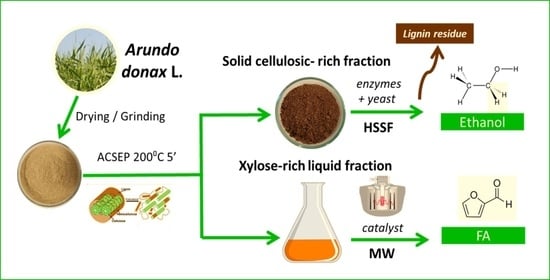
Arundo donax Refining to Second Generation Bioethanol and Furfural
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Pretreatment
2.2. Hydrolysis Tests
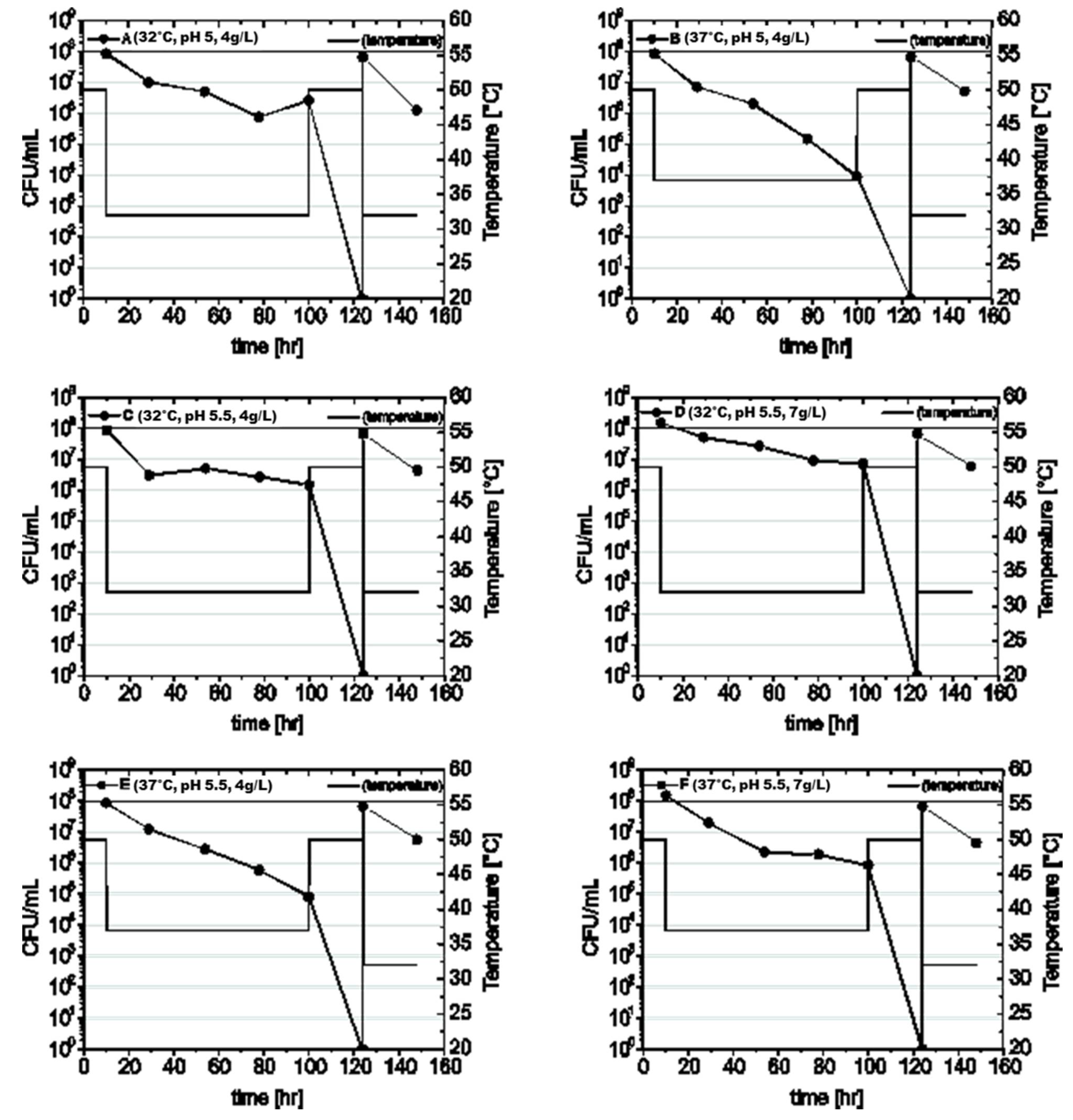
2.3. Fermentation Tests
2.4. Dehydration Tests
3. Result and Discussion
