NOx Emission Reduction by Advanced Reburning in Grate-Rotary Kiln for the Iron Ore Pelletizing Production
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Reaction Parameters on the NOx Reduction
3.2. Effects of Reductants on the NOx Reduction
3.3. Effects of Additives on the NOx Reduction
3.4. Effects of Process Conditions on the NOx Reduction
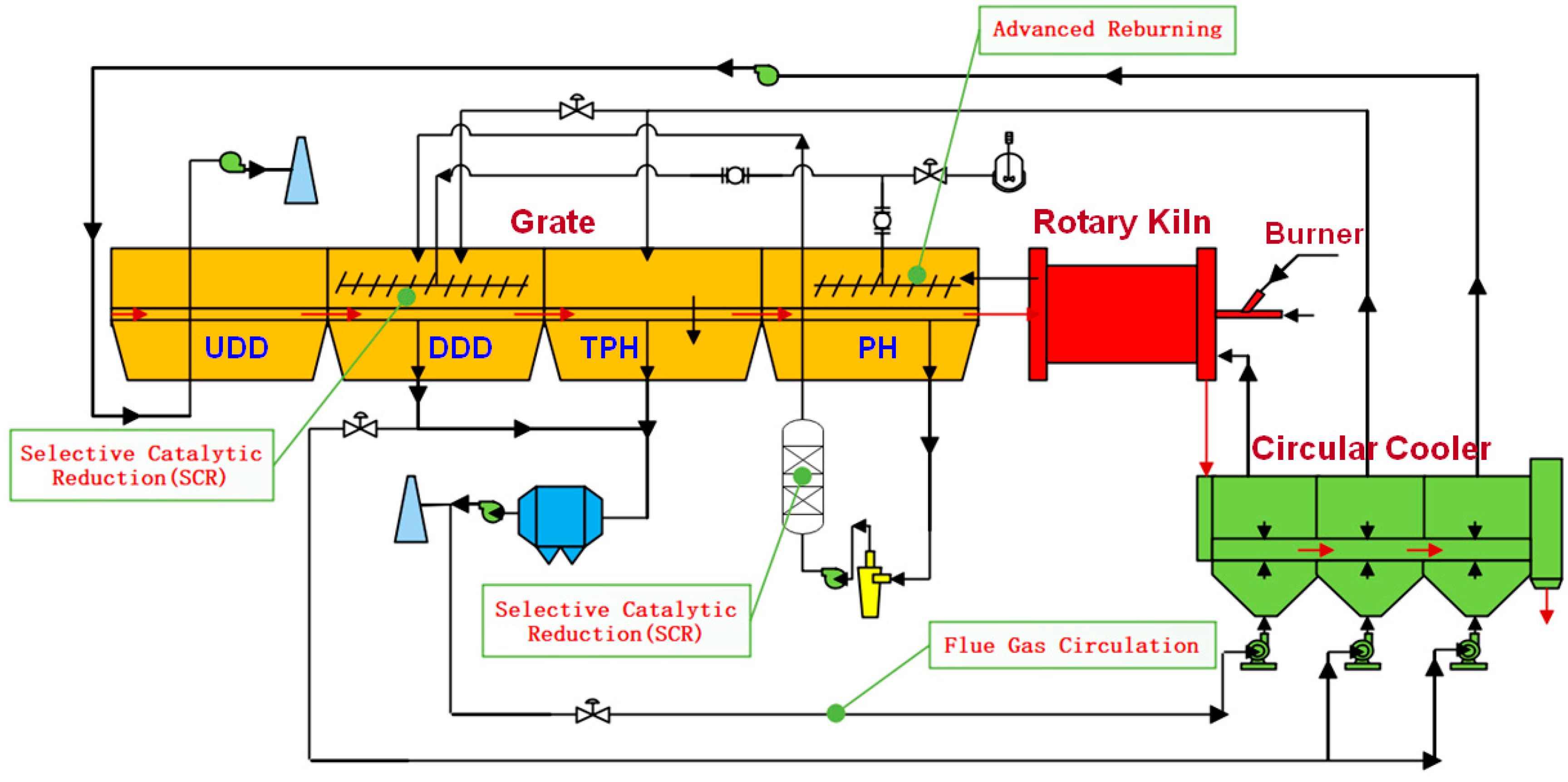
4. NOx Emission Behavior and Prospects of Denitrification Technology
4.1. NOx Emission Behavior
4.2. Prospects of Denitrification Technology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.J.; She, X.F.; Han, Y.H.; Wang, J.S.; Zeng, F.B.; Xue, Q.G. Softening and Melting Behavior of Ferrous Burden under Simulated Oxygen Blast Furnace Condition. J. Iron Steel Res. Int. 2015, 22, 297–303. [Google Scholar] [CrossRef]
- Bolen, J. Modern air pollution control for iron ore induration. Min. Metall. Explor. 2014, 31, 103–114. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, D.; Pan, J.; Guo, Z.; Yang, C. Effect of basicity on the structure characteristics of chromium-nickel bearing iron ore pellets. Powder Technol. 2019, 342, 409–417. [Google Scholar] [CrossRef]
- Gan, M.; Ji, Z.; Fan, X.; Chen, X.; Zheng, R.; Gao, L.; Wang, G.; Jiang, T. Value-added utilization of waste silica powder into high-quality chromite pellets preparation process. Powder Technol. 2018, 328, 122–129. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Jiang, T.; Zhong, R.; Liang, Z. Iron ore pellet disintegration mechanism in simulated shaft furnace conditions. Powder Technol. 2017, 317, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Stjernberg, J.; Ion, J.C.; Antti, M.L.; Nordin, L.O.; Lindblom, B.; Odén, M. Extended studies of degradation mechanisms in the refractory lining of a rotary kiln for iron ore pellet production. J. Eur. Ceram. Soc. 2012, 32, 1519–1528. [Google Scholar] [CrossRef]
