Impact of Effluent from the Leachate Treatment Plant of Taman Beringin Solid Waste Transfer Station on the Quality of Jinjang River
Abstract
1. Introduction
2. Materials and Methods
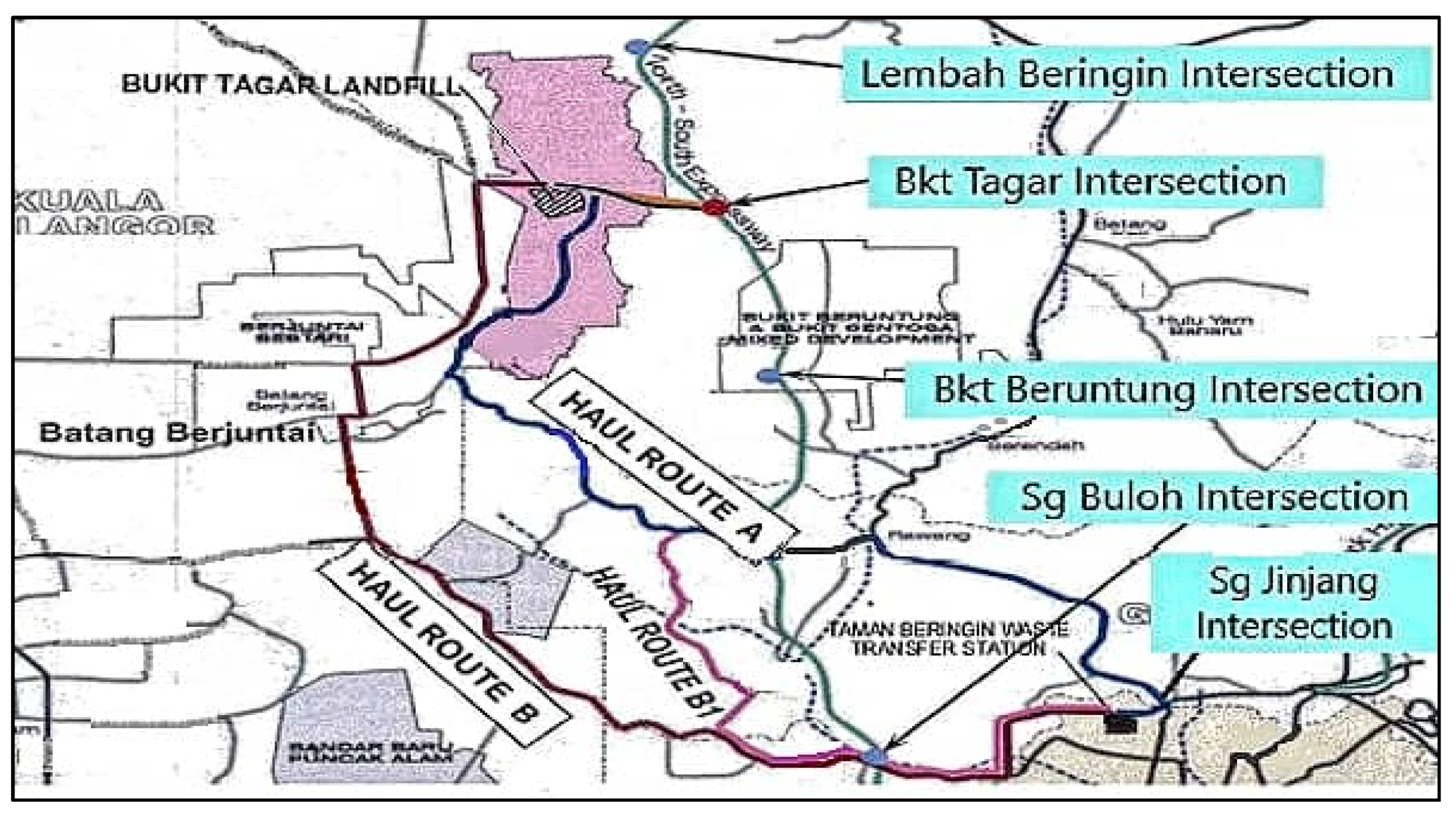
2.1. Study Site
2.2. Sampling Points
2.3. Experimental Procedures
2.4. Data Analysis
2.4.1. Parameter of Activated Sludge System
2.4.2. Jinjang River Water Quality Analysis
3. Results and Discussion
3.1. Raw Leachate Characteristics
3.2. Performance in Removal of Pollutants in Leachate
3.3. Health of the Activated Sludge System
3.3.1. Operational Parameter
3.3.2. Design Parameter
3.3.3. Kinetic Parameter
3.4. Water Quality Analysis of Jinjang River
3.5. Impact of Discharged Treated Leachate on Water Quality of Jinjang River
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoornweg, D.; Bhada-Tata, P. What A Waste: A Global Review of Solid Waste Management; World Bank Group: Washington, DC, USA, 2012. [Google Scholar]
- Ghasimi, S.M.D.; Tey, B.T.; Suyot, R. Municipal solid waste management at Taman Beringin Transfer Station in Malaysia. Waste Manag. 2010, 30, 357–359. [Google Scholar]
- Ashraf, M.A.; Balkhair, K.S.; Chowdhury, A.J.K.; Hanafiah, M.M. Treatment of Taman Beringin landfill leachate using the column technique. Desal. Water Treat. 2019, 149, 370–387. [Google Scholar] [CrossRef]
- Aziz, H.A.; Adlan, M.N.; Zahari, M.S.M.; Alias, S. Removal of ammoniacal nitrogen (N-NH3) from municipal solid waste leachate by using activated carbon and limestone. Waste Manag. Res. 2004, 22, 371–375. [Google Scholar] [CrossRef]
- Ofomola, M.; Umayah, O.; Akpoyibo, O. Contamination assessment of dumpsites in Ughelli, Nigeria using the Leachate Pollution Index method. J. Appl. Sci. Environ. Manag. 2017, 21, 77. [Google Scholar] [CrossRef]
- Gomes, A.I.; Foco, M.L.; Vieira, E.; Cassidy, J.; Silva, T.F.; Fonseca, A.; Saraiva, I.; Boaventura, R.A.; Vilar, V.J. Multistage treatment technology for leachate from mature urban landfill: Full scale operation performance and challenges. Chem. Eng. J. 2019, 376, 120573. [Google Scholar] [CrossRef]
- Hussein, M.; Yoneda, K.; Zaki, Z.M.; Othman, N.; Amir, A. Leachate characterizations and pollution indices of active and closed unlined landfills in Malaysia. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100232. [Google Scholar] [CrossRef]
- Atta, M.; Yaacob, W.Z.W.; Bin Jaafar, O. The potential impact of leachate-contaminated groundwater of an ex-landfill site at Taman Beringin Kuala Lumpur, Malaysia. Environ. Earth Sci. 2014, 73, 3913–3923. [Google Scholar] [CrossRef]