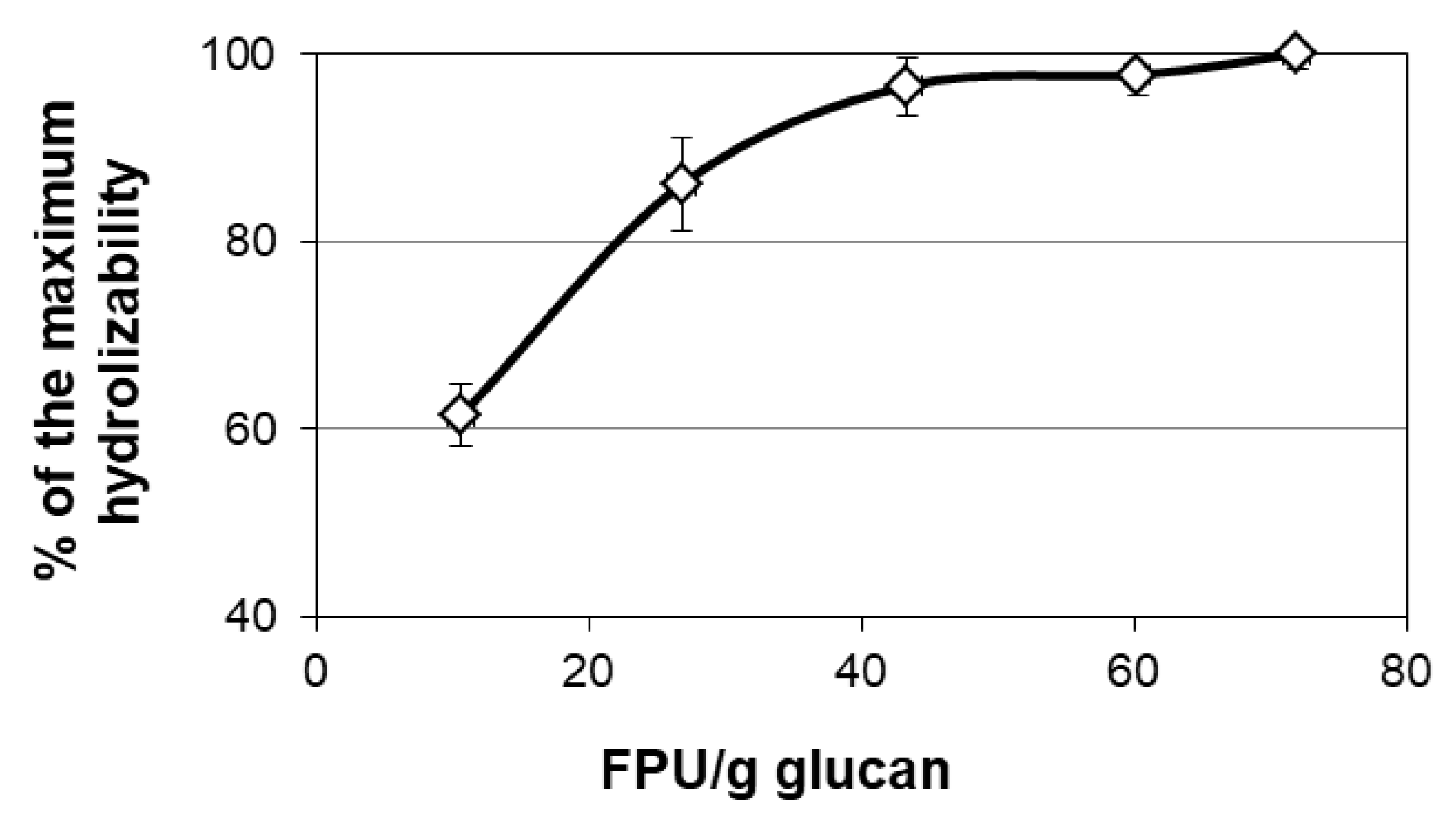
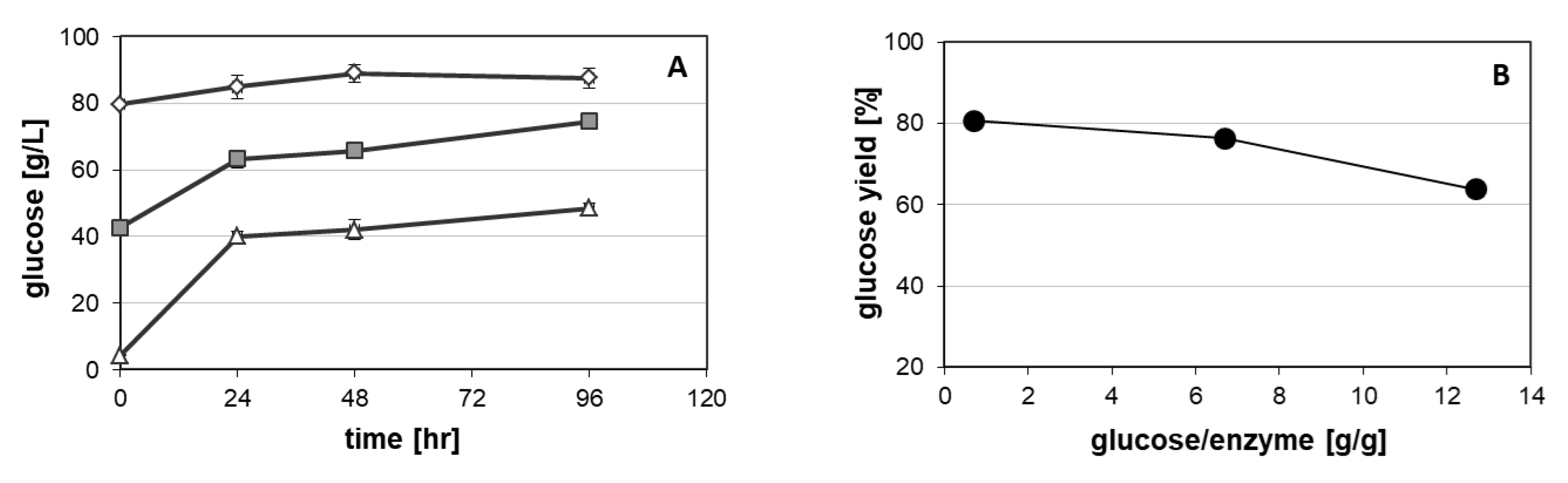
3.1. Enzymatic Hydrolysis of Giant Reed
3.2. Hybrid SSF of Arundo Donax
3.3. Conversion of Hemicellulose
4. Conclusions
- Cellic® CTec3 inhibition thresholds by glucose and ethanol were assessed under high gravity process conditions.
- pH 5.5 resulted in the optimal condition because at this pH, S. cerevisiae cells demonstrated higher viability at 37 °C.
- Ethanol concentration of 51 g/L was finally achieved by using an optimized hybrid process.
- The maximum yield of FA obtained by using a regenerable Amberlyst solid catalyst was 3.8 g per 100 g of original dry A. donax.