- Stjernberg, J.; Isaksson, O.; Ion, J.C. The grate-kiln induration machine: History, advantages, and drawbacks, and outline for the future. J. S. Afr. Inst. Min. Metall. 2015, 115, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zhou, Y.; Lv, J.; Wu, J. Assessment of multi-air emissions: Case of particulate matter (dust), SO2, NOx and CO2 from iron and steel industry of China. J. Clean. Prod. 2019, 232, 350–358. [Google Scholar] [CrossRef]
- Lv, W.; Sun, Z.; Su, Z. Life cycle energy consumption and greenhouse gas emissions of iron pelletizing process in China, a case study. J. Clean. Prod. 2019, 233, 1314–1321. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Y.; Yan, L.; Liu, T.; Zhang, Q.; He, K. A unit-based emission inventory of SO2, NOx and PM for the Chinese iron and steel industry from 2010 to 2015. Sci. Total Environ. 2019, 676, 18–30. [Google Scholar] [CrossRef]
- Fan, X.H.; Yang, G.M.; Chen, X.L.; Gao, L.; Huang, X.X.; Li, X. Predictive models and operation guidance system for iron ore pellet induration in traveling grate–rotary kiln process. Comput. Chem. Eng. 2015, 79, 80–90. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Lu, H.; Ni, T.; Li, Y. Waste energy recovery and energy efficiency improvement in China’s iron and steel industry. Appl. Energy 2017, 191, 502–520. [Google Scholar] [CrossRef]
- Zhu, T.; Hu, Y.; Tang, C.; Wang, L.; Liu, X.; Deng, L.; Che, D. Experimental study on NOx formation and burnout characteristics of pulverized coal in oxygen enriched and deep-staging combustion. Fuel 2020, 272, 117639. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Jin, Q.; Chen, Q.; Zhou, Y. Mechanism analysis on the pulverized coal combustion flame stability and NOx emission in a swirl burner with deep air staging. J. Energy Inst. 2019, 92, 298–310. [Google Scholar] [CrossRef]