- Nor Nazrieza, M.S.; Siti Rohana, M.Y.; Subramaniam, K.; Hazilia, H.; Amir Herberd, A. Characterization of leachate from panchang bedena landfill, batang padang landfill and matang landfill: A comparative study. Malays. J. Sci. 2015, 34, 69–77. [Google Scholar] [CrossRef]
- De Albuquerque, E.M.; Pozzi, E.; Sakamoto, I.K.; Jurandyr, P. Treatability of landfill leachate combined with sanitary sewage in an activated sludge system. J. Water Process. Eng. 2018, 23, 119–128. [Google Scholar] [CrossRef]
- Naveen, B.; Mahapatra, D.M.; Sitharam, T.; Sivapullaiah, P.; Ramachandra, T. Physico-chemical and biological characterization of urban municipal landfill leachate. Environ. Pollut. 2017, 220, 1–12. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Elbl, J.; Koda, E.; Adamcová, D.; Bilgin, A.; Lukas, V.; Podlasek, A.; Kintl, A.; Wdowska, M.; Brtnický, M.; et al. Chemical Composition and Hazardous Effects of Leachate from the Active Municipal Solid Waste Landfill Surrounded by Farmlands. Sustainability 2020, 12, 4531. [Google Scholar] [CrossRef]
- Seow, T.W.; Er, X.Y.; Lim, C.K.; Ibrahim, Z.; Mat Sarip, S.H. Leachate Management: Biological Approach. In Proceedings of the 4th International Conference on Civil and Environmental Engineering for Sustainability, Langkawi, Malaysia, 4–5 December 2018. [Google Scholar]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and Conventional Technologies for Landfill Leachates Treatment: A Review. Sustainability 2016, 9, 9. [Google Scholar] [CrossRef]
- Gotvajn, A. Žgajnar; Pavko, A. Žgajnar G.A.A. Perspectives on Biological Treatment of Sanitary Landfill Leachate. Wastewater Treat. Eng. 2015, 1, 115–152. [Google Scholar] [CrossRef]
- Yong, Z.J.; Bashir, M.J.; Ng, C.A.; Sethupathi, S.; Lim, J.W. A sequential treatment of intermediate tropical landfill leachate using a sequencing batch reactor (SBR) and coagulation. J. Environ. Manag. 2018, 205, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, L.P.; Arana, V.A.; Trilleras, J.; Torne, G.B.; González-Solano, A.J.; Maury, H. Efficiency of Combined Processes Coagulation/Solar Photo Fenton in the Treatment of Landfill Leachate. Water 2019, 11, 1351. [Google Scholar] [CrossRef]
- Shamin, A.A.R.; Gasim, M.B.; Toriman, M.E.; Juahir, H.; Kamarudin, M.K.A.; Azid, A.; Aziz, N.A.B. Water quality deterioration of jinjang river, kuala lumpur: Urban risk case water pollution. Arab World Geogr. 2014, 16, 349–362. [Google Scholar]
- Rodger, B.B.; Andrew, D.E.; Eugene, W.R. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Enitan, I.T.; Enitan, A.M.; Odiyo, J.O.; Alhassan, M.M. Human Health Risk Assessment of Trace Metals in Surface Water Due to Leachate from the Municipal Dumpsite by Pollution Index: A Case Study from Ndawuse River, Abuja, Nigeria. Open Chem. 2018, 16, 214–227. [Google Scholar] [CrossRef]