- Under the process conditions tested in the present paper, the catalyst maintained a significant residual activity after three batch processes.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACSEP | acid-catalyzed steam explosion pretreatment |
| SHF | separated hydrolysis and fermentation |
| SSF | simultaneous saccharification and fermentation |
| SSCF | simultaneous saccharification and co-fermentation |
| HSSF | hybrid simultaneous saccharification and fermentation |
| FPU | filter paper unit |
References
- Jahromi, R.; Rezaei, M.; Samadi, S.H.; Jahromi, H. Biomass gasification in a downdraft fixed-bed gasifier: Optimization of operating conditions. Chem. Eng. Sci. 2020, 116249. [Google Scholar] [CrossRef]
- Ghadiri, S.K.; Alidadi, H.; Nezhad, N.T.; Javid, A.; Roudbari, A.; Talebi, S.S.; Mohammadi, A.A.; Shams, M.; Rezania, S. Valorization of biomass into amine- functionalized bio graphene for efficient ciprofloxacin adsorption in water-modeling and optimization study. PLoS ONE 2020, 15, e0231045. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Noghani, F.; Dehghan, A.; Van Der Hoek, J.P.; Giannakoudakis, D.A.; Ghadiri, S.K.; Anastopoulos, I.; Sarkhosh, M.; Colmenares, J.C.; Shams, M. Biomass-derived porous aminated graphitic nanosheets for removal of the pharmaceutical metronidazole: Optimization of physicochemical features and exploration of process mechanisms. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 125791. [Google Scholar] [CrossRef]
- Ibarra-Gonzalez, P.; Rong, B.-G. Integrated Methodology for the Optimal Synthesis of Lignocellulosic Biomass-to-Liquid Fuel Production Processes: 1. Simulation-Based Superstructure Synthesis and Development. Ind. Eng. Chem. Res. 2020, 59, 14881–14897. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Moreno, A.D.; Alvira, P.; Ibarra, D.; Tomás-Pejó, E. Production of ethanol from lignocellulosic biomass. In Production of Platform Chemicals from Sustainable Resources; Springer: Singapore, 2017; pp. 375–410. [Google Scholar]
- Zabed, H.; Sahu, J.; Suely, A.; Boyce, A.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Kapoor, M.; Semwal, S.; Gaur, R.; Kumar, R.; Gupta, R.P.; Puri, S.K. The pretreatment technologies for deconstruction of lignocellulosic biomass. In Waste to Wealth; Springer: Singapore, 2018; pp. 395–421. [Google Scholar]
- De Bari, I.; Nanna, F.; Braccio, G. SO2-Catalyzed Steam Fractionation of Aspen Chips for Bioethanol Production: Optimization of the Catalyst Impregnation. Ind. Eng. Chem. Res. 2007, 46, 7711–7720. [Google Scholar] [CrossRef]
