Experimental and Numerical Analysis of a Low Environmental Impact Pyro-Gasification System for the Energetic Valorization of Waste through a Biomass Steam Power Plant
Abstract
:1. Introduction
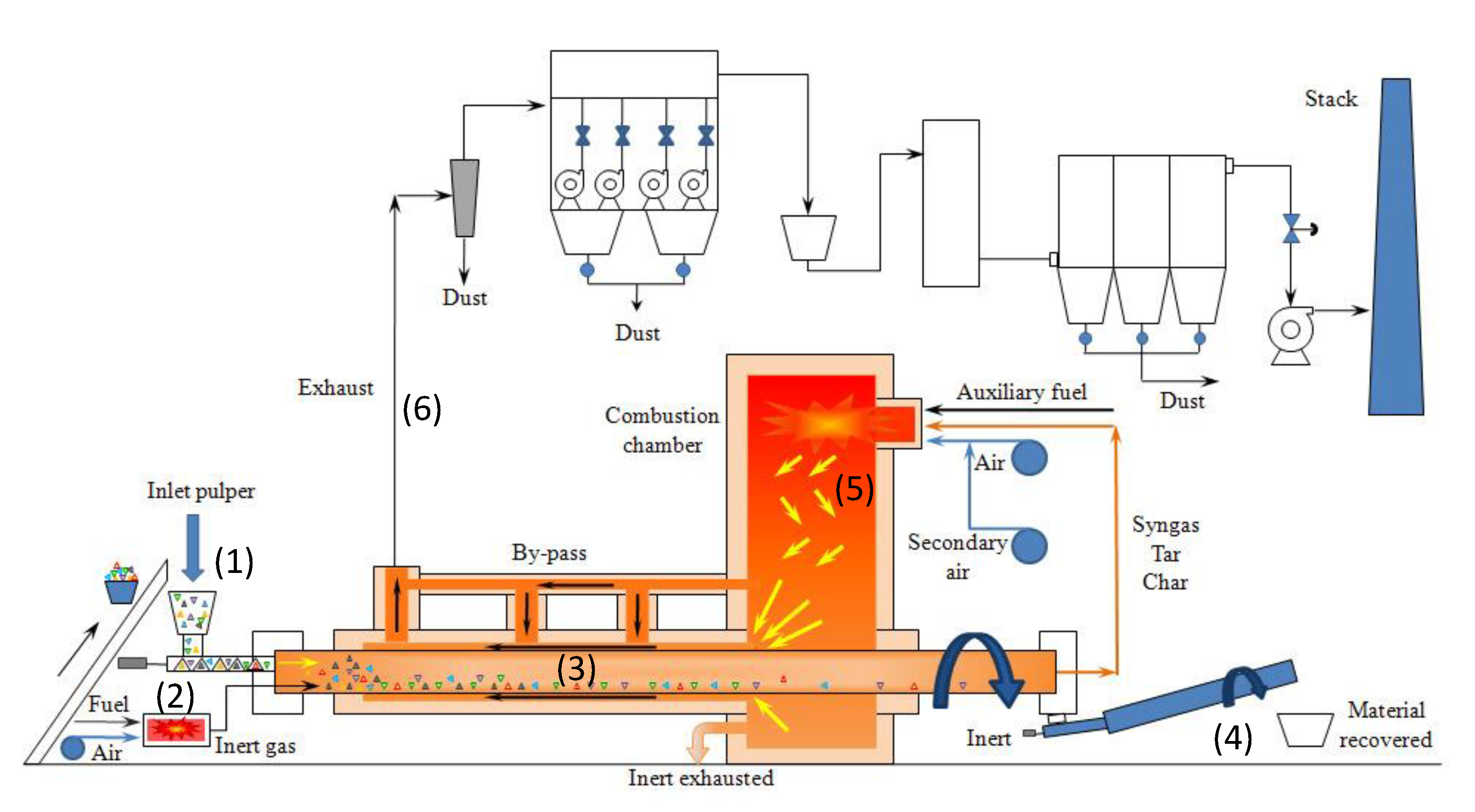
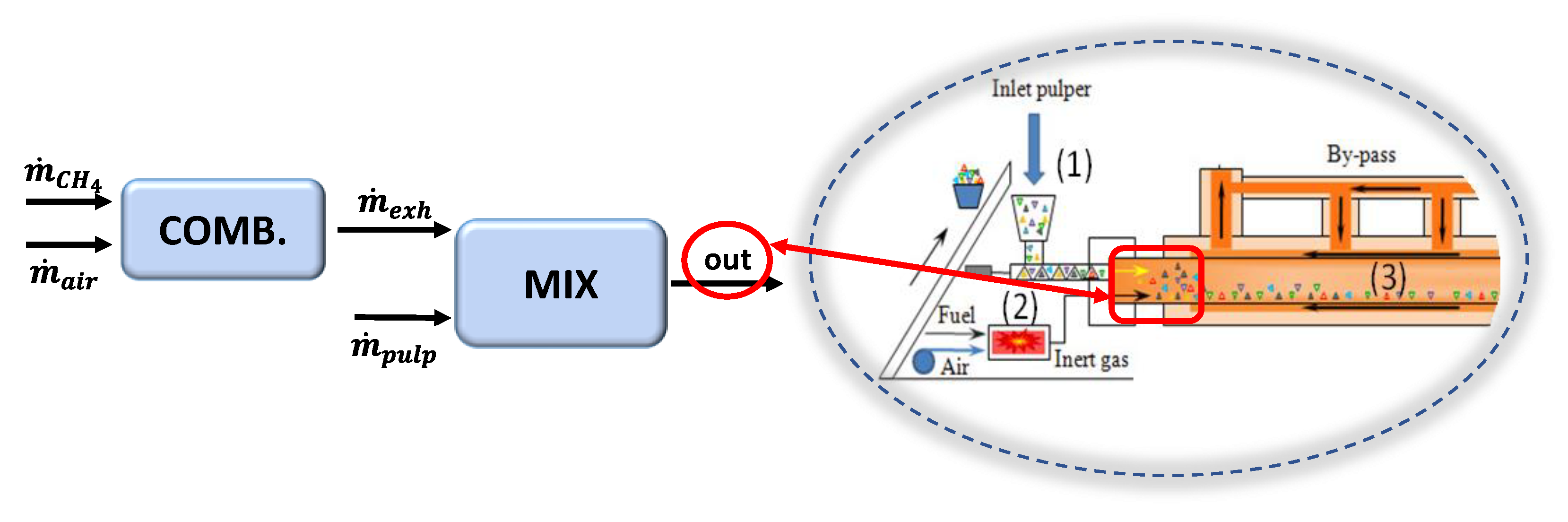
2. The MR System: Experimental Setup and Test
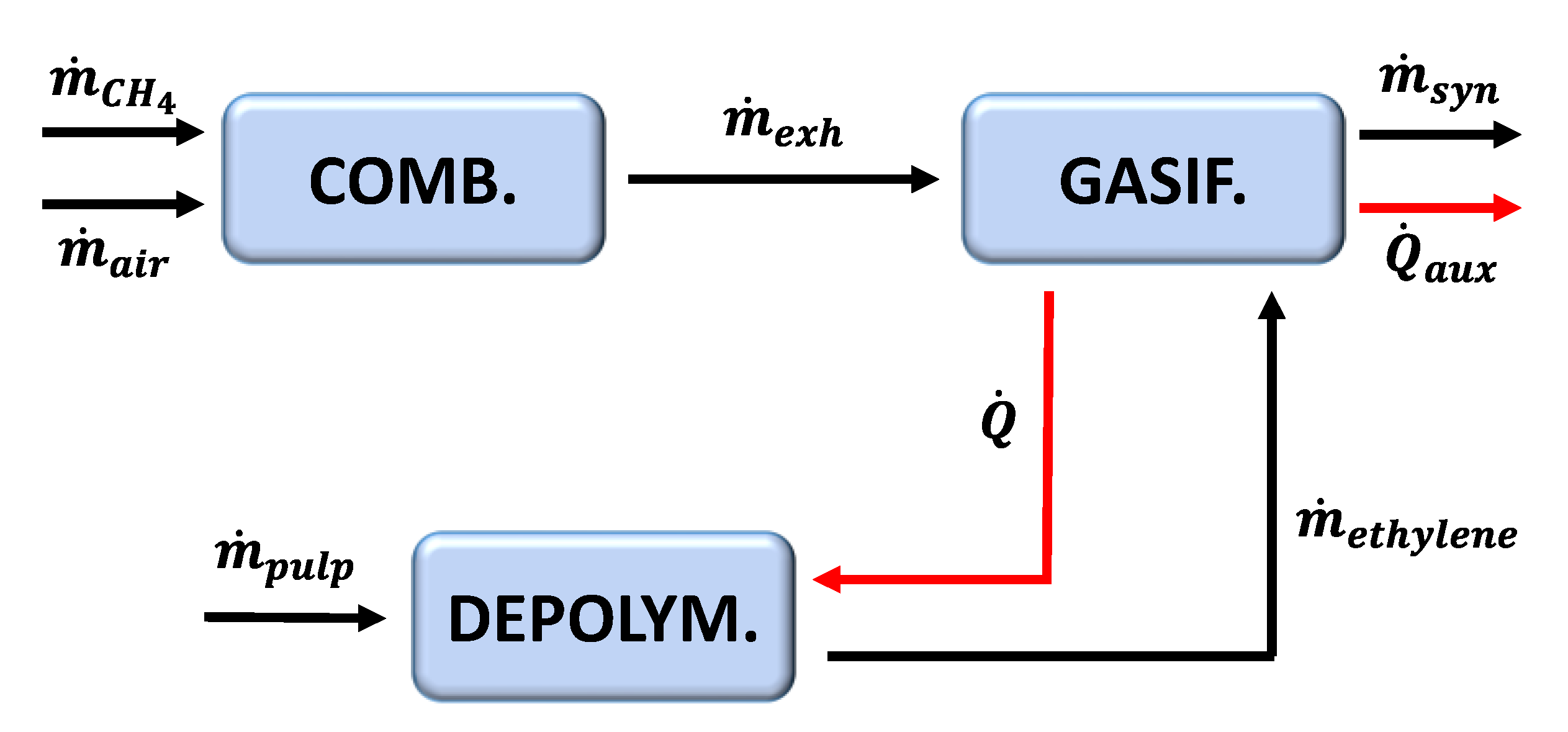
3. The Thermochemical Model
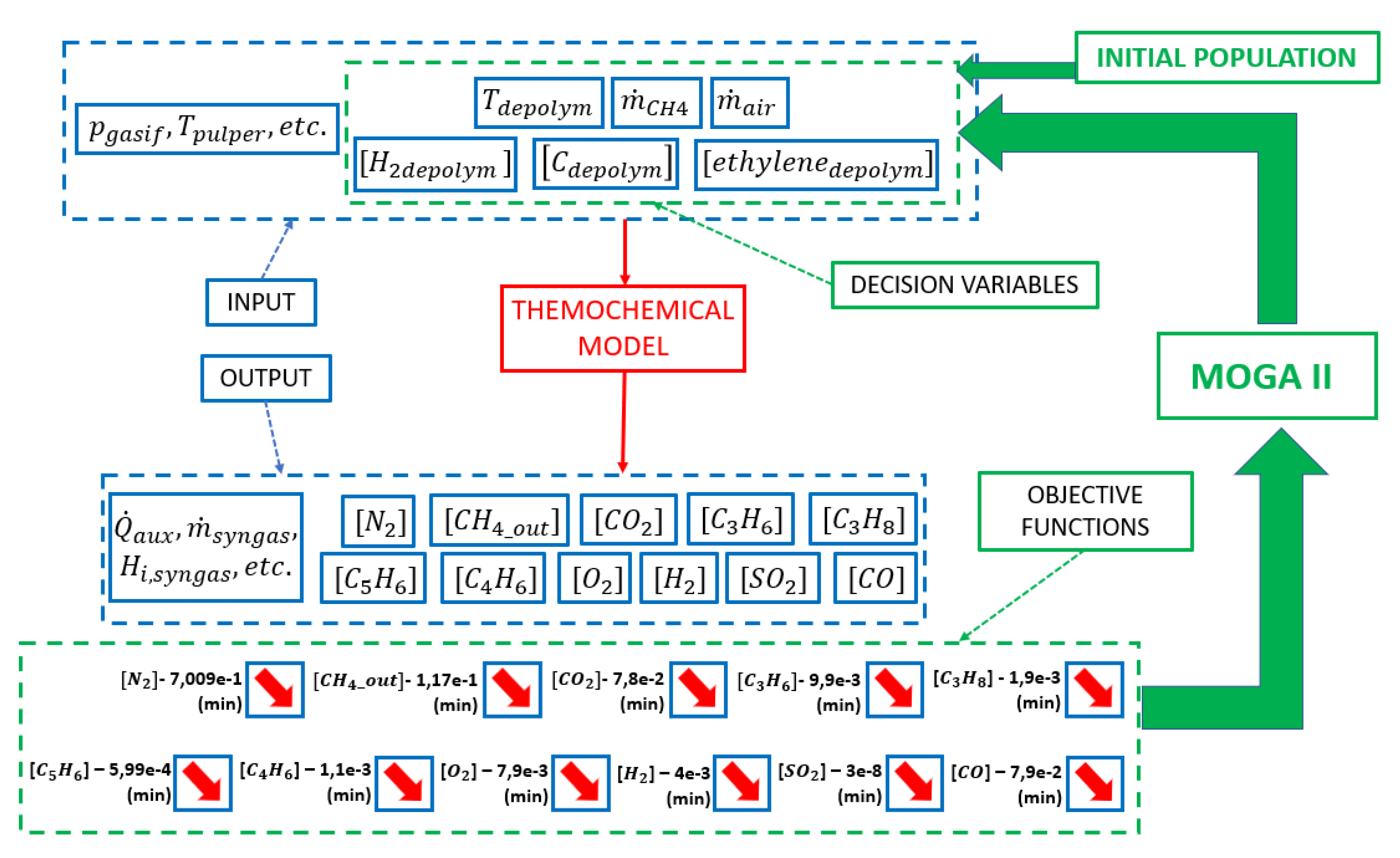
4. Thermochemical Model Calibration: The Proposed Multi-Objective Optimization Approach
5. Optimization Results and Decision-Making Problem
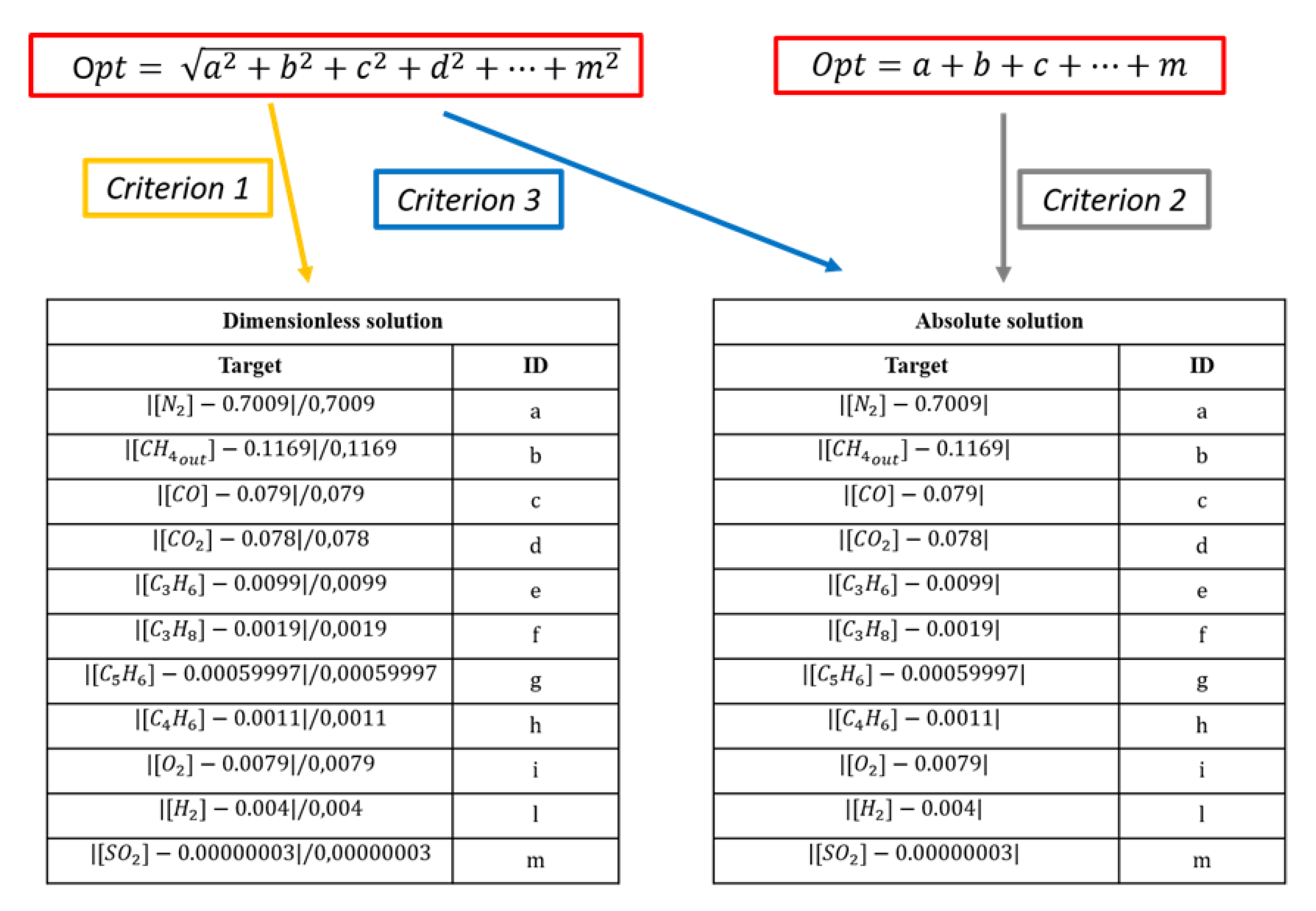
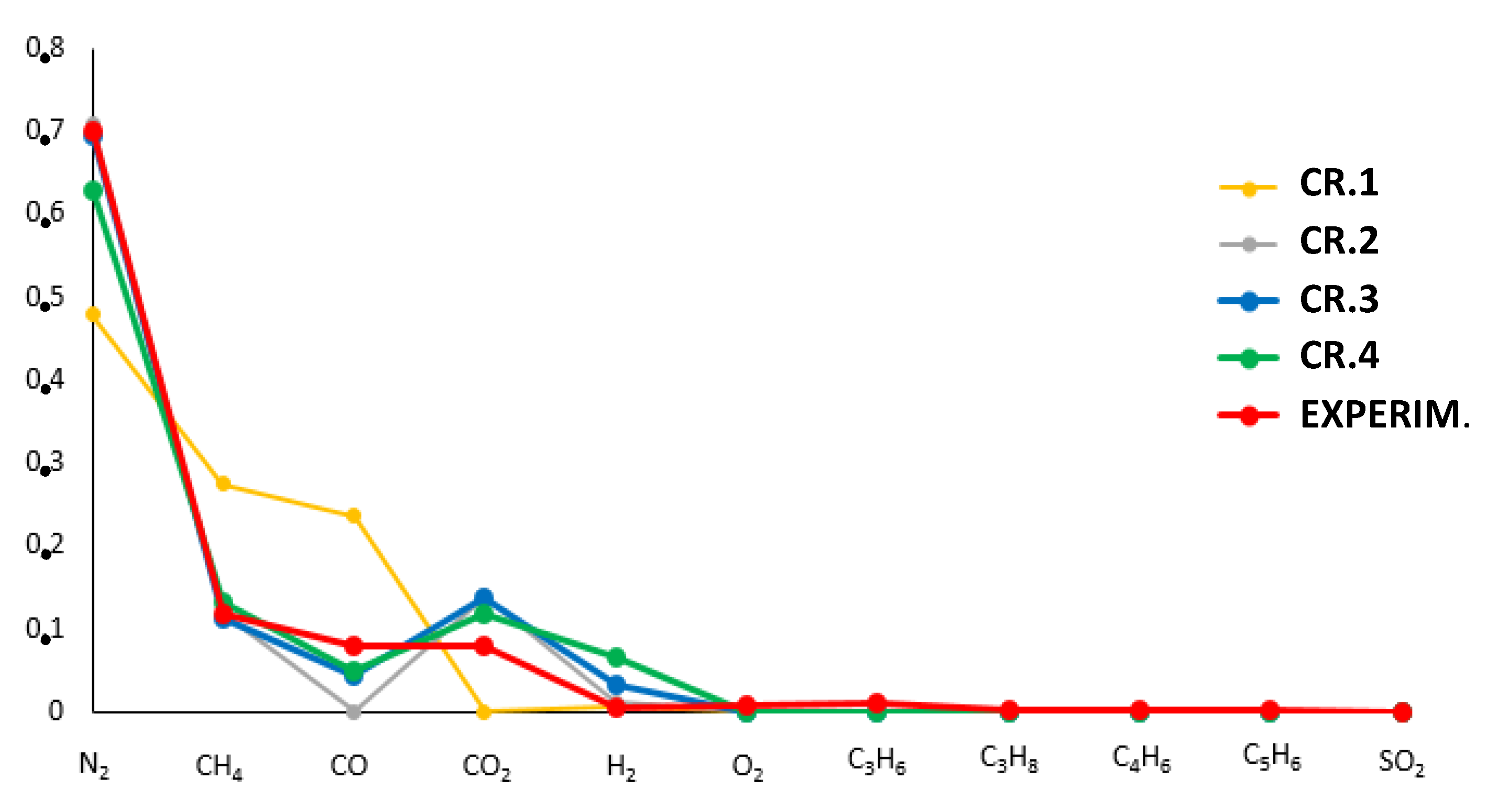
- CR.1: Minimum Euclidean norm of the relative errors vector (i.e., dimensionless objective functions vector, Figure 7).
- CR.2: Minimum total sum of the absolute errors vector (i.e., objective functions vector, Figure 7).
- CR.3: Minimum Euclidean norm of the absolute errors vector (i.e., objective functions vector, Figure 7).
- CR.4: A combination of the criterion n. 3 with the minimum absolute difference between calculated and experimental LHV of the syngas: .
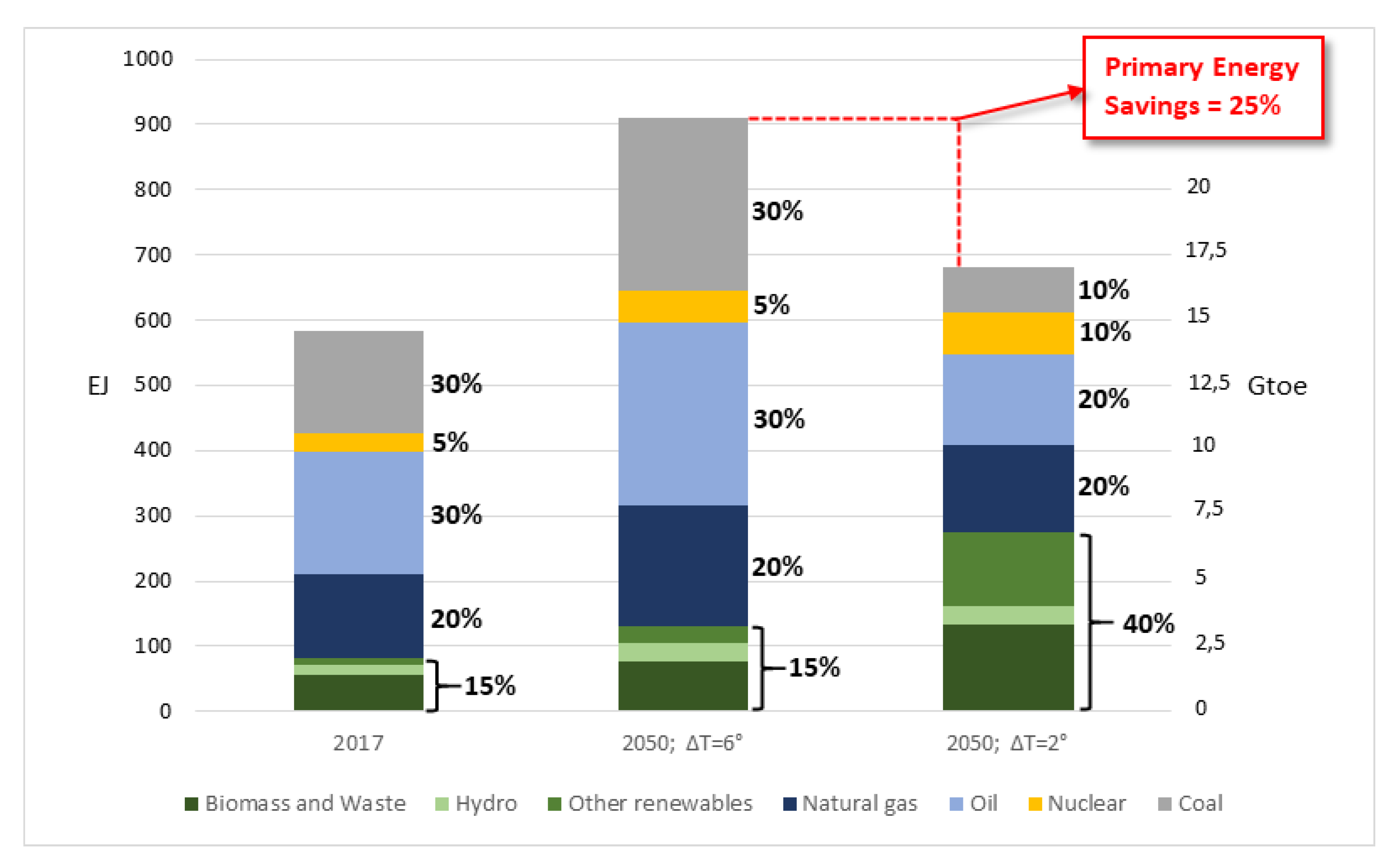
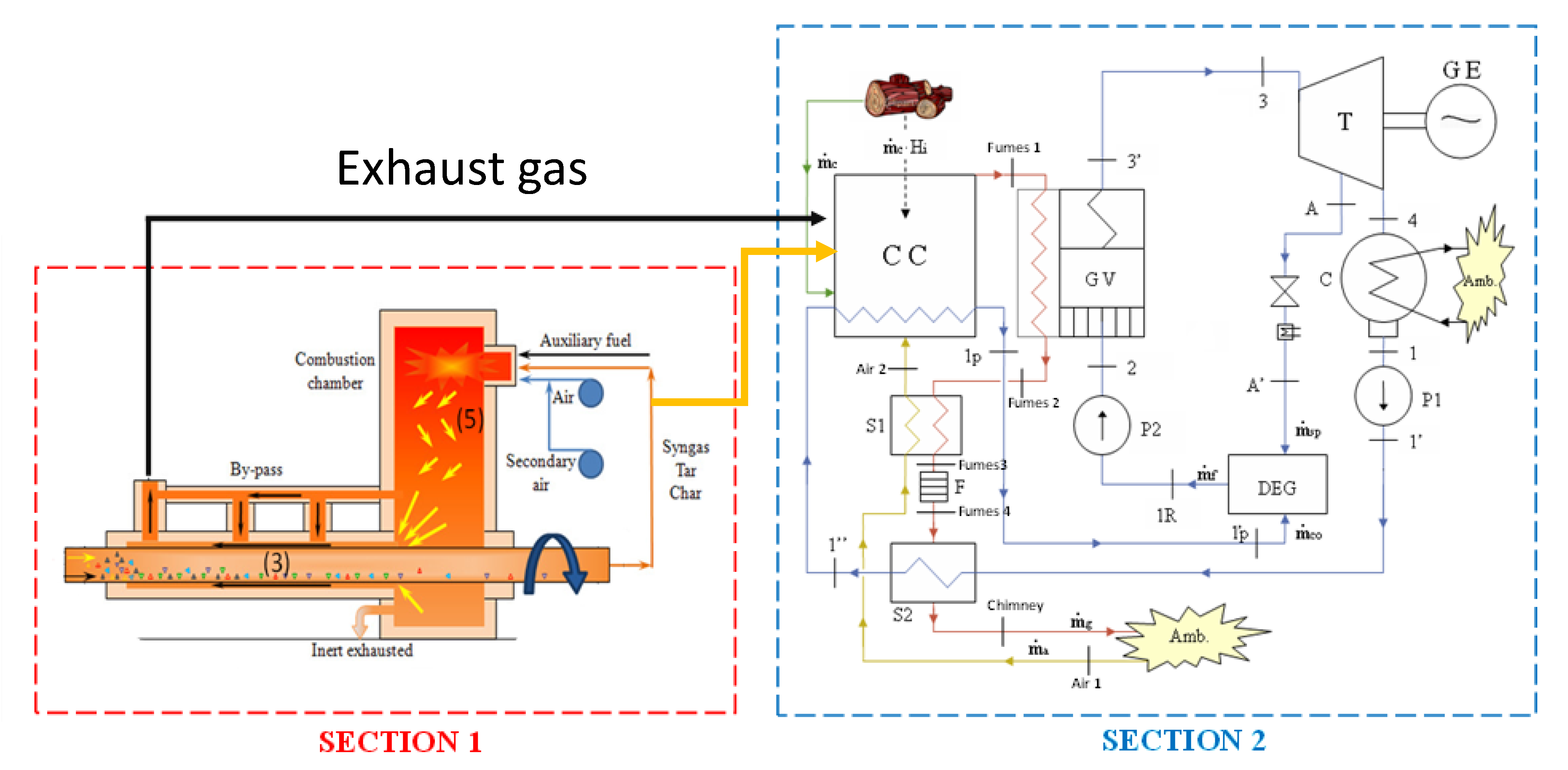
6. Application: Coupling the Pyro-Gasification Plant with a Biomass Steam Power Plant
