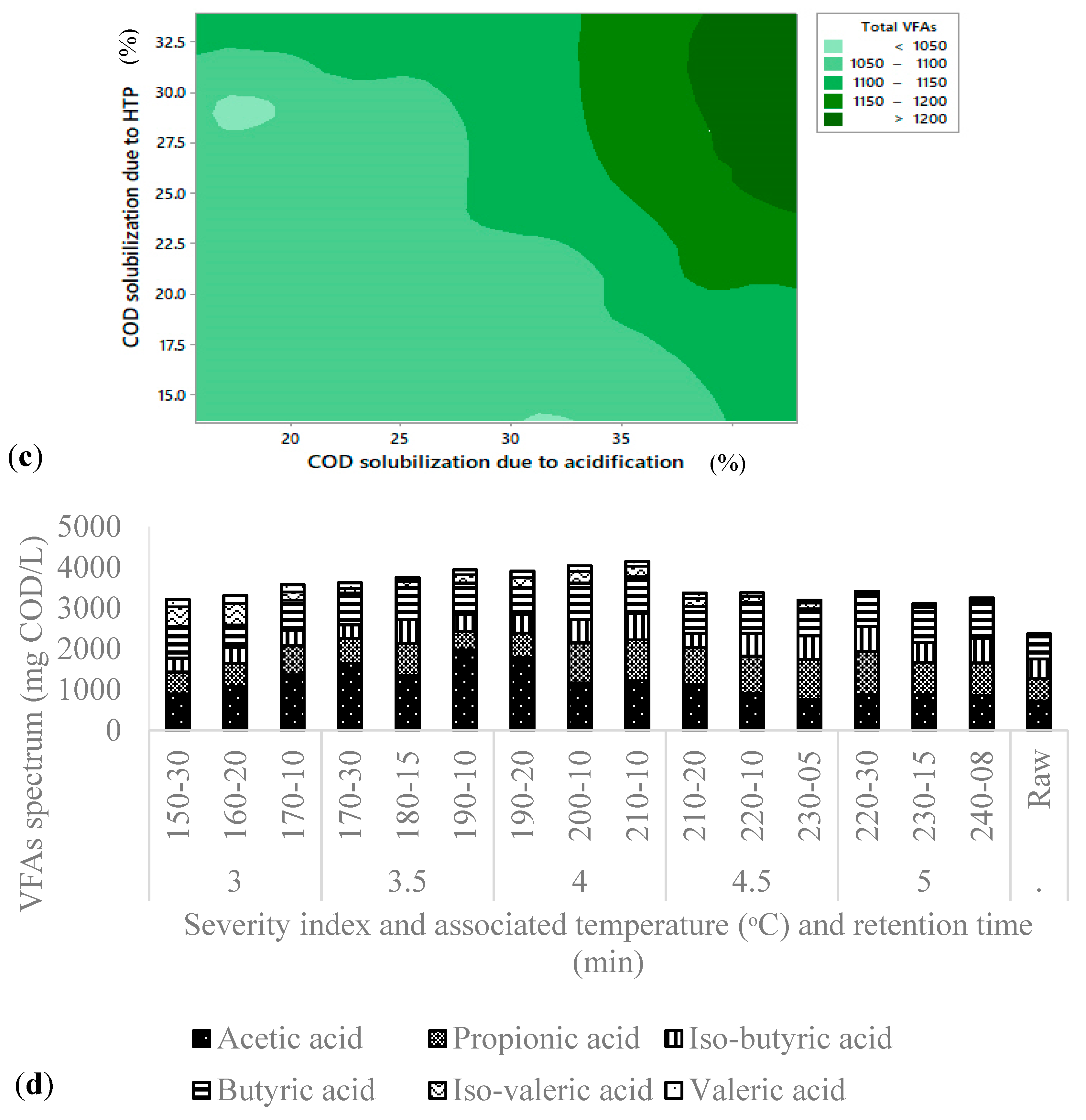
3.3. Volatile Fatty Acids Production
Product Spectrum
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grizzetti, B.; Pretato, U.; Lassaletta, L.; Billen, G.; Garnier, J. The contribution of food waste to global and European nitrogen pollution. Environ. Sci. Policy 2013, 33, 186–195. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Li, J. Influence of thermal pretreatment on physical and chemical properties of kitchen waste and the efficiency of anaerobic digestion. J. Environ. Manag. 2016, 180, 291–300. [Google Scholar] [CrossRef] [PubMed]
- TORONTO DISCO ROAD Canada Disco Road Organics Processing Facility (DROPF). Available online: https://www.ccibioenergy.com/wp-content/uploads/2018/01/CCI-Fact-Sheet_Disco_mar-2015_email.pdf (accessed on 7 July 2019).
