Determination of Dissolved CO2 Concentration in Culture Media: Evaluation of pH Value and Mathematical Data
Abstract
1. Introduction
2. Materials and Methods
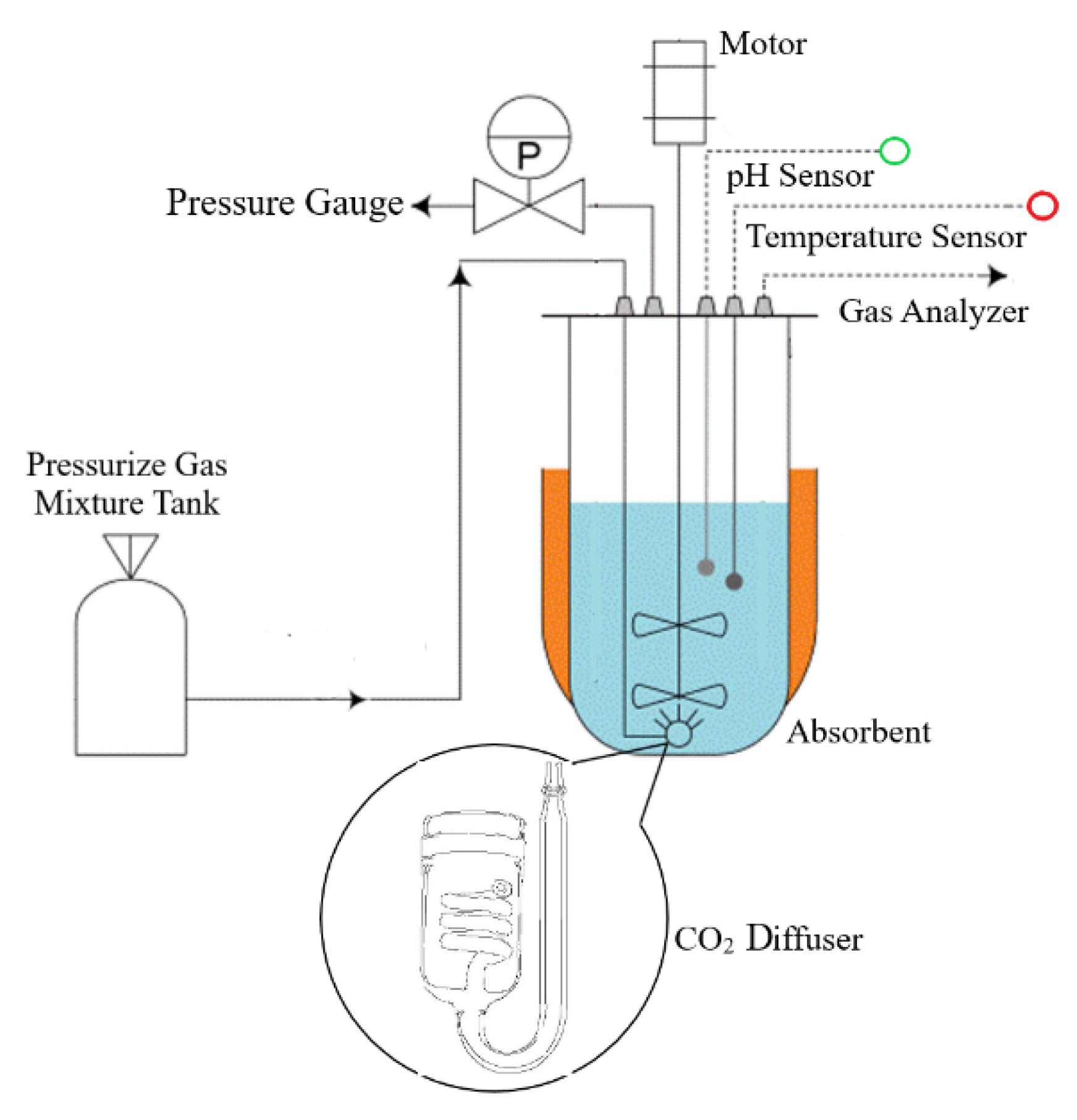
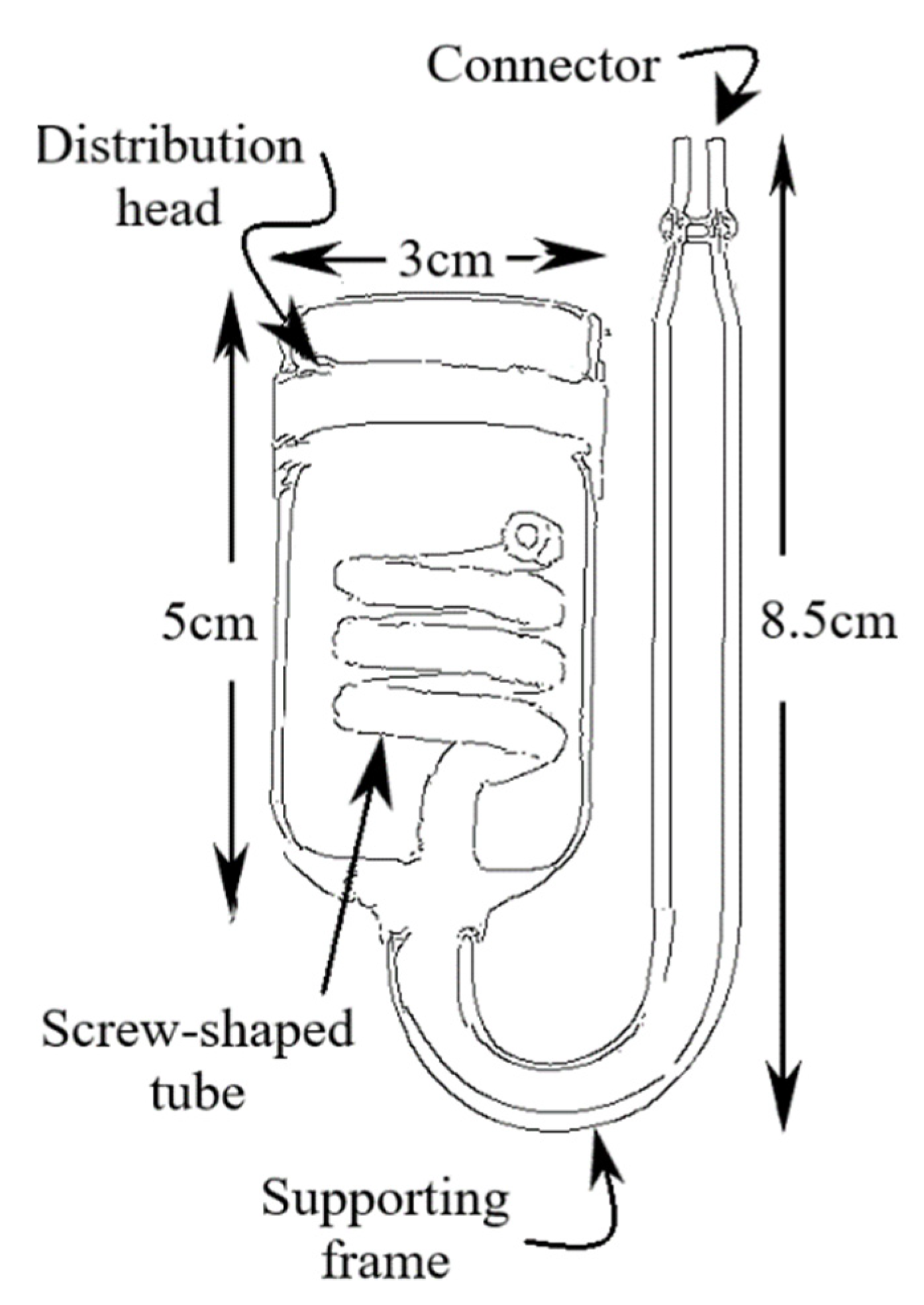
2.1. Carbon Dioxide (CO2) Delivery System
2.2. Time Measurement of The Maximum Carbon Dioxide (CO2) Dissolved
2.3. Optimization through Surface Response Methodology
2.4. Analytical Methods
2.5. Model Description and Calculation
3. Results and Discussion
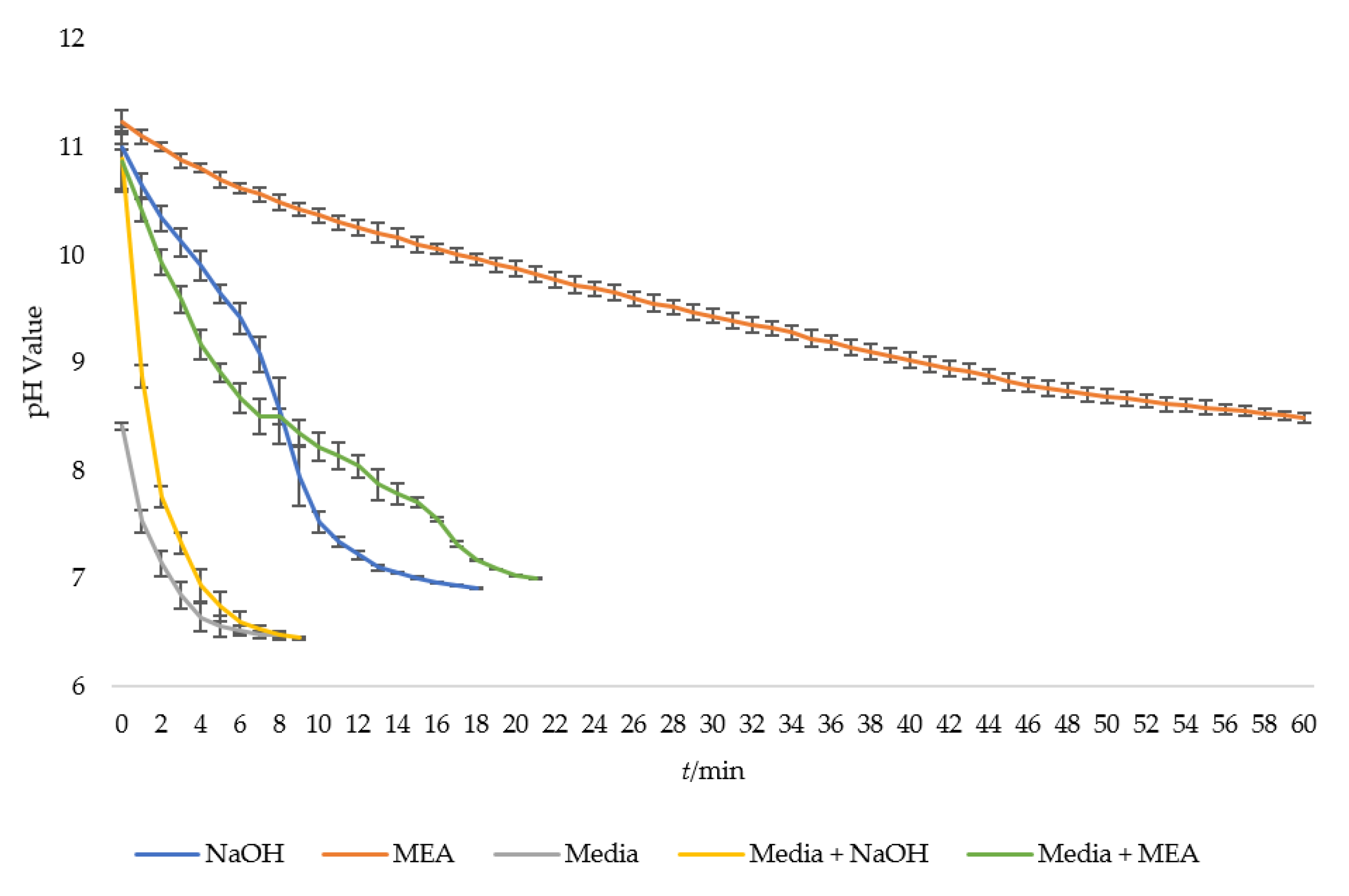
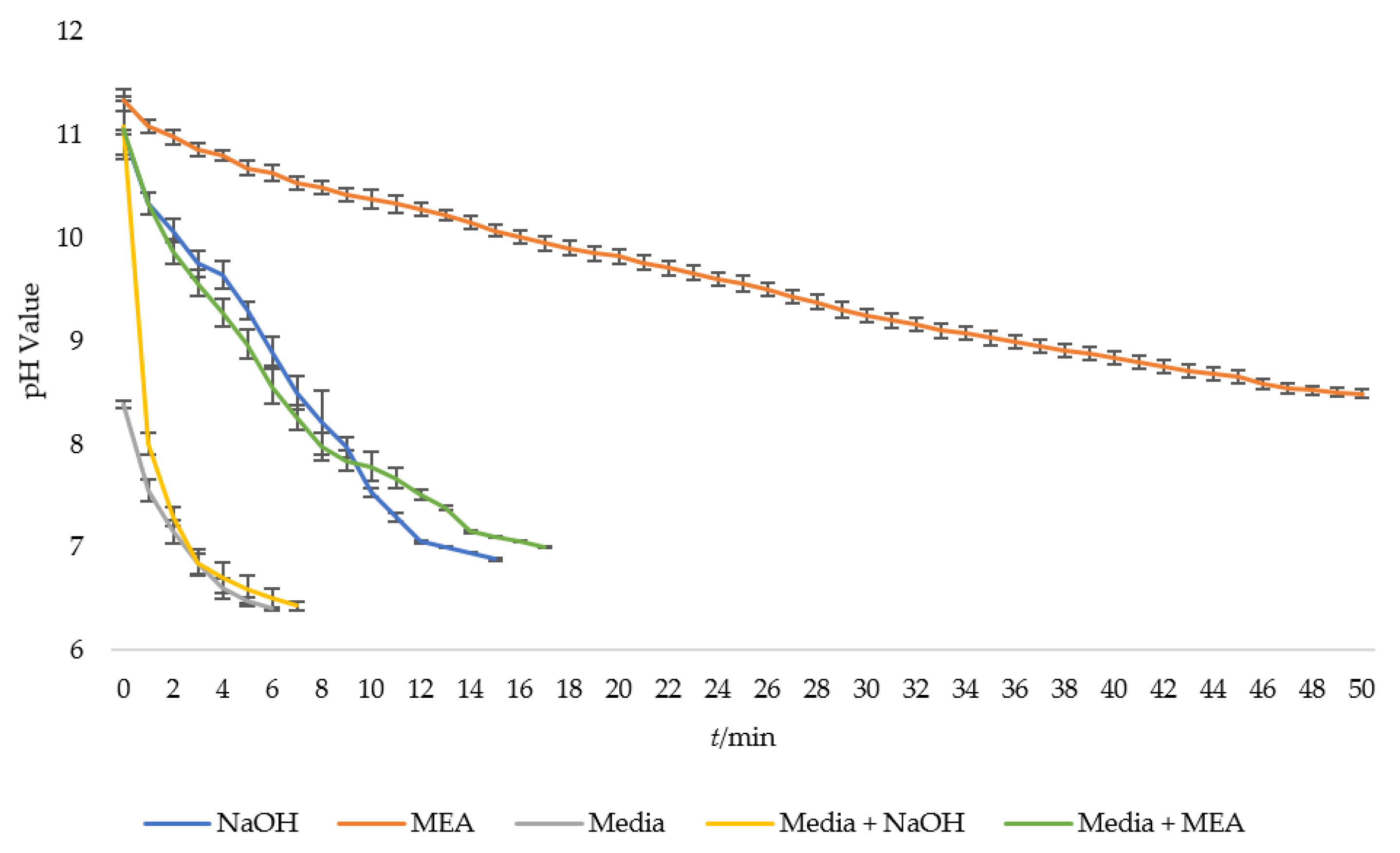
3.1. Relationship between Carbon Dioxide (CO2) Concentration and pH
3.2. System Performance with Deployment of Carbon Dioxide (CO2) Diffuser
3.3. Factors Affecting Carbon Dioxide (CO2) Solubilities in Culture Media