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Acronyms and Abbreviations
| 11-D | Eleven Dimensional |
| CES | Circular Economy System |
| CHP | Combined Heat and Power |
| MR | Material Recovery |
| MSW | Municipal Solid Waste |
| PES | Primary Energy Saving |
| WtE | Waste to Energy |
| BSPP | Biomass Steam Power Plant |
| CC | Combustion Chamber |
Nomenclature
| volumetric fraction of carbon in the substance produced by the DEPOLYM block | |
| specific heat of air | |
| specific heat of methane | |
| specific heat of exhaust flow | |
| specific heat of pulper | |
| volumetric fraction of ethylene in the substance produced by the DEPOLYM block | |
| volumetric fraction of hydrogen in the substance produced by the DEPOLYM block | |
| lower heating value of methane | |
| lower heating value of ethylene | |
| lower heating value of pulper | |
| lower heating value of syngas | |
| lower heating value of biomass | |
| LHV | lower heating value flow |
| air mass flow | |
| methane mass flow | |
| mass leaving DEPOLYM block | |
| exhaust combustor mass flow | |
| individual species involved in combustion mass flow | |
| pulper mass flow | |
| syngas mass flow | |
| total mass flow out | |
| biomass mass flow | |
| heat exchanged between DEPOLYM and GASIF blocks | |