- Karthikeyan, P.O.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2017, 249, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Meng, H.; Nie, Z.; Zhang, M. Polyhydroxyalkanoate production from fermented volatile fatty acids: Effect of pH and feeding regimes. Bioresour. Technol. 2013, 128, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Venkata Mohan, S.; Devi, P.M.; Peri, D.; Sarma, P.N. Acetate and butyrate as substrates for hydrogen production through photo-fermentation: Process optimization and combined performance evaluation. Int. J. Hydrogen Energy 2009, 34, 7513–7522. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Spasiano, D.; Luongo, V.; Petrella, A.; Alfè, M.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. Preliminary study on the adoption of dark fermentation as pretreatment for a sustainable hydrothermal denaturation of cement-asbestos composites. J. Clean. Prod. 2017, 166, 172–180. [Google Scholar] [CrossRef]
- Spasiano, D. Dark fermentation process as pretreatment for a sustainable denaturation of asbestos containing wastes. J. Hazard. Mater. 2018, 349, 45–50. [Google Scholar] [CrossRef]
- Huang, Y.L.; Wu, Z.; Zhang, L.; Cheung, C.M.; Yang, S. Production of carboxylic acids from hydrolyzed corn meal by immobilized cell fermentation in a fibrous-bed bioreactor. Bioresour. Technol. 2002, 82, 51–59. [Google Scholar] [CrossRef]
- Luongo, V.; Palma, A.; Rene, E.R.; Fontana, A.; Pirozzi, F.; Esposito, G.; Lens, P.N. Lactic acid recovery from a model of Thermotoga neapolitana fermentation broth using ion exchange resins in batch and fixed-bed reactors. Sep. Sci. Technol. 2019, 54, 1008–1025. [Google Scholar] [CrossRef]
- Li, Y.; Noike, T. Upgrading of anaerobic digestion of waste activated sludge by thermal pretreatment. Water Sci. Technol. 1992, 26, 857–866. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Dwyer, J.; Starrenburg, D.; Tait, S.; Barr, K.; Batstone, D.J.; Lant, P. Decreasing activated sludge thermal hydrolysis temperature reduces product colour, without decreasing degradability. Water Res. 2008, 42, 4699–4709. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, J.; Chen, T.; Long, Y.; Shen, D. Influence of melanoidins on acidogenic fermentation of food waste to produce volatility fatty acids. Bioresour. Technol. 2019, 284, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Cheng, J.; Qiao, D.; Yue, L.; Li, Y.Y.; Zhou, J.; Cen, K. Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresour. Technol. 2017, 241, 491–499. [Google Scholar] [CrossRef]
- Menon, A.; Ren, F.; Wang, J.Y.; Giannis, A. Effect of pretreatment techniques on food waste solubilization and biogas production during thermophilic batch anaerobic digestion. J. Mater. Cycles Waste Manag. 2016, 18, 222–230. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y. Effects of thermal pretreatment on acidification phase during two-phase batch anaerobic digestion of kitchen waste. Renew. Energy 2015, 77, 550–557. [Google Scholar] [CrossRef]
- City of Toronto Ashbridges Bay Wastewater Treatment Plant 2018 Annual Report. Available online: https://www.toronto.ca/wp-content/uploads/2019/05/8f0f-2018-TAB-Annual-Report-FINAL-ecopy.pdf (accessed on 7 July 2019).
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2008, 100, 10–18. [Google Scholar] [CrossRef]
- Higgins, M.J.; Beightol, S.; Mandahar, U.; Suzuki, R.; Xiao, S.; Lu, H.W.; Le, T.; Mah, J.; Pathak, B.; DeClippeleir, H.; et al. Pretreatment of a primary and secondary sludge blend at different thermal hydrolysis temperatures: Impacts on anaerobic digestion, dewatering and filtrate characteristics. Water Res. 2017, 122, 557–569. [Google Scholar] [CrossRef]
- Standard Methods for the Examination of Water and Wastewater Part 4000 INORGANIC. Nonmetallic Constituents Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1999.
- Ampules, A.S. Bradford Protein Assay Kit Bradford. Available online: www.thermoscientific.com/pierce (accessed on 7 July 2019).
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- HACH DR2900 Procedure Manual. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwiHnum5tqvkAhUHSN8KHaa-CGIQFjAAegQIAhAC&url=https%3A%2F%2Fwww.hach.com%2Fasset-get.download.jsa%3Fid%3D7639982436&usg=AOvVaw3LdGgPYQQQsdIJmWcCCZPS (accessed on 7 July 2019).