- Sassner, P.; Mårtensson, C.-G.; Galbe, M.; Zacchi, G. Steam pretreatment of H2SO4-impregnated Salix for the production of bioethanol. Bioresour. Technol. 2008, 99, 137–145. [Google Scholar] [CrossRef]
- De Bari, I.; Liuzzi, F.; Villone, A.; Braccio, G. Hydrolysis of concentrated suspensions of steam pretreated Arundo donax. Appl. Energy 2013, 102, 179–189. [Google Scholar] [CrossRef]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Radhika, K.; Ravinder, R.; Ravindra, P. Bioconversion of pentose sugars into ethanol: A review and future directions. Biotechnol. Mol. Biol. Rev. 2011, 6, 8–20. [Google Scholar]
- Nakasu, P.; Ienczak, J.L.; Costa, A.C.; Rabelo, S.C. Acid post-hydrolysis of xylooligosaccharides from hydrothermal pretreatment for pentose ethanol production. Fuel 2016, 185, 73–84. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Pretreatment and hydrolysis of lignocellulosic wastes for butanol production: Challenges and perspectives. Bioresour. Technol. 2018, 270, 702–721. [Google Scholar] [CrossRef]
- Bura, R.; Vajzovic, A.; Doty, S.L. Novel endophytic yeast Rhodotorula mucilaginosa strain PTD3 I: Production of xylitol and ethanol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1003–1011. [Google Scholar] [CrossRef]
- Dhar, K.S.; Wendisch, V.F.; Nampoothiri, K.M. Engineering of Corynebacterium glutamicum for xylitol production from lignocellulosic pentose sugars. J. Biotechnol. 2016, 230, 63–71. [Google Scholar] [CrossRef]
- Clauser, N.M.; Gutiérrez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Techno-economic assessment of carboxylic acids, furfural, and pellet production in a pine sawdust biorefinery. Biofuels Bioprod. Biorefining 2018, 12, 997–1012. [Google Scholar] [CrossRef]
- Corma, A.; De La Torre, O.; Renz, M.; Villandier, N. Production of High-Quality Diesel from Biomass Waste Products. Angew. Chem. Int. Ed. 2011, 50, 2375–2378. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Saha, B.; Alam, I. Advances in conversion of hemicellulosic biomass to furfural and upgrading to biofuels. Catal. Sci. Technol. 2012, 2, 2025–2036. [Google Scholar] [CrossRef]
- Cho, H.; Schäfer, C.; Török, B. Microwave-assisted solid acid catalysis. In Microwaves in Catalysis: Methodology and Applications, 1st ed.; Horikoshi, S., Serpone, N., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 193–212. [Google Scholar]