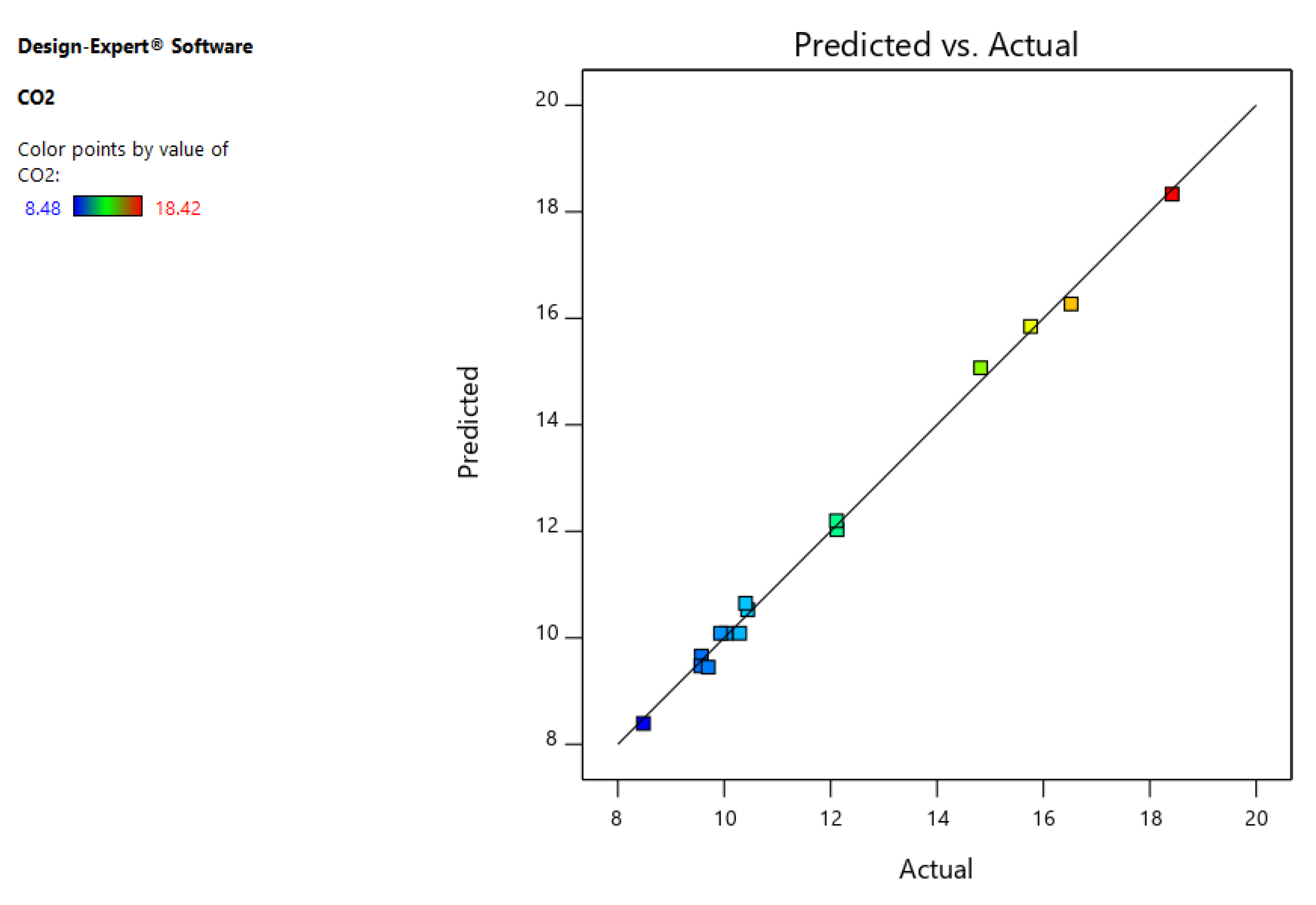
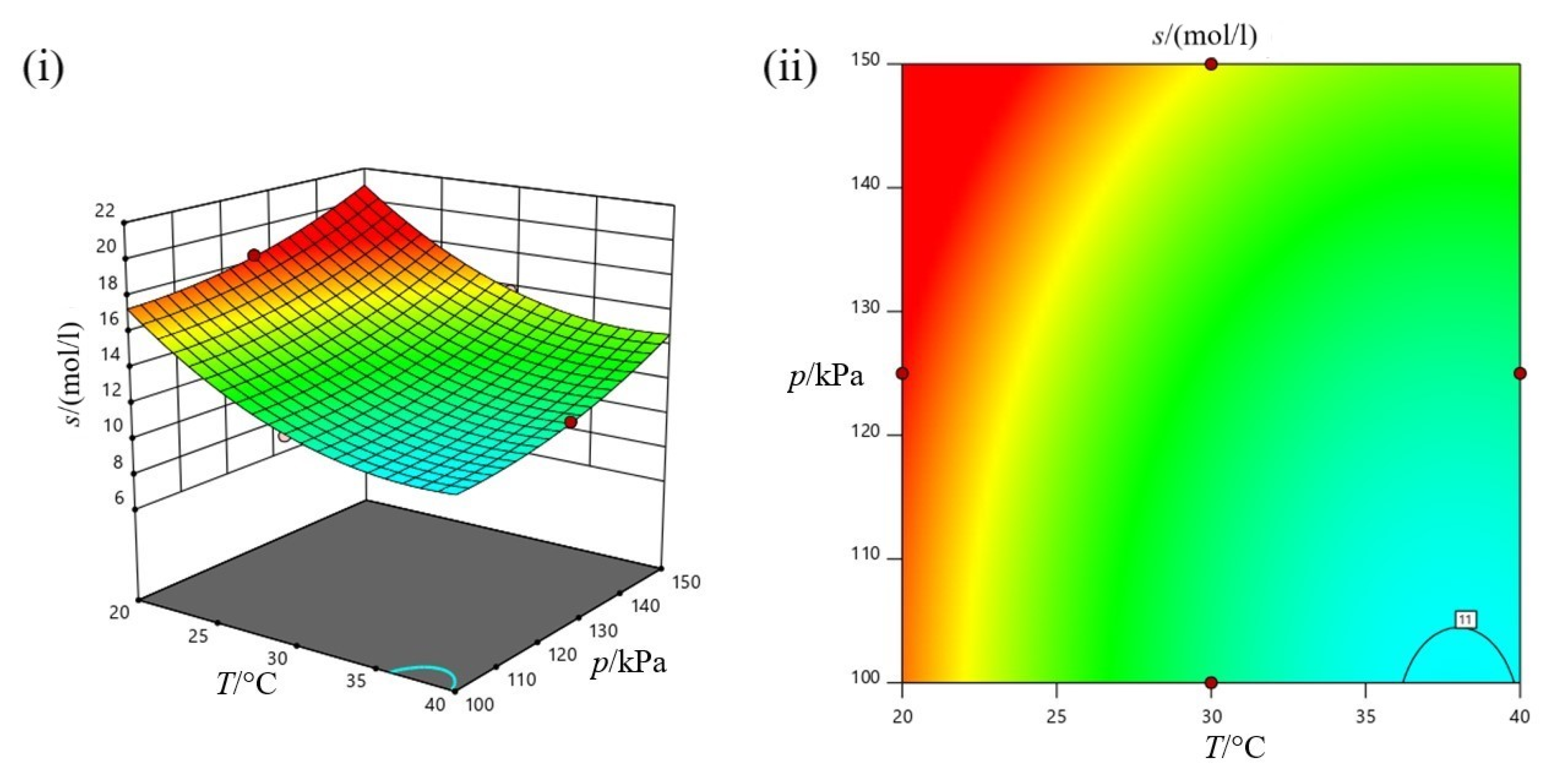
3.3.1. Design of Experiment Analysis
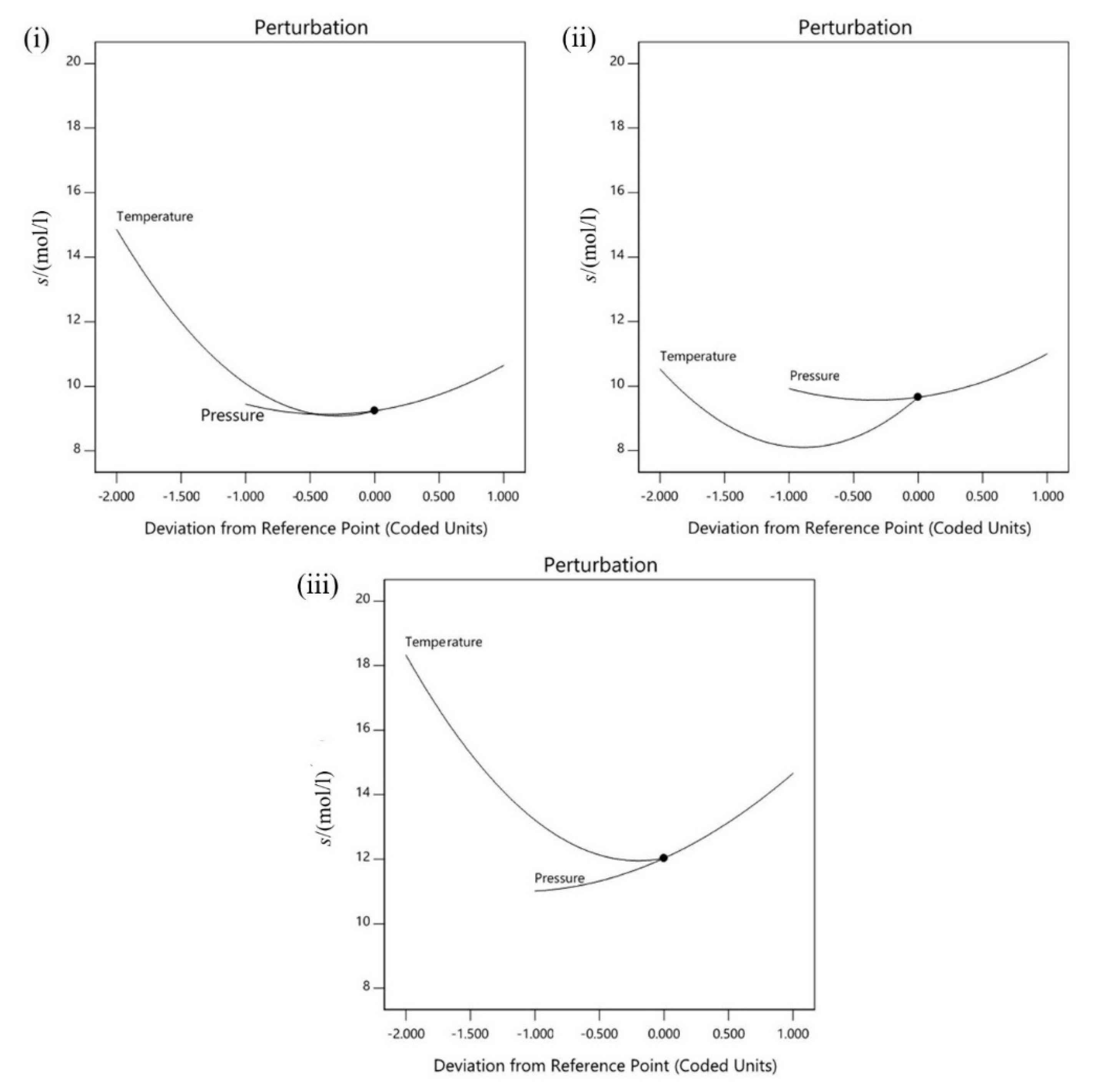
3.3.2. Individual Effect of Experiment Parameters on Carbon Dioxide (CO2) Absorption
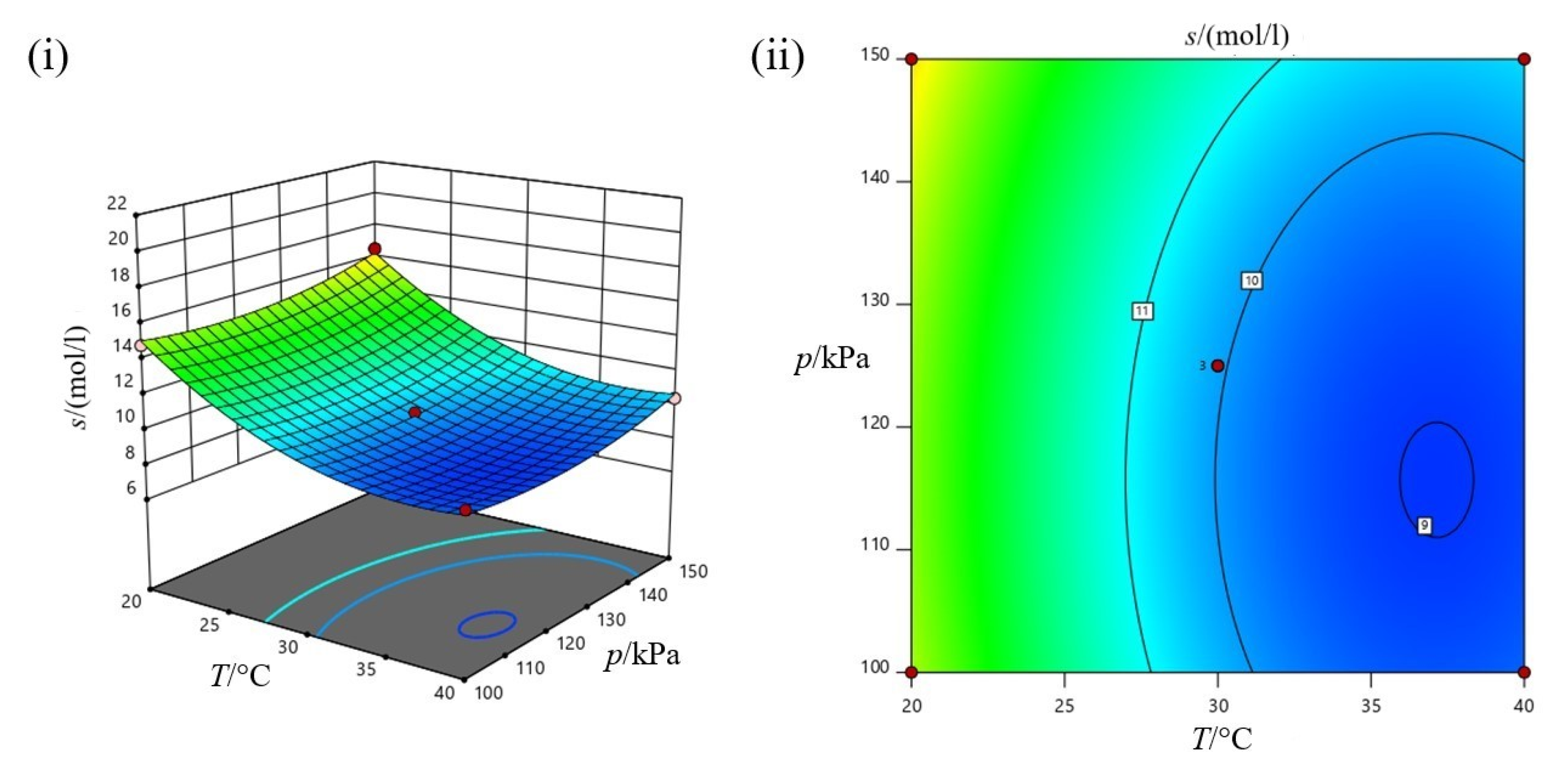
3.3.3. Collective Effect of Experiment Parameters on Carbon Dioxide (CO2) Absorption
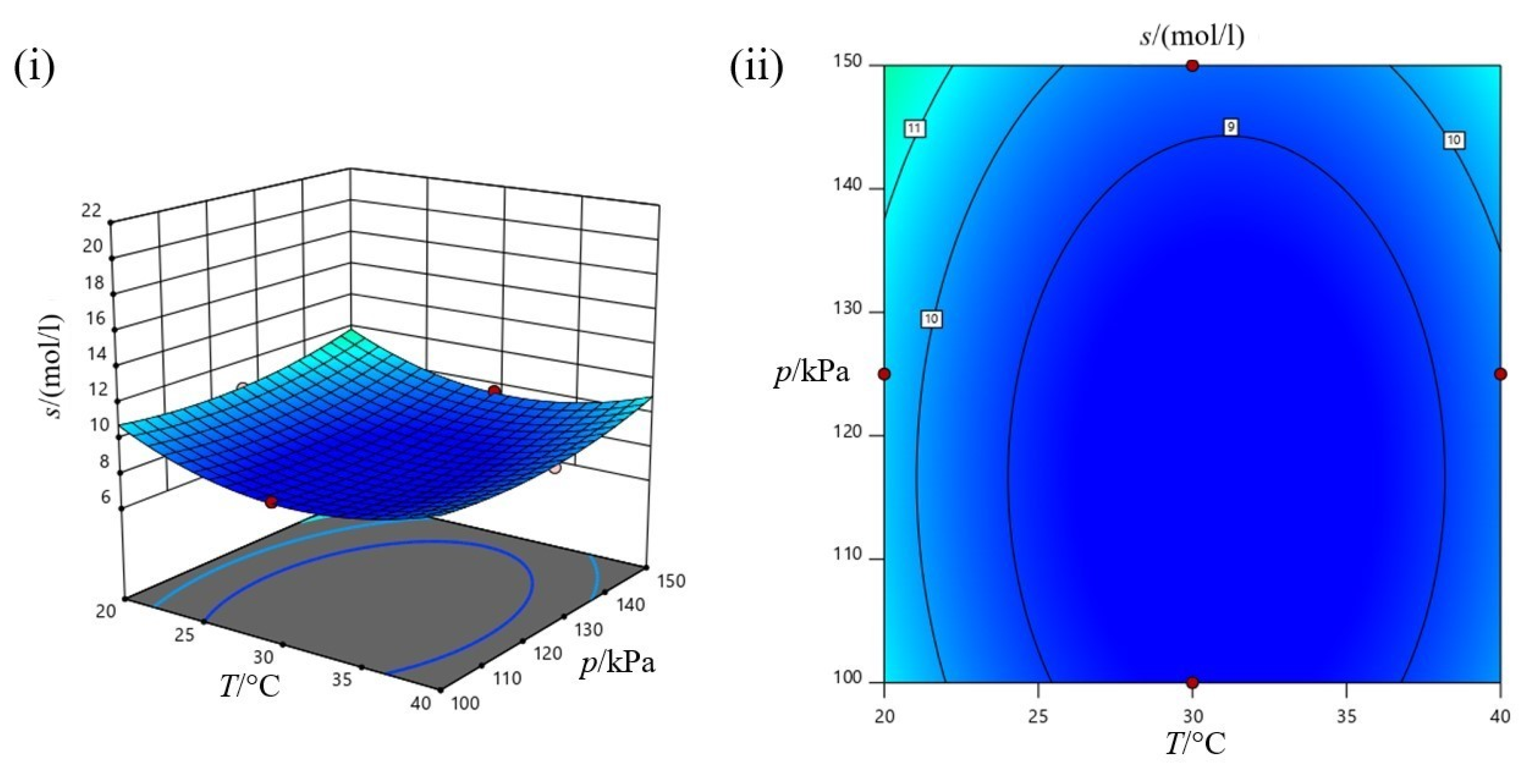
3.3.4. Process Optimization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- John, J.C.; Paul, D.H.; James, R.D.; Sam, A.N.; AMichael, S.; James, T.T.; Lynn, D.W. U.S. Energy Information, an Annual Energy Outlook 2015 with projections to 2040. Off. Integr. Int. Energy Anal. 2015, 1, 1–244. [Google Scholar]
- Al-mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef]