- Liu, Z.; Zhang, C.; Lu, Y.; Wu, X.; Wang, L.; Wang, L.; Han, B.; Xing, X.H. States and challenges for high-value biohythane production from waste biomass by dark fermentation technology. Bioresour. Technol. 2013, 135, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, L.; An, Y.; He, W.; Themelis, N.J.; Li, G. Hydrothermal liquefaction of three kinds of starches into reducing sugars. J. Clean. Prod. 2016, 112, 1049–1054. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Frunzo, L.; Esposito, G.; Lens, P.N.L.; Pirozzi, F. Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. J. Environ. Manag. 2014, 146, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, F.; Gong, Y.; Wang, Y.; Xu, H.; Yuan, F.; Gao, Y. Investigation into the Maillard reaction between ε-polylysine and dextran in subcritical water and evaluation of the functional properties of the conjugates. LWT Food Sci. Technol. 2014, 57, 612–617. [Google Scholar] [CrossRef]
- Takata, E.; Tsutsumi, K.; Tsutsumi, Y.; Tabata, K. Production of monosaccharides from napier grass by hydrothermal process with phosphoric acid. Bioresour. Technol. 2013, 143, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, W.; Gao, X.; Zhou, Y.; Shen, R. Effect of thermal pretreatment on the physical and chemical properties of municipal biomass waste. Waste Manag. 2012, 32, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, H.; Chen, S.; Dichtl, N.; Dai, X.; Li, N. Effects of thermal hydrolysis on organic matter solubilization and anaerobic digestion of high solid sludge. Chem. Eng. J. 2015, 264, 174–180. [Google Scholar] [CrossRef]
- Matsakas, L.; Kekos, D.; Loizidou, M.; Christakopoulos, P. Utilization of household food waste for the production of ethanol at high dry material content. Biotechnol. Biofuels 2014, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.; Müller, U.; Sauer, T.; Augner, K.; Pischetsrieder, M. Maillard reaction products as antimicrobial components for packaging films. Food Chem. 2014, 145, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Elbeshbishy, E.E. Enhancement of Biohydrogen and Biomethane Production from Wastes Using Ultrasonication. Ph.D. Thesis, The University of Western Ontario, London, ON, Canada, 2011. [Google Scholar]
- Ozkan, L.; Erguder, T.H.; Demirer, G.N. Effects of pretreatment methods on solubilization of beet-pulp and bio-hydrogen production yield. Int. J. Hydrogen Energy. 2011, 36, 382–389. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Dhar, B.R.; Nakhla, G.; Lee, H.S. A critical review on inhibition of dark biohydrogen fermentation. Renew. Sustain. Energy Rev. 2017, 79, 656–668. [Google Scholar] [CrossRef]
- Luo, K.; Pang, Y.; Yang, Q.; Wang, D.; Li, X.; Lei, M.; Huang, Q. A critical review of volatile fatty acids produced from waste activated sludge: enhanced strategies and its applications. Environ. Sci. Pollut. Res. 2019, 26, 13984–13998. [Google Scholar] [CrossRef] [PubMed]







| Studies on Thermal Pretreatment of Food Waste Following Fermentation. | |||||
|---|---|---|---|---|---|
| Reference | Pretreatment Condition | Effect of Hydrothermal Pretreatment | |||
| Temperature (°C) | Retention Time (min) | Increase in SCOD | Increase in VFAs/Methane Compared to the Raw Sample | Solid Reduction | |
| [1] | 100–200 | 5–30 | Highest COD solubilization 70% at 180 °C | 85% at 160 °C-20 min, | NA * |
| [2] | 55–160 | 50–70 | NA | 63% at 120 °C-50 min | Highest vs. solubilization of 49% at 120 °C-50 min |
| [3] | 100–220 | 30 | 43% more soluble COD than the control at 180 °C-30 min | 35% at 160 °C-50 min | 31% decrease in vs. after HTP at 220 °C |