| total heat exiting the GASIF block | |
| environment temperature | |
| electric efficiency | |
| net electric power |
References
- Gimelli, A.; Muccillo, M. Optimization criteria for cogeneration systems: Multi-objective approach and application in a hospital facility. Appl. Energy 2013, 104, 910–923. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2018. Available online: https://www.iea.org/reports/world-energy-outlook-2018 (accessed on April 2020).
- Kumar, A.; Samadder, S.R. A review on technological options of waste to energy for effective management of municipal solid waste. Waste Manag. 2017, 69, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Tyagi, V.V.; Pathak, A. Waste-to-energy: A way from renewable energy sources to sustainable development. Renew. Sustain. Energy Rev. 2010, 14, 3164–3170. [Google Scholar] [CrossRef]
- Ouda, O.K.M.; Raza, S.A.; Nizami, A.S.; Rehan, M.; Al-Waked, R.; Korres, N.E. Waste to energy potential: A case study of Saudi Arabia. Renew. Sustain. Energy Rev. 2016, 61, 328–340. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook 2016. Available online: https://www.iea.org/reports/world-energy-outlook-2016 (accessed on November 2017).
- International Energy Agency. Energy Technology Perspectives; International Energy Agency: Paris, France, 2014; ISBN 978-92-64-20800-1. [Google Scholar]
- Gimelli, A.; Muccillo, M. The key role of the vector optimization algorithm and robust design approach for the design of polygeneration systems. Energies 2018, 11, 821. [Google Scholar] [CrossRef] [Green Version]
- Charters, W.W.S. Developing markets for renewable energy technologies. Renew. Energy 2001, 22, 217–222. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Urban Development; World Bank ©: Washington, DC, USA, 2018. [Google Scholar]
- Palmiotto, M.; Fattore, E.; Paiano, V.; Celeste, G.; Colombo, A.; Davoli, E. Influence of a municipal solid waste landfill in the surrounding environment: Toxicological risk and odor nuisance effects. Environ. Int. 2014, 68, 16–24. [Google Scholar] [CrossRef]
- Tan, S.T.; Hashim, H.; Lim, J.S.; Ho, W.S.; Lee, C.T.; Yan, J. Energy and emissions benefits of renewable energy derived from municipal solid waste: Analysis of a low carbon scenario in Malaysia. Appl. Energy 2014, 136, 797–804. [Google Scholar] [CrossRef]
- Baggio, P.; Baratieri, M.; Fiori, L.; Grigiante, M.; Avi, D.; Tosi, P. Experimental and modelling analysis of a batch gasification/pyrolysis reactor. Energy Convers. Manag. 2009, 50, 1426–1435. [Google Scholar] [CrossRef]