- Gerard, D.; Wilson, E.J. Environmental bonds and the challenge of long-term carbon sequestration. J. Environ. Manag. 2009, 90, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130. [Google Scholar] [CrossRef]
- Styring, P.; Jansen, D.; de Coninck, H.; Reith, H.; Armstrong, K. Carbon Capture and Utilisation in the Green Economy; The Centre for Low Carbon Futures 2011 and CO2Chem Publishing 2012: Sheffield, UK, 2011; ISBN 9780957258815. [Google Scholar]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Gong, F.; Cai, Z.; Li, Y. Synthetic biology for CO2 fixation. Sci. China Life Sci. 2016, 59, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Al-Anezi, K.; Somerfield, C.; Mee, D.; Hilal, N. Parameters affecting the solubility of carbon dioxide in seawater at the conditions encountered in MSF desalination plants. Desalination 2008, 222, 548–571. [Google Scholar] [CrossRef]
- Yincheng, G.; Zhenqi, N.; Wenyi, L. Energy Procedia Comparison of removal efficiencies of carbon dioxide between aqueous ammonia and NaOH solution in a fine spray column. Energy Procedia 2011, 4, 512–518. [Google Scholar] [CrossRef]
- Da Rosa, G.M.; de Morais, M.G.; Costa, J.A. Green alga cultivation with monoethanolamine: Evaluation of CO2 fixation and macromolecule production. Bioresour. Technol. 2018, 261, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Han, S.; Wee, J. Carbon dioxide capture capacity of sodium hydroxide aqueous solution. J. Environ. Manag. 2013, 114, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Shirayama, Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 2004, 274, 161–169. [Google Scholar] [CrossRef]