- Galia, A.; Schiavo, B.; Antonetti, C.; Galletti, A.M.R.; Interrante, L.; Lessi, M.; Scialdone, O.; Valenti, M.G. Autohydrolysis pretreatment of Arundo donax: A comparison between microwave-assisted batch and fast heating rate flow-through reaction systems. Biotechnol. Biofuels 2015, 8, 218. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Len, C. Various carbohydrate precursors dehydration to 5-HMF in an acidic biphasic system under microwave heating using betaine as a co-catalyst. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Millán, G.G.; Phiri, J.; Mäkelä, M.; Maloney, T.; Balu, A.M.; Pineda, A.; Llorca, J.; Sixta, H. Furfural production in a biphasic system using a carbonaceous solid acid catalyst. Appl. Catal. A Gen. 2019, 585, 117180. [Google Scholar] [CrossRef]
- Di Fidio, N.; Antonetti, C.; Galletti, A.M.R. Microwave-assisted cascade exploitation of giant reed (Arundo donax L.) to xylose and levulinic acid catalysed by ferric chloride. Bioresour. Technol. 2019, 293, 122050. [Google Scholar] [CrossRef]
- Di Fidio, N.; Fulignati, S.; De Bari, I.; Antonetti, C.; Galletti, A.M.R. Optimisation of glucose and levulinic acid production from the cellulose fraction of giant reed (Arundo donax L.) performed in the presence of ferric chloride under microwave heating. Bioresour. Technol. 2020, 313, 123650. [Google Scholar] [CrossRef]
- Bondesson, P.-M.; Galbe, M. Process design of SSCF for ethanol production from steam-pretreated, acetic-acid-impregnated wheat straw. Biotechnol. Biofuels 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Palmqvist, B.; Lidén, G. Combining the effects of process design and pH for improved xylose conversion in high solid ethanol production from Arundo donax. AMB Express 2014, 4, 41. [Google Scholar] [CrossRef]
- Cassells, B.; Karhumaa, K.; Nogué, V.S.I.; Lidén, G. Hybrid SSF/SHF Processing of SO2 Pretreated Wheat Straw—Tuning Co-fermentation by Yeast Inoculum Size and Hydrolysis Time. Appl. Biochem. Biotechnol. 2017, 181, 536–547. [Google Scholar] [CrossRef]
- Nielsen, F.; Galbe, M.; Zacchi, G.; Wallberg, O. The effect of mixed agricultural feedstocks on steam pretreatment, enzymatic hydrolysis, and cofermentation in the lignocellulose-to-ethanol process. Biomass Convers. Biorefinery 2019, 10, 253–266. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Vasco-Correa, J.; Li, Y. Giant reed: A competitive energy crop in comparison with miscanthus. Renew. Sustain. Energy Rev. 2016, 54, 350–362. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Chen, S.; Wojcieszak, R.; Dumeignil, F.Y.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.-H.; Xu, G.; Chu, J.; Zhuang, Y.; Zhang, S. Influence of High Solid Concentration on Enzymatic Hydrolysis and Fermentation of Steam-Exploded Corn Stover Biomass. Appl. Biochem. Biotechnol. 2008, 160, 360–369. [Google Scholar] [CrossRef]
- Battista, F.; Bolzonella, D. Some critical aspects of the enzymatic hydrolysis at high dry-matter content: A review. Biofuels Bioprod. Biorefining 2018, 12, 711–723. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef]