- Poulsen, T.G.; Hansen, J.A. Assessing the impacts of changes in treatment technology on energy and greenhouse gas balances for organic waste and wastewater treatment using historical data. Waste Manag. Res. 2009, 27, 861–870. [Google Scholar] [CrossRef]
- Ionescu, G.; Merler, G.; Rada, E.C.; Ragazzi, M. Integrated valorization system of MSW: Metropolitan area case study. In Proceedings of the 5th International Conference on Energy and Environment, Bucharest, Romania, 3–4 November 2011. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 3rd ed.; Academic Press Elsevier: Cambridge, MA, USA, 2018; ISBN 978-0-12-812992-0. [Google Scholar]
- Leckner, B. Process aspects in combustion and gasification Waste-to-Energy (WtE) units. Waste Manag. 2015, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Panepinto, D.; Tedesco, V.; Brizio, E.; Genon, G. Environmental performances and energy efficiency for MSW gasification treatment. Waste Biomass Valoriz. 2015, 6, 123–135. [Google Scholar] [CrossRef]
- Belgiorno, V.; De Feo, G.; Della Rocca, C.; Napoli, D.R. Energy from gasification of solid wastes. Waste Manag. 2003, 23, 1–15. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M. Life cycle assessment of pyrolysis, gasification and incineration waste-to-energy technologies: Theoretical analysis and case study of commercial plants. Sci. Total Environ. 2018, 626, 744–753. [Google Scholar] [CrossRef]