- Fleischer, C.; Becker, S.; Eigenberger, G. Detailed modeling of the chemisorption of CO2 into NaOH in a bubble column. Chem. Eng. Sci. 1996, 51, 1715–1724. [Google Scholar] [CrossRef]
- Knoche, W. Chemical Reactions of CO2 in Water BT—Biophysics and Physiology of Carbon Dioxide; Bauer, C., Gros, G., Bartels, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1980; pp. 3–11. [Google Scholar]
- Fundamentals of Environmental Measurements. pH of Water. Available online: https://www.fondriest.com/environmental-measurements/parameters/water-quality/ph/ (accessed on 23 April 2020).
- Bureau, I. Method and Apparatus for Rapid Carbonanation of a Fluid. WO Patent 2016/122718, 4 August 2016. [Google Scholar]
- Geng, H.; Chen, Q.; Zhao, G. The Experiment Study of Biogas Atomization Upgrading with Water Scrubbing at Atmospheric Pressure. In 2015 International Conference on Industrial Technology and Management Science; Atlantis Press: Paris, France, 2015. [Google Scholar]
- Martínez, I.; Casas, P.A. Simple model for CO2 absorption in a bubbling water column. Braz. J. Chem. Eng. 2012, 29, 107–111. [Google Scholar]
- Song, H.; Lee, J.W.; Choi, S.; You, J.K.; Hong, W.H.; Lee, S.Y. Effects of dissolved CO2 levels on the growth of Mannheimia succiniciproducens and succinic acid production. Biotechnol. Bioeng. 2007, 98, 1296–1304. [Google Scholar] [CrossRef]
- Xi, Y.; Chen, K.; Li, J.; Fang, X.; Zheng, X.; Sui, S.; Jiang, M.; Wei, P. Optimization of culture conditions in CO2 fixation for succinic acid production using Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 2011, 38, 1605–1612. [Google Scholar] [CrossRef]
- Hauser, C.; Thielmann, J.; Muranyi, P. Chapter 46—Organic Acids: Usage and Potential in Antimicrobial Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 563–580. ISBN 978-0-12-800723-5. [Google Scholar]
- Schumpe, A.; Quicker, G. Gas Solubilities in Microbial Culture Media. In Reaction Engineering; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Gunnarsson, I.B.; Alvarado-Morales, M.; Angelidaki, I. Utilization of CO2 fixating bacterium Actinobacillus succinogenes 130Z for simultaneous biogas upgrading and biosuccinic acid production. Environ. Sci. Technol. 2014, 48, 12464–12468. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- LibreTexts 13.4: Effects of Temperature and Pressure on Solubility—Chemistry LibreTexts; Department of Education Open Textbook Pilot Project: Washington, DC, USA, 2019; pp. 1–5.