- Saini, J.K.; Patel, A.K.; Adsul, M.; Singhania, R.R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Liuzzi, F.; Mastrolitti, S.; De Bari, I. Hydrolysis of Corn Stover by Talaromyces cellulolyticus Enzymes: Evaluation of the Residual Enzymes Activities through the Process. Appl. Biochem. Biotechnol. 2019, 188, 690–705. [Google Scholar] [CrossRef]
- Viikari, L.; Vehmaanperä, J.; Koivula, A. Lignocellulosic ethanol: From science to industry. Biomass Bioenergy 2012, 46, 13–24. [Google Scholar] [CrossRef]
- Drissen, R.E.T.; Maas, R.H.W.; Tramper, J.; Beeftink, H.H. Modelling ethanol production from cellulose: Separate hydrolysis and fermentation versus simultaneous saccharification and fermentation. Biocatal. Biotransform. 2009, 27, 27–35. [Google Scholar] [CrossRef]
- Chen, H.; Jin, S. Effect of ethanol and yeast on cellulase activity and hydrolysis of crystalline cellulose. Enzym. Microb. Technol. 2006, 39, 1430–1432. [Google Scholar] [CrossRef]
- Mutturi, S.; Lidén, G. Effect of Temperature on Simultaneous Saccharification and Fermentation of Pretreated Spruce and Arundo. Ind. Eng. Chem. Res. 2013, 52, 1244–1251. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Wang, M.; He, Q.; Zhang, H. The inhibition of Saccharomyces cerevisiae cells by acetic acid quantified by electrochemistry and fluorescence. Bioelectrochemistry 2008, 72, 117–121. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of Hemicellulose and Derivatives—A Review of Recent Advances in the Production of Furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef]
- Di Fidio, N.; Galletti, A.M.R.; Fulignati, S.; Licursi, D.; Liuzzi, F.; De Bari, I.; Antonetti, C. Multi-Step Exploitation of Raw Arundo donax L. for the Selective Synthesis of Second-Generation Sugars by Chemical and Biological Route. Catalysts 2020, 10, 79. [Google Scholar] [CrossRef]
- Hykkerud, A.; Marchetti, J. Esterification of oleic acid with ethanol in the presence of Amberlyst 15. Biomass Bioenergy 2016, 95, 340–343. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Dhepe, P.L. Effects of cations, anions and H+ concentration of acidic ionic liquids on the valorization of polysaccharides into furfural. New J. Chem. 2017, 41, 6137–6144. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; D’Alessio, A.; Licursi, D.; Antonetti, C.; Valentini, G.; Galia, A.; Di Nasso, N.N.O. Midinfrared FT-IR as a Tool for Monitoring Herbaceous Biomass Composition and Its Conversion to Furfural. J. Spectrosc. 2015, 2015, 719042. [Google Scholar] [CrossRef]