- Naje, A.S.; Ajeel, M.A.; Ali, I.M.; Al-Zubaidi, H.A.M.; Alaba, P.A. Raw landfill leachate treatment using an electrocoagulation process with a novel rotating electrode reactor. Water Sci. Technol. 2019, 80, 458–465. [Google Scholar] [CrossRef]
- Demirbilek, D.; Önal, A. Öztüfekçi; Demir, V.; Uslu, G.; Arslanoglu-Isık, H. Characterization and pollution potential assessment of Tunceli, Turkey municipal solid waste open dumping site leachates. Environ. Monit. Assess. 2013, 185, 9435–9449. [Google Scholar] [CrossRef]
- De, S.; Maiti, S.K.; Hazra, T.; Debsarkar, A.; Dutta, A. Leachate characterization and identification of dominant pollutants using leachate pollution index for an uncontrolled landfill site. Glob. J. Environ. Sci. Manag. 2016, 2, 177–186. [Google Scholar]
- Theses, C.E.; Branigan, J. Lincoln Development of a Field Test for Total Suspended Solids Analysis. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, December 2013. [Google Scholar]
- Nagarajan, R.; Subramani, T.; Lakshumanan, E. Impact of leachate on groundwater pollution due to non-engineered municipal solid waste landfill sites of erode city, Tamil Nadu, India. Iran. J. Environ. Health Sci. Eng. 2012, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.C.; Surampalli, R.Y. Landfill Leachate Collection and Treatment. Sustain. Solid Waste Manag. 2016, 149, 605–631. [Google Scholar]
- Kamaruddin, M.A.; Yusoff, M.S.; Aziz, H.A.; Alrozi, R. Current status of Pulau Burung Sanitary Landfill leachate treatment, Penang Malaysia. AIP Conf. Proc. 2016, 1774, 030014. [Google Scholar] [CrossRef]
- Mohamad, W.L.; S, Z.N.; Zin, M. Characterization of Leachate at Simpang Renggam Landfill Site, Johor Malaysia. J. Phys. Conf. Ser. 2018, 1049, 012040. [Google Scholar] [CrossRef]
- El-Gawad, S.A.A.; El-Aziz, H.M.A. Effective removal of chemical oxygen demand and phosphates from aqueous medium using entrapped activated carbon in alginate. MOJ Biol. Med. 2018, 3, 6. [Google Scholar]
- Hazzaa, R. Removal of Ammoniacal Nitrogen from Synthetic Wastewater Using Granular Activated Carbon and Limestone. J. High Inst. Public Health 2012, 42, 169–184. [Google Scholar] [CrossRef]
- Nayl, A.E.A.; Elkhashab, R.A.; El Malah, T.; Yakout, S.M.; El-Khateeb, M.A.; Ali, M.M.S.; Ali, H.M. Adsorption studies on the removal of COD and BOD from treated sewage using activated carbon prepared from date palm waste. Environ. Sci. Pollut. Res. 2017, 24, 22284–22293. [Google Scholar] [CrossRef]