- Zeng, R.; Wang, S.; Cai, J.; Kuang, C. A review of pyrolysis gasification of MSW. Adv. Eng. Res. 2018, 163. [Google Scholar] [CrossRef] [Green Version]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Sarkar, J.K.; Wang, Q. Characterization of pyrolysis products and kinetic analysis of waste jute stick biomass. Processes 2020, 8, 837. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Ryms, M.; Kosakowski, W. Thermal biomass conversion: A review. Processes 2020, 8, 516. [Google Scholar] [CrossRef]
- Costa, M.; Di Blasio, G.; Prati, M.V.; Costagliola, M.A.; Cirillo, D.; La Villetta, M.; Caputo, C.; Martoriello, G. Multi-objective optimization of a syngas powered reciprocating engine equipping a combined heat and power unit. Appl. Energy 2020, 275. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.L.; Zhao, X.Y.; Cao, J.P. Biomass thermochemical conversion: A review on tar elimination from biomass catalytic gasification. J. Energy Inst. 2020, 93, 1083–1098. [Google Scholar] [CrossRef]
- Hu, B.; Huang, Q.; Buekens, A.; Chi, Y.; Yan, J. Co-gasification of municipal solid waste with high alkali coal char in a three-stage gasifier. Energy Convers. Manag. 2017, 153, 473–481. [Google Scholar] [CrossRef]