| [4] | 90–200 | 30 | Highest COD solubilization of 26% at 150 °C | NA | NA |
| Studies on Thermal Pretreatment of Food Waste Following AD | |||||
| [5] | 90 | 30 | NA | 29% | NA |
| [6] | 55–160 | 15–120 | NA | 15% at 120 °C | 29% increase in vs. proportion at 120 °C-15 min |
| [7] | 70–150 | 30–60 | NA | 90% at 80 °C | NA |
| [10] | 175 | 60 | SCOD increased significantly after HTP (No Numbers) | decreased by 7.9% at 175 °C-60 min | VSS solubilization ratio increased by 39% |
| Parameter | SSO | Inoculum |
|---|---|---|
| TCOD (mg/L) | 144,050 ± 17,254 | 16,267 ± 1595 |
| SCOD (mg/L) | 42,167 ± 400 | 575 ± 50 |
| TSS (mg/L) | 66,183 ± 860 | 15,100 ± 600 |
| VSS (mg/L) | 49,250 ± 330 | 10,500 ± 800 |
| Total carbohydrates (mg/L) | 11,408 ± 1506 | 5707 ± 991 |
| Soluble carbohydrates (mg/L) | 302 ± 58 | 3 ± 0.03 |
| Total Protein (mg/L) | 986 ± 113 | 410 ± 93 |
| Soluble Protein (mg/L) | 221 ± 9 | 33 ± 3 |
| Ammonia nitrogen NH3-N (mg/L) | 1716 ± 45 | 543 ± 12 |
| Alkalinity (mg CaCO3/L) | 5183 ± 226 | 1545 ± 30 |
| pH | 5.9 | 7.00 |
| Severity Index (SI) | 3.0 ± 0.05 | 3.5 ± 0.05 | 4.0 ± 0.05 | 4.5 ± 0.05 | 5.0 ± 0.05 |
|---|---|---|---|---|---|
| Pretreatment Parameters | (kPa) (°C) (min) | (kPa) (°C) (min) | (kPa) (°C) (min) | (kPa) (°C) (min) | (kPa) (°C) (min) |
| Pretreatment Scenario 1 | 476-150-30 | 786-170-30 | 1247-190-20 | 1565-210-20 | 1909-220-30 |
| Pretreatment Scenario 2 | 613-160-20 | 999-180-15 | 1551-200-10 | 1909-220-10 | 2323-230-15 |
| Pretreatment Scenario 3 | 786-170-10 | 1247-190-10 | 1565-210-5 | 2323-230-5 | 2806-240-8 |
| Soluble-Carbohydrate (mg/L) | Soluble-Protein (mg/L) | Ammonia (mg/L) | TVFAs/SCOD Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| SI | Temp (°C)-Time (min) | Average | STD | Average | STD | Average | STD | ° |
| 3.00 | 150-30 | 236 | 11 | 15 | 2 | 767 | 19 | 0.55 |
| 160-20 | 277 | 16 | 14 | 1 | 696 | 36 | 0.56 | |
| 170-10 | 293 | 3 | 19 | 3 | 761 | 9 | 0.60 | |
| 3.50 | 170-30 | 271 | 27 | 19 | 2 | 769 | 35 | 0.61 |
| 180-15 | 272 | 16 | 21 | 2 | 761 | 18 | 0.62 | |
| 190-10 | 223 | 13 | 32 | 4 | 757 | 14 | 0.63 | |
| 4.00 | 190-20 | 155 | 12 | 35 | 1 | 780 | 35 | 0.65 |
| 200-10 | 160 | 20 | 38 | 4 | 789 | 42 | 0.66 | |
| 210-10 | 170 | 6 | 36 | 1 | 775 | 13 | 0.68 | |
| 4.50 | 210-20 | 157 | 13 | 34 | 1 | 834 | 55 | 0.57 |
| 220-10 | 120 | 19 | 39 | 3 | 707 | 56 | 0.53 | |
| 230-05 | 137 | 15 | 41 | 3 | 690 | 42 | 0.52 | |
| 5.00 | 220-30 | 145 | 22 | 49 | 1 | 779 | 14 | 0.58 |
| 230-15 | 105 | 25 | 44 | 4 | 791 | 17 | 0.53 | |
| 240-08 | 112 | 26 | 38 | 2 | 757 | 17 | 0.56 | |
| . | Raw | 160 | 15 | 44 | 3 | 893 | 15 | 0.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakar, F.l.; Koupaie, E.H.; Hafez, H.; Elbeshbishy, E. Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Source-Separated Organics. Processes 2019, 7, 576. https://doi.org/10.3390/pr7090576
Kakar Fl, Koupaie EH, Hafez H, Elbeshbishy E. Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Source-Separated Organics. Processes. 2019; 7(9):576. https://doi.org/10.3390/pr7090576
Chicago/Turabian StyleKakar, Farokh laqa, Ehssan Hosseini Koupaie, Hisham Hafez, and Elsayed Elbeshbishy. 2019. "Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Source-Separated Organics" Processes 7, no. 9: 576. https://doi.org/10.3390/pr7090576
APA StyleKakar, F. l., Koupaie, E. H., Hafez, H., & Elbeshbishy, E. (2019). Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Source-Separated Organics. Processes, 7(9), 576. https://doi.org/10.3390/pr7090576