- Mukhtar, G.; Retno, I.; M, T.; Fajar, N.; Satria, A. The Reduction of COD Levels in Domestic Waste Water Using Combination of Activated Sludge Methode—Activated Carbon Continously. J. Physics: Conf. Ser. 2019, 1295, 012040. [Google Scholar] [CrossRef]
- Fleming, G. Wastewater Treatment Plant Operator Certification Training Module 16—The Activated Sludge Process Part II. 2014. Available online: http://files.dep.state.pa.us/Water/BSDW/OperatorCertification/TrainingModules/ww16_sludge_2_wb.pdf (accessed on 5 April 2020).
- Al-Hussieny, A.A.; Obeid, S.H.; Baqer, N.N.; Hussain, S.F.; Ayad Gheni Al-Hussieny, A.A.; Obeid, S.S.F.; Mohammed, A.G. The Role of Formed Microorganism in Sludge on Processing of Wastewater Treatment. J. Life Sci. 2015, 10, 10. [Google Scholar] [CrossRef][Green Version]
- Burnashova, E.N.; Semyonov, S.Y.; Sibataev, A.K. Controlled nitrification in wastewater treatment. IOP Conf. Ser. Earth Environ. Sci. 2018, 201, 012002. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Jegatheesan, V.; Shi, G. Dissolved Oxygen Control in Activated Sludge Process Using a Neural Network-Based Adaptive PID Algorithm. Appl. Sci. 2018, 8, 261. [Google Scholar] [CrossRef]
- EMERSON. Process Management, Dissolved Oxygen Measurement. In Wastewater Treatment. Water and Wastewater Industry; Rosemount Analytical Inc.: Irvine, CA, USA, 2009. [Google Scholar]
- Zhang, X.; Li, X.; Zhang, Q.; Peng, Q.; Zhang, W.; Gao, F. New insight into the biological treatment by activated sludge: The role of adsorption process. Bioresour. Technol. 2014, 153, 160–164. [Google Scholar] [CrossRef]
- Nawaz, M.S.; Ahsan, M. Comparison of physico-chemical, advanced oxidation and biological techniques for the textile wastewater treatment. Alex. Eng. J. 2014, 53, 717–722. [Google Scholar] [CrossRef]
- Pishgar, R.; Hamza, R.A.; Tay, J.H. Augmenting Lagoon Process Using Reactivated Freeze-dried Biogranules. Appl. Biochem. Biotechnol. 2017, 183, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, E.; Tchobanoglous, G.; Stensel, H.; Burton, F. Wasterwater Engineering—Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Faye, M.C.A.S.; Zhang, Y.; Yang, J. Extracellular polymeric substances and sludge solid/liquid separation under Moringa oleifera and chitosan conditioning: A review. Environ. Technol. Rev. 2017, 6, 59–73. [Google Scholar] [CrossRef]
- Fleming, G. Wastewater Treatment Plant Operator Certification Training Module 17 —The Activated Sludge Process Part III. 2014. Available online: http://files.dep.state.pa.us/Water/BSDW/OperatorCertification/TrainingModules/ww17_sludge_3_wb.pdf (accessed on 25 April 2020).