- Edland, R.; Normann, F.; Fredriksson, C.; Andersson, K. Implications of fuel choice and burner settings for combustion efficiency and NOx formation in PF-fired iron ore rotary kilns. Energy Fuels 2017, 31, 3253–3261. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, Y.; Mo, G.; Liao, Z.; Cen, K. Experimental investigations of the conversion of fuel-N, volatile-N and char-N to NOx and N2O during single coal particle fluidized bed combustion. J. Energy Inst. 2017, 90, 62–72. [Google Scholar] [CrossRef]
- Edland, R.; Smith, N.; Allgurén, T.; Fredriksson, C.; Normann, F.; Haycock, D.; Johnson, C.; Frandsen, J.; Fletcher, T.H.; Andersson, K. Evaluation of NOx-Reduction Measures for Iron-Ore Rotary Kilns. Energy Fuels 2020, 34, 4934–4948. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Fan, W.; Wu, X.; Guo, H.; Zhu, J.; Liu, P.; Chen, C.; Wang, Y. Experimental study on the impact of adding NH3 on NO production in coal combustion and the effects of char, coal ash, and additives on NH3 reducing NO under high temperature. Energy 2019, 173, 109–120. [Google Scholar] [CrossRef]
- Fu, S.; Song, Q.; Yao, Q. Mechanism study on the adsorption and reactions of NH3, NO, and O2 on the CaO surface in the SNCR deNOx process. Chem. Eng. J. 2016, 285, 137–143. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Lim, Y.I.; Kim, S.J.; Eom, W.H.; Yoo, K.S. Experiment and computational fluid dynamics (CFD) simulation of urea-based selective noncatalytic reduction (SNCR) in a pilot-scale flow reactor. Energy Fuels 2008, 22, 3864–3876. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, S.; You, C.; Wang, H. Experimental Study on the Enhancement of SNCR Denitrification Process with Methane and Propane in A Circulating Fluidized Bed. Ind. Eng. Chem. Res. 2019, 58, 7825–7833. [Google Scholar] [CrossRef]