- Wiebe, R.; Gaddy, V.L. The Solubility of Carbon Dioxide in Water at Various Temperatures from 12 to 40° and at Pressures to 500 Atmospheres. Critical Phenomena. J. Am. Chem. Soc. 1940, 62, 815–817. [Google Scholar] [CrossRef]
| Level and Range | ||||
|---|---|---|---|---|
| Parameters | Symbols | Low | Centre | High |
| Absorbent | X | Media + NaOH | NaOH | Media + MEA |
| Pressure, (kPa) | p | 100 | 125 | 150 |
| Temperature, (°C) | T | 20 | 30 | 40 |
| Parameters | Model | Remark | Ref. |
|---|---|---|---|
| Composition | Empirical model of Henry’s Law | Solubility depends on ion and organic substance appearing in solvent | [26] |
| whereas H: Henry’s constant in solvent mixtures; sG: CO2 solubility in solvent mixtures; subscript 0: value at pure solvent (e.g., water); h: ion-specific parameter; Kn,j: substance specific model parameter; ci: concentration of ion; cn,j: concentration of an organic substance | |||
| Pressure | Henry’s Law: | Solubility is directly proportional to the pressure | [26] |
| whereas pCO2: CO2 partial pressure; H0: Henry’s constant; cCO2: concentration of dissolved CO2. | |||
| Temperature | Henry’s Law and Van’t Hoff equation: | Solubility is inversely proportional to temperature. | [21,22] |
| whereas kh: temperature-dependent Henry’s constant; k298K: Henry’s constant at 298K; ΔHdiss: dissolution enthalpy; R: ideal gas constant |
| Independent Input Variables [X] | Response [Y] | |||
|---|---|---|---|---|
| Exp. | Absorbent | T/°C | p/kPa | s/(mol/L) |
| 1 | NaOH | 20 | 100 | 14.82 |
| 2 | NaOH | 40 | 100 | 9.7 |
| 3 | Culture media with MEA | 20 | 125 | 18.42 |
| 4 | Culture media with NaOH | 20 | 125 | 10.44 |
| 5 * | NaOH | 30 | 125 | 10.04 |
| 6 | Culture media with MEA | 30 | 100 | 12.11 |
| 7 | Culture media with MEA | 40 | 125 | 12.12 |
| 8 | NaOH | 20 | 150 | 16.52 |
| 9 | Culture media with MEA | 30 | 150 | 15.76 |
| 10 | NaOH | 40 | 150 | 10.4 |
| 11 | Culture media with NaOH | 30 | 100 | 8.48 |
| 12 * | NaOH | 30 | 125 | 10.29 |
| 13 | Culture media with NaOH | 30 | 150 | 9.56 |
| 14 * | NaOH | 30 | 125 | 9.93 |
| 15 | Culture media with NaOH | 40 | 125 | 9.57 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 128.74 | 10 | 12.87 | 135.76 | 0.0001 | significant |
| X-Medium | 53.08 | 2 | 26.54 | 279.85 | <0.0001 | |
| T-Temperature | 42.367 | 1 | 42.37 | 446.76 | <0.0001 | |
| p-Pressure | 6.35 | 1 | 6.35 | 67.01 | 0.0012 | |
| XT | 9.44 | 2 | 4.72 | 49.78 | 0.0015 | |
| Xp | 2.33 | 2 | 1.16 | 12.28 | 0.0196 | |
| T2 | 14.28 | 1 | 14.28 | 150.60 | 0.0003 | |
| p2 | 2.40 | 1 | 2.40 | 25.34 | 0.0073 | |
| Residual | 0.3793 | 4 | 0.0948 | |||
| Lack of Fit | 0.3112 | 2 | 0.1556 | 4.57 | 0.1794 | not significant |
| Pure Error | 0.0681 | 2 | 0.0340 | |||
| Corrected Total Sum of Squares | 129.12 | 14 |
| No. | Absorbent | T/°C | p/kPa | s/(mol/L) |
|---|---|---|---|---|
| 1 | Culture media with MEA | 20.483 | 136.728 | 19.029 |
| 2 | Culture media with MEA | 20.702 | 133.270 | 18.537 |
| 3 | Culture media with MEA | 20.557 | 132.113 | 18.529 |
| 4 | Culture media with MEA | 21.951 | 143.633 | 18.834 |
| 5 | Culture media with MEA | 20.125 | 132.344 | 18.850 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Show, P.L. Determination of Dissolved CO2 Concentration in Culture Media: Evaluation of pH Value and Mathematical Data. Processes 2020, 8, 1373. https://doi.org/10.3390/pr8111373
Adnan AI, Ong MY, Nomanbhay S, Show PL. Determination of Dissolved CO2 Concentration in Culture Media: Evaluation of pH Value and Mathematical Data. Processes. 2020; 8(11):1373. https://doi.org/10.3390/pr8111373
Chicago/Turabian StyleAdnan, Amir Izzuddin, Mei Yin Ong, Saifuddin Nomanbhay, and Pau Loke Show. 2020. "Determination of Dissolved CO2 Concentration in Culture Media: Evaluation of pH Value and Mathematical Data" Processes 8, no. 11: 1373. https://doi.org/10.3390/pr8111373
APA StyleAdnan, A. I., Ong, M. Y., Nomanbhay, S., & Show, P. L. (2020). Determination of Dissolved CO2 Concentration in Culture Media: Evaluation of pH Value and Mathematical Data. Processes, 8(11), 1373. https://doi.org/10.3390/pr8111373