- El-Fadel, M.; Sleem, F.; Hashisho, J.; Saikaly, P.; Alameddine, I.; Ghanimeh, S. Impact of SRT on the performance of MBRs for the treatment of high strength landfill leachate. Waste Manag. 2018, 73, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K.; Nakajima, F.; Fukushi, K. Ecalcitrant Industrial Wastewater Treatment by Membrane Bioreactor (MBR); Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Hashemi, H.; Jasemizad, T.; Derakhshan, Z.; Ebrahimi, A.A. Determination of Sequencing Batch Reactor (SBR) Performance in Treatment of Composting Plant Leachate. Health Scope 2017. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Amin, M.M.; Bina, B.; Ebrahimi, A.; Pourzamani, H.; Hajizadeh, Y.; Hashemi, H. Evaluate the effects of organic loading rate from windrow composting leachate on the performance of an anaerobic migrating blanket reactor. Int. J. Environ. Health Eng. 2015, 4, 30. [Google Scholar]
- Chae, K.-J.; Oh, S.E.; Lee, S.T.; Bae, J.-W.; Kim, I.S. The optimum substrate to biomass ratio to reduce net biomass yields and inert compounds in biological leachate treatment under pure-oxygen conditions. Bioprocess Biosyst. Eng. 2000, 23, 0235–0243. [Google Scholar] [CrossRef]
- Emerald, F.M.E.; A Prasad, D.S.; Ravindra, M.R.; Pushpadass, H.A. Performance and biomass kinetics of activated sludge system treating dairy wastewater. Int. J. Dairy Technol. 2012, 65, 609–615. [Google Scholar] [CrossRef]
- Hadei, M.; Aalipour, M.; Fatehizadeh, A.; Safavi, H.R.; Ghasemian, M.; Sahbaei, A.R.; Mousavi, S.M.; Zadeh, M.A.; Amin, M.M. Determination of Biokinetic Coefficients for an adsorption/bio-oxidation process on municipal wastewater in pilot-scale. Iranian J. Environ. Health Sci. Eng. 2011, 8, 25–34. [Google Scholar]
- Lateef, A.; Chaudhry, M.N.; Ilyas, S. Biological treatment of dairy wastewater using activated sludge. Science 2013, 39, 179. [Google Scholar] [CrossRef]
- Setiadi, T.; Fairus, S. Hazardous waste landfill leachate treatment using an activated sludge-membrane system. Water Sci. Technol. 2003, 48, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yin, H.; Chapra, S.C.; Zhou, Q. Modelling Dissolved Oxygen Depression in an Urban River in China. Water 2017, 9, 520. [Google Scholar] [CrossRef]
- Zheng, L.; Paul, M.J. Effects of Eutrophication on Stream Ecosystems; Tetra Tech, Inc.: Pasadena, CA, USA, 2007. [Google Scholar]









| Types of Leachate | Young | Intermediate | Old |
|---|---|---|---|
| Landfill age (years) | <5 | 5–10 | >10 |
| pH | 6.5–7.5 (7) | 7.0–8.0 (7.5) | 7.5–8.5 (8) |
| Chemical Oxygen Demand(COD) (g/L) | 10–30 (15) | 3–10 (5) | <3 (2) |
| Biochemical Oxygen Demand/Chemical Oxygen Demand (BOD/COD) | 0.5–0.7 (0.6) | 0.3–0.5 (0.4) | <0.3 (0.2) |
| Nitrogen Ammonia (mg/L) | 500–1000 (700) | 800–2000 (1000) | 1000–3000 (2000) |