- Du, L.; Jin, B.; Zheng, X.; Niu, M. Effect of reburning zone conditions on no reduction efficiency in an online precalciner-type kiln system. Environ. Prog. Sustain. Energy 2016, 35, 439–446. [Google Scholar] [CrossRef]
- Fan, W.; Zhu, T.; Sun, Y.; Lv, D. Effects of gas compositions on NOx reduction by selective non-catalytic reduction with ammonia in a simulated cement precalciner atmosphere. Chemosphere 2014, 113, 182–187. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Wei, X. Experiment on NO x reduction by advanced reburning in cement precalciner. Fuel 2018, 224, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Niu, Y.; Gong, Y.; Zhang, Y.; Zhang, Y.; Hui, S. Influence of Biomass Reburning on NOx Reductions during Pulverized Coal Combustion. Energy Fuels 2017, 31, 5597–5602. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kajimura, S. Kinetic Study on NO Reduction Using Dimethyl Ether as a Reburning Fuel. Energy Fuels 2017, 31, 12500–12507. [Google Scholar] [CrossRef]
- Kang, Z.; Yuan, Q.; Zhao, L.; Dai, Y.; Sun, B.; Wang, T. Study of the performance, simplification and characteristics of SNCR de-NOx in large-scale cyclone separator. Appl. Therm. Eng. 2017, 123, 635–645. [Google Scholar] [CrossRef]
- Fu, S.; Song, Q.; Yao, Q. Mechanism of the Reaction between HNCO and CaO in the Urea-Selective Non-catalytic Reduction deNOx Process. Energy Fuels 2017, 31, 5318–5323. [Google Scholar] [CrossRef]
- Forsmo, S.P.E.; Forsmo, S.E.; Samskog, P.O.; Björkman, B.M.T. Mechanisms in oxidation and sintering of magnetite iron ore green pellets. Powder Technol. 2008, 183, 247–259. [Google Scholar] [CrossRef]
- Lyon, R.K. Thermal DeNOx controlling nitrogen oxides emissions by a noncatalytic process. Environ. Sci. Technol. 1987, 21, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Shon, B.H.; Yoo, J.G.; Jung, J.H.; Oh, K.J. The influence of mixing between NH3 and NO for a De-NOx reaction in the SNCR process. J. Ind. Eng. Chem. 2008, 14, 457–467. [Google Scholar] [CrossRef]