- Sá, L.C.R.; Loureiro, L.M.E.F.; Nunes, L.J.R.; Mendes, A.M.M. Torrefaction as a pretreatment technology for chlorine elimination from biomass: A case study using eucalyptus globulus labill. Resources 2020, 9, 54. [Google Scholar] [CrossRef]
- Hu, M.; Cui, B.; Xiao, B.; Luo, S.; Guo, D. Insight into the Ex Situ catalytic pyrolysis of biomass over char supported metals catalyst: Syngas production and tar decomposition. Nanomaterials 2020, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Recari, J.; Berrueco, C.; Abelló, S.; Montané, D.; Farriol, X. Gasification of two solid recovered fuels (SRFs) in a lab-scale fluidized bed reactor: Influence of experimental conditions on process performance and release of HCl, H2S, HCN and NH3. Fuel Process. Technol. 2016, 142, 107–114. [Google Scholar] [CrossRef]
- Vaish, B.; Sharma, B.; Srivastava, V.; Singh, P.; Ibrahim, M.H.; Singh, R.P. Energy recovery potential and environmental impact of gasification for municipal solid waste. Biofuel 2017, 10, 87–100. [Google Scholar] [CrossRef]
- Yuan, P.S.; Du, M.A.; Te Huang, I.; Liu, I.H.; Chang, E.E. Strategies on implementation of waste-to-energy (WTE) supply chain for circular economy system: A review. J. Clean. Prod. 2015, 108, 409–421. [Google Scholar] [CrossRef]