| Chemical Oxygen Demand/Nitrogen Ammonia (COD/N) | 5–10 (6) | 3–4 (3) | <3 (1.5) |
| In-Situ Test | Laboratory Tests |
|---|---|
| pH | Biochemical Oxygen Demand ( BOD), (APHA 5210B) |
| Dissolved Oxygen (DO) | Chemical Oxygen Demand (COD) (USEPA Reactor Digestion Method—Method 8000) |
| Total Suspended Solid, TSS (APHA 2540D) | |
| Ammonia Nitrogen (Salicylate Method—Method 10031) | |
| Total Kjeldahl Nitrogen, TKN (APHA 4500-NorgB) | |
| Mixed Liquor Suspended Solid, MLSS (APHA 2540E) |
| Parameter | Sub-Index | |
|---|---|---|
| DO (SIDO) (in % saturation) | for for for | |
| BOD (SIBOD) | for for | |
| COD (SICOD) | for for | |
| NH3-N (SIAN) | for for for | |
| TSS (SISS) | for for for | |
| pH (SIPH) | for for for for |
| Parameters | Unit | Classes | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Ammoniacal Nitrogen | mg/L | <0.1 | 0.1–0.3 | 0.3–0.9 | 0.9–2.7 | >2.7 |
| Biochemical Oxygen Demand (BOD5) | mg/L | <1 | 1–3 | 3–6 | 6–12 | >12 |
| Chemical Oxygen Demand (COD) | mg/L | <10 | 10–25 | 25–50 | 50–100 | >100 |
| Dissolved Oxygen | mg/L | >7 | 5–7 | 3–5 | 1–3 | <1 |
| pH | mg/L | >7 | 6–7 | 5–6 | <5 | >5 |
| Total Suspended Solid (TSS) | mg/L | <25 | 25–50 | 50–150 | 150–300 | >300 |
| Water Quality Index (WQI) | mg/L | >92.7 | 76.5–92.7 | 51.9–76.5 | 31.0–51.9 | <31.0 |
| Parameters | Index Range | ||
|---|---|---|---|
| Clean | Slightly Polluted | Polluted | |
| SIBOD | 91–100 | 80–90 | 0–79 |
| SIAN | 92–100 | 71–91 | 0–70 |
| SISS | 76–100 | 70–75 | 0–69 |
| WQI | 81–100 | 60–80 | 0–59 |
| No | Parameter | Value (Average ± SD) | Environmental Quality (Sewage) Regulation 2009 |
|---|---|---|---|
| 1 | pH | 7.73 ± 0.08 | 6–9 |
| 2 | Temperature, °C | 32.7 ± 0.79 | 40 |
| 3 | Dissolved oxygen (DO), mg/L | 0.19 ± 0.08 | - |
| 4 | Biochemical Oxygen Demand (BOD), mg/L | 697.50 ± 127.94 | 20 |
| 5 | Chemical Oxygen Demand (COD), mg/L | 2419.75 ± 1155.22 | 400 |
| 6 | Total Suspended Solid (TSS), mg/L | 2710.00 ± 334.79 | 50 |
| 7 | Nitrogen (Ammonia), mg/L | 317.08 ± 35.45 | 5 |
| 8 | Total Kjeldahl Nitrogen (TKN), mg/L | 339.50 ± 94.11 | - |
| No | Parameter | Value (Average ± SD) | Monitoring Point (Fleming 2014a) |
|---|---|---|---|
| 1 | pH | 8.40 ± 0.07 | 6.5–7.5 (Bacteria growth) 7.8–8.2 (Nitrification process) |
| 2 | Dissolved Oxygen (DO), mg/L | 0.16 ± 0.02 | 2–4 |
| 3 | Mixed liquor suspended solid (MLSS), mg/L | 2335 ± 174.24 | 2000–4000 |
| 4 | Chemical Oxygen Demand: Nitrogen (COD:N) | 100:13 | 100:5 |
| Coefficient | Unit | Value (This Study) | Value from (Chae et al. 2000) [48] |
|---|---|---|---|
| 5.655 | 0.36 | ||
| −0.247 | 0.022 | ||
| 500 | 0.56 | ||
| −0.005 | 612 |
| Parameters (Sub-Index) | Concentration (mg/L) | Classes (Index Range) | ||||
|---|---|---|---|---|---|---|
| Sample 1 (9 December) | Sample 2 (16 December) | Sample 3 (13 January) | Sample 4 (20 January) | Average ± SD | ||