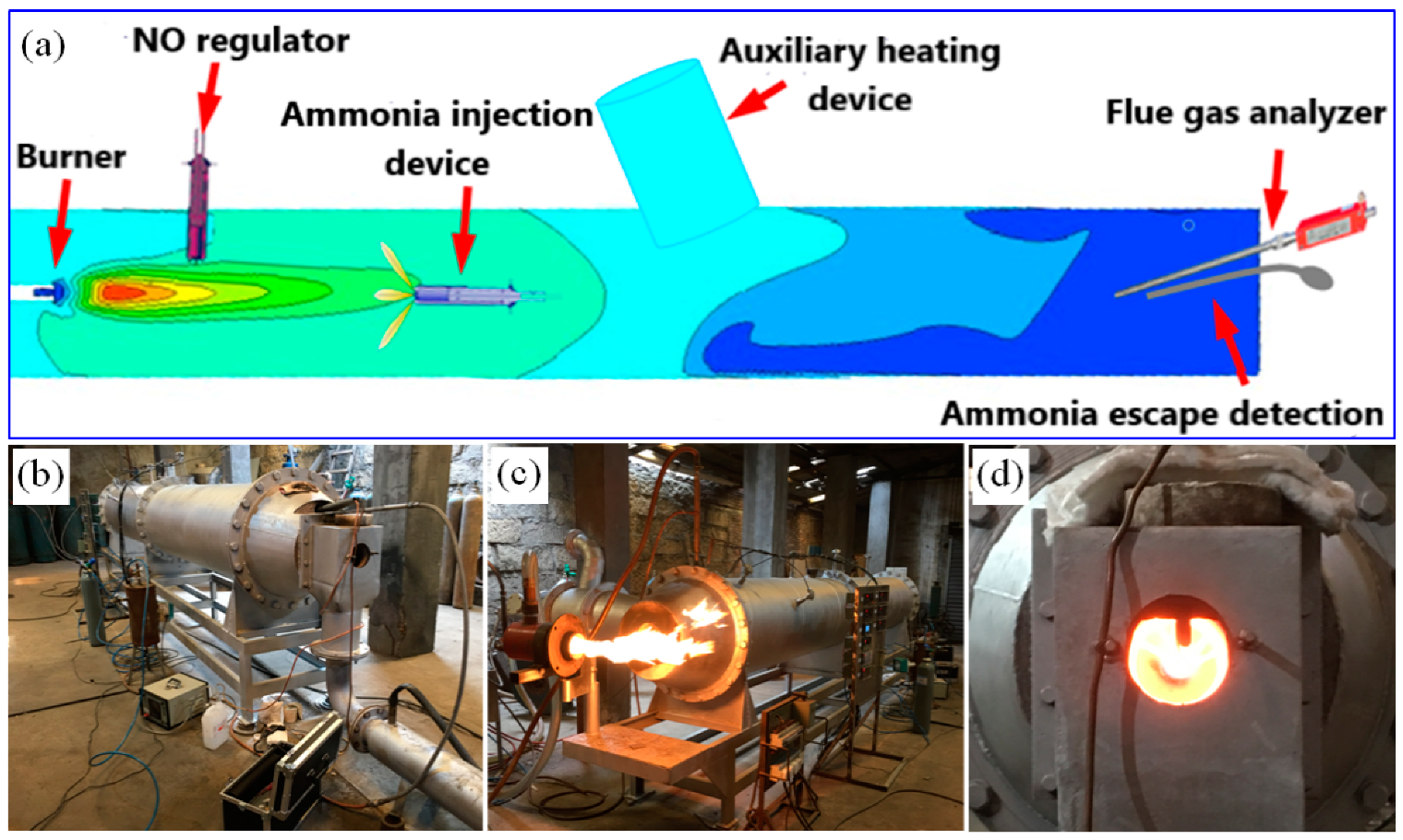

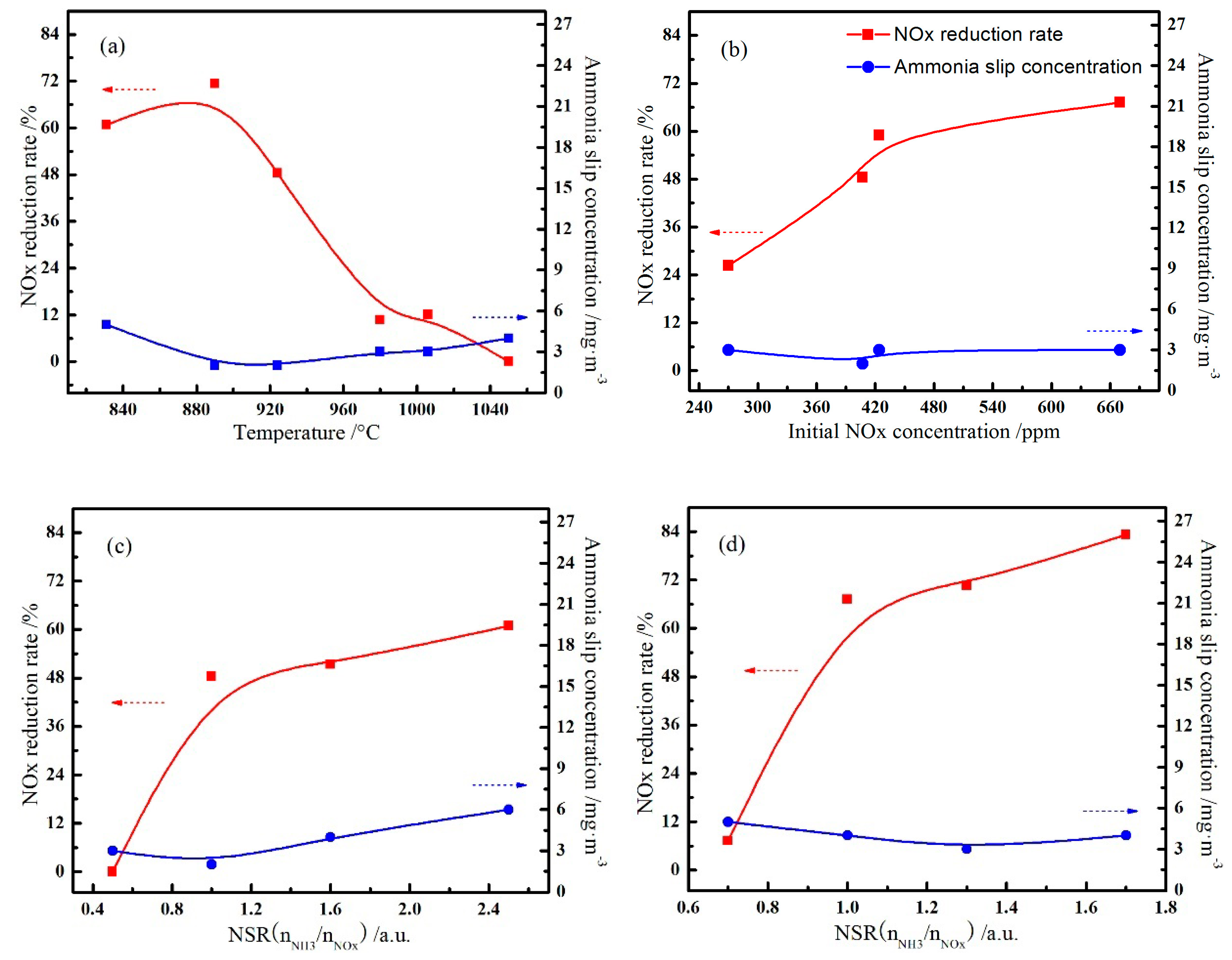

| NSR | Temperature /°C | Pre-Flue Gas/ppm | Post-Flue Gas/ppm | NOx Reduction Rate | Ammonia Slip Concentration/mg m−3 | Reductants | ||
|---|---|---|---|---|---|---|---|---|
| O2 | NOx | O2 | NOx | |||||
| 0.5 | 933 | 7.6 | 519 | 8.7 | 525 | - | <3.0 | NH4HCO3 solution |
| 1.0 | 911 | 7.6 | 519 | 9.0 | 253 | 51.3% | <3.0 | |
| 1.5 | 960 | 7.6 | 519 | 8.8 | 181 | 65.1% | <5.0 | |
| 0.9 | 923 | 7.6 | 457 | 8.9 | 174 | 66.5% | <3.0 | urea solution |
| 2.0 | 933 | 7.6 | 457 | 9.4 | 142 | 72.6% | <6.0 | |
| NSR | Temperature /°C | Pre-Flue Gas/ppm | Post-Flue Gas/ppm | NOx Reduction Rate | Ammonia Slip Concentration/mg m−3 | Additives | ||
|---|---|---|---|---|---|---|---|---|
| O2 | NOx | O2 | NOx | |||||
| 1.0 | 913 | 7.8 | 421 | 8.3 | 173 | 58.9% | <2.0 | NaCl |
| 2.0 | 903 | 7.8 | 421 | 8.8 | 167 | 60.3% | <5.0 | |
| 2.5 | 900 | 7.8 | 421 | 9.1 | 155 | 63.2% | <7.0 | |
| 0.9 | 927 | 7.8 | 421 | 8.6 | 300 | 28.7% | <3.0 | ethanol |
| 1.7 | 912 | 7.8 | 421 | 8.6 | 136 | 67.7% | <4.0 | |
| 2.5 | 960 | 7.8 | 421 | 8.5 | 168 | 60.1% | <5.0 | |
| 1.0 | 930 | 8.7 | 544 | 9.5 | 228 | 58.1% | <3.0 | vanadium- titanium catalyst |
| 1.4 | 928 | 8.7 | 544 | 9.9 | 177 | 67.5% | <4.0 | |
| 2.5 | 922 | 8.7 | 544 | 9.7 | 144 | 73.5% | <7.0 | |
| NSR | Temperature /°C | Pre-Flue Gas/ppm | Post-Flue Gas/ppm | NOx Reduction Rate | Ammonia Slip Concentration/mg m−3 | Process Conditions | |||
|---|---|---|---|---|---|---|---|---|---|
| O2 | NOx | O2 | NOx | ||||||
| 1.0 | 901 | 11.2 | 415 | 11.2 | 121 | 70.8% | <2.0 | 11.2 | O2/% |
| 1.1 | 918 | 9.0 | 424 | 9.3 | 174 | 59.0% | <3.0 | 9.0 | |
| 1.1 | 900 | 3.8 | 416 | 4.0 | 167 | 60.0% | <4.0 | 3.8 | |