- Jeon, W.; Ban, C.; Kim, J.E.; Woo, H.C.; Kim, D.H. Production of furfural from macroalgae-derived alginic acid over Amberlyst-15. J. Mol. Catal. A Chem. 2016, 423, 264–269. [Google Scholar] [CrossRef]

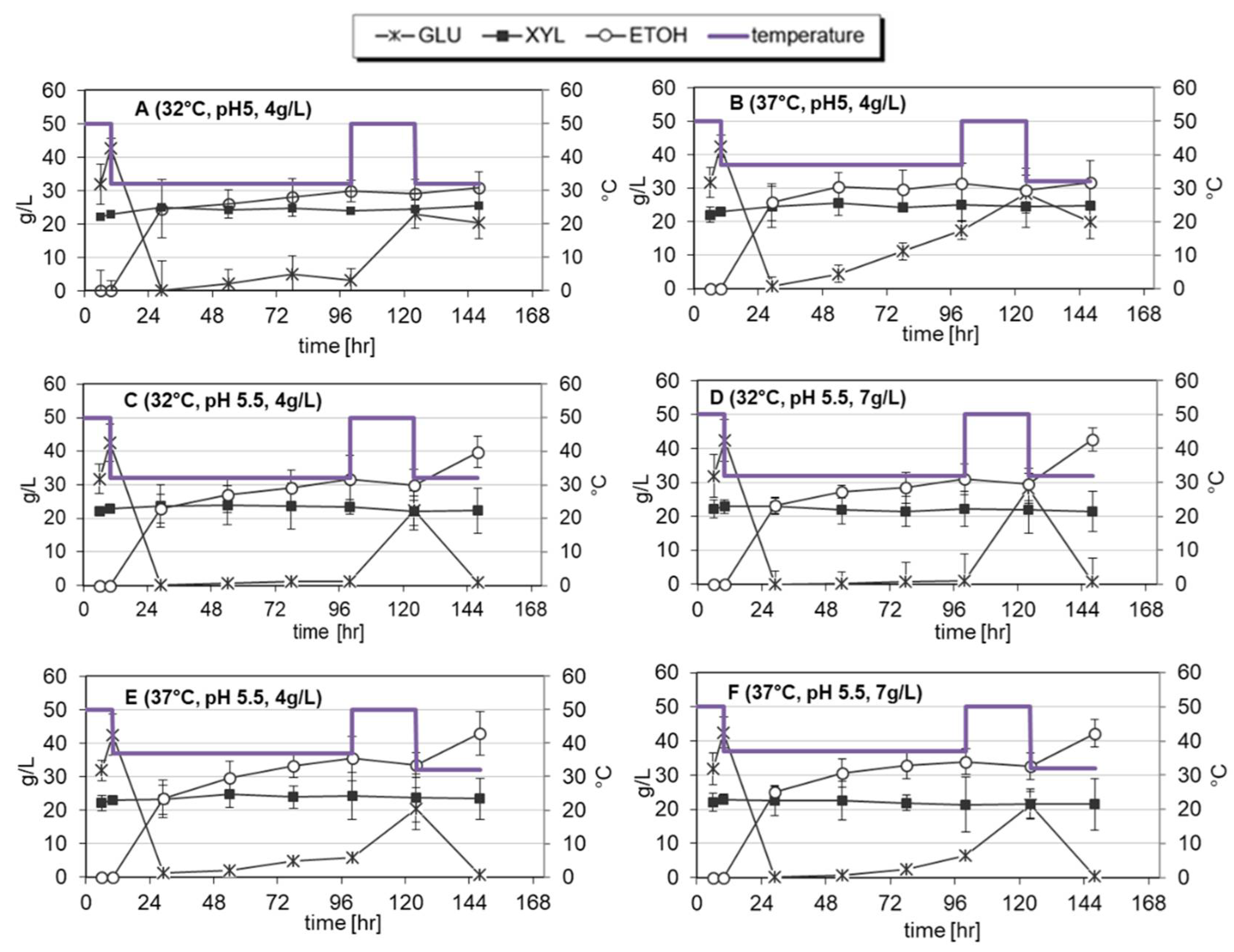


| Test | Yeast Dosage [g/L] a | T (°C) b | Fermentation Mode | pH | EtOH [g/L] | Overall Converted Cellulose c |
|---|---|---|---|---|---|---|
| 1 | 4 | 37 | control | 5 | 29.8 ± 1.0 | 88 ± 2 |
| 2 | 4 | 32 | control | 5 | 30.1 ± 1.2 | 62 ± 2 |
| 3 | 7 | 32 | control | 5.5 | 30.8 ± 1.4 | 62 ± 3 |
| 4 (A) | 4 | 32 | T step | 5 | 30.9 ± 1.7 | 81 ± 3 |
| 5 | 4 | 32 | control | 5.5 | 31.6 ± 1.8 | 63 ± 4 |
| 6 (B) | 4 | 37 | T step | 5 | 31.7 ± 1.0 | 82 ± 2 |
| 7 | 7 | 37 | control | 5.5 | 33.9 ± 0.8 | 82.0 ± 1.8 |
| 8 | 4 | 37 | control | 5.5 | 35.4 ± 1.5 | 78 ± 3 |
| 9 (C) | 4 | 32 | T step | 5.5 | 39.8 ± 1.8 | 79 ± 4 |
| 10 (F) | 7 | 37 | T step | 5.5 | 42.3 ± 0.9 | 83.0 ± 1.8 |
| 11 (D) | 7 | 32 | T step | 5.5 | 42.7 ± 1.4 | 85 ± 3 |
| 12 (E) | 4 | 37 | T step | 5.5 | 42.9 ± 1.7 | 85 ± 3 |
| T [°C] | Catalyst [meq/gXYLOSE] * | FA [%] | Xylose Conversion [%] | Selectivity [%] |
|---|---|---|---|---|
| Solid catalyst (a) | ||||
| 132 | 9 | 9.9 ± 0.5 | 24 ± 3 | 65 ± 5 |
| 132 | 14 | 17.6 ± 0.9 | 54 ± 3 | 51 ± 3 |
| 132 | 17 | 19.9 ± 0.6 | 61 ± 3 | 51 ± 2 |
| 157 | 9 | 27.0 ± 1.1 | 86 ± 3 | 49 ± 3 |
| 157 | 14 | 33.5 ± 1.7 | 91 ± 3 | 57 ± 3 |
| 157 | 17 | 32.0 ± 0.6 | 92 ± 3 | 54 ± 3 |
| 179 | 9 | 35.4 ± 1.1 | 96 ± 3 | 58 ± 2 |
| 179 | 14 | 31.3 ± 1.3 | 99 ± 3 | 49 ± 2 |
| 179 | 17 | 31.0 ± 0.9 | 99 ± 3 | 49 ± 2 |
| Mineral acid (b) | ||||
| 157 | 1.6 | 3.7 ± 0.3 | 48.6 ± 1.5 | 11.7 ± 1.0 |
| 157 | 2.4 | 3.4 ± 0.2 | 41.9 ± 1.7 | 12.5 ± 0.7 |
| 157 | 3.0 | 4.7 ± 0.2 | 40.3 ± 1.1 | 17.9 ± 1.2 |
| 157 | 6.0 | 24.1 ± 0.7 | 44.6 ± 1.4 | 83 ± 4 |
| 157 | 10 | 27.7 ± 1.1 | 58.0 ± 1.5 | 74 ± 4 |
| 157 | 26 | 37.4 ± 1.0 | 79 ± 2 | 73 ± 3 |
| 179 | 6 | 49.4 ± 0.8 | 95 ± 3 | 80 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bari, I.; Liuzzi, F.; Ambrico, A.; Trupo, M. Arundo donax Refining to Second Generation Bioethanol and Furfural. Processes 2020, 8, 1591. https://doi.org/10.3390/pr8121591
De Bari I, Liuzzi F, Ambrico A, Trupo M. Arundo donax Refining to Second Generation Bioethanol and Furfural. Processes. 2020; 8(12):1591. https://doi.org/10.3390/pr8121591
Chicago/Turabian StyleDe Bari, Isabella, Federico Liuzzi, Alfredo Ambrico, and Mario Trupo. 2020. "Arundo donax Refining to Second Generation Bioethanol and Furfural" Processes 8, no. 12: 1591. https://doi.org/10.3390/pr8121591
APA StyleDe Bari, I., Liuzzi, F., Ambrico, A., & Trupo, M. (2020). Arundo donax Refining to Second Generation Bioethanol and Furfural. Processes, 8(12), 1591. https://doi.org/10.3390/pr8121591