| pH | 7.14 | 7.95 | 7.89 | 8.22 | 7.80 ± 0.40 | I |
| (SIPH) | 98.91 | 91.70 | 92.51 | 87.54 | 92.67 ± 4.07 | - |
| Dissolved Oxygen | 2.2 | 2.19 | 2.1 | 2.91 | 2.35 ± 0.33 | IV |
| (SIDO) | 18.57 | 17.66 | 15.33 | 28.26 | 19.96 ± 4.94 | - |
| Biochemical Oxygen Demand, (BOD5) | 0.20 | 0.50 | 0.70 | 0.60 | 0.50 ± 0.19 | I |
| (SIBOD) | 99.55 | 98.29 | 97.45 | 97.86 | 98.29 ± 0.79 | Clean |
| Chemical Oxygen Demand, COD | 23.67 | 46.00 | 23.00 | 19.33 | 28.00 ± 10.52 | III |
| (SICOD) | 67.62 | 37.92 | 68.51 | 75.27 | 62.33 ± 14.40 | - |
| Ammoniacal Nitrogen | 5.27 | 5.70 | 4.80 | 5.00 | 5.19 ± 0.34 | V |
| (SIAN) | 0 | 0 | 0 | 0 | 0 | Polluted |
| Water Quality Index (WQI) | 41.71 | 35.70 | 40.05 | 43.44 | 40.23 ± 2.87 | Polluted |
| Parameters (Sub-Index) | Concentration (mg/L) | Classes (Index Range) | ||||
|---|---|---|---|---|---|---|
| Sample 1 (9 December) | Sample 2 (16 December) | Sample 3 (13 January) | Sample 4 (20 January) | Average ± SD | ||
| pH | 7.20 | 7.99 | 7.81 | 8.44 | 7.86 ± 0.44 | I |
| (SIPH) | 98.65 | 91.14 | 93.52 | 83.49 | 91.70 ± 5.46 | - |
| Dissolved Oxygen | 4.55 | 3.80 | 3.62 | 5.1 | 4.27 ± 0.59 | III |
| (SIDO) | 58.80 | 43.86 | 39.42 | 67.36 | 52.36 ± 11.25 | - |
| Biochemical Oxygen Demand (BOD5) | 0.60 | 1.90 | 1.00 | 1.50 | 1.25 ± 0.49 | II |
| (SIBOD) | 97.86 | 92.36 | 96.17 | 94.06 | 95.11 ± 2.08 | Clean |
| Chemical Oxygen Demand, COD | 37.00 | 65.67 | 27.33 | 34.00 | 41.00 ± 14.67 | III |
| (SICOD) | 49.89 | 11.76 | 62.75 | 53.88 | 44.57 ± 19.51 | - |
| Ammoniacal Nitrogen | 4.97 | 4.30 | 3.70 | 5.80 | 4.69 ± 0.78 | V |
| (SIAN) | 0 | 0 | 2.78 | 0 | 0.70 ± 1.20 | Polluted |
| Water Quality Index, WQI | 47.44 | 36.32 | 44.78 | 47.57 | 44.03 ± 4.59 | Polluted |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, P.M.; Naim, A.N.; Suja, F.; Ahmad Usul, M.F. Impact of Effluent from the Leachate Treatment Plant of Taman Beringin Solid Waste Transfer Station on the Quality of Jinjang River. Processes 2020, 8, 1553. https://doi.org/10.3390/pr8121553
Chin PM, Naim AN, Suja F, Ahmad Usul MF. Impact of Effluent from the Leachate Treatment Plant of Taman Beringin Solid Waste Transfer Station on the Quality of Jinjang River. Processes. 2020; 8(12):1553. https://doi.org/10.3390/pr8121553
Chicago/Turabian StyleChin, Pui Mun, Aine Nazira Naim, Fatihah Suja, and Muhammad Fadly Ahmad Usul. 2020. "Impact of Effluent from the Leachate Treatment Plant of Taman Beringin Solid Waste Transfer Station on the Quality of Jinjang River" Processes 8, no. 12: 1553. https://doi.org/10.3390/pr8121553
APA StyleChin, P. M., Naim, A. N., Suja, F., & Ahmad Usul, M. F. (2020). Impact of Effluent from the Leachate Treatment Plant of Taman Beringin Solid Waste Transfer Station on the Quality of Jinjang River. Processes, 8(12), 1553. https://doi.org/10.3390/pr8121553