| 1.0 | 924 | 10.0 | 407 | 9.8 | 210 | 48.4% | <2.0 | 1.0–1.3 | Reaction time/s |
| 1.1 | 920 | 12.0 | 363 | 12.1 | 271 | 25.3% | <6.0 | 0.4–0.6 | |
| 1.3 | 918 | 8.0 | 424 | 9.3 | 174 | 59.0% | <3.0 | 75 | Injection pressure/kPa |
| 1.3 | 910 | 8.0 | 424 | 10.8 | 168 | 60.4% | <3.0 | 45 | |
| 0.6 | 914 | 9.0 | 531 | 9.3 | 445 | 16.2% | <3.0 | 7.0 | NH3·H2O concentration /vol.% |
| 1.0 | 927 | 9.0 | 531 | 9.4 | 227 | 57.3% | <3.0 | ||
| 1.6 | 924 | 9.0 | 531 | 8.7 | 178 | 66.5% | <5.0 | ||
| Components | Kiln Tail Gas | System Exhaust Gas | ||||
|---|---|---|---|---|---|---|
| NOx /mg Nm−3 | O2/% | NOx Annual Emissions/t a−1 | NOx /mg Nm−3 | O2/% | NH3 /mg Nm−3 | |
| Before process | 680~810 | 15.4~17.2 | ≈939 | 275~296 | 17.3~18.1 | - |
| After process | - | - | ≈582 | 163~188 | 17.2~17.9 | 7.1~9.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Hu, P.; Lu, B.; Xie, Z.; Liu, L.; Cheng, G.; Wei, J. NOx Emission Reduction by Advanced Reburning in Grate-Rotary Kiln for the Iron Ore Pelletizing Production. Processes 2020, 8, 1470. https://doi.org/10.3390/pr8111470
Hu B, Hu P, Lu B, Xie Z, Liu L, Cheng G, Wei J. NOx Emission Reduction by Advanced Reburning in Grate-Rotary Kiln for the Iron Ore Pelletizing Production. Processes. 2020; 8(11):1470. https://doi.org/10.3390/pr8111470
Chicago/Turabian StyleHu, Bing, Peiwei Hu, Biao Lu, Zhicheng Xie, Liu Liu, Gangli Cheng, and Jiaoyang Wei. 2020. "NOx Emission Reduction by Advanced Reburning in Grate-Rotary Kiln for the Iron Ore Pelletizing Production" Processes 8, no. 11: 1470. https://doi.org/10.3390/pr8111470
APA StyleHu, B., Hu, P., Lu, B., Xie, Z., Liu, L., Cheng, G., & Wei, J. (2020). NOx Emission Reduction by Advanced Reburning in Grate-Rotary Kiln for the Iron Ore Pelletizing Production. Processes, 8(11), 1470. https://doi.org/10.3390/pr8111470