- Singh, J.; Ordonez, I. Resource recovery from post-consumer waste: Important lessons for the upcoming circular economy. J. Clean. Prod. 2016, 134, 342–353. [Google Scholar] [CrossRef]
- Tisserant, A.; Fry, J.; Merciai, S.; Pauliuk, S.; Schmidt, J.; Tukker, A.; Wood, R. Solid waste and the circular economy: A global analysis of waste treatment and waste footprints. J. Ind. Ecol. 2017, 21, 628–640. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, P.; Tyagi, R. Advanced simulation of biomass gasification in a fluidized bed reactor using aspen plus. Renew. Energy 2017, 101, 629–636. [Google Scholar] [CrossRef]
- Eikeland, M.S.; Thapa, R.K.; Halvorsen, B. Aspen plus simulation of biomass gasification with known reaction kinetic. In Proceedings of the Linköping Electronic Conference, Wroclaw, Poland, 14–16 October 2015; Volume 119, pp. 149–155. [Google Scholar] [CrossRef] [Green Version]
- Gagliano, A.; Nocera, F.; Bruno, M.; Cardillo, G. Development of an equilibrium-based model of gasification of biomass by Aspen Plus. Energy Procedia 2017, 111, 1010–1019. [Google Scholar] [CrossRef]
- Nikoo, M.B.; Mahinpeya, N. Simulation of biomass gasification in fluidized bed reactor using aspen plus. Biomass Bioenergy 2008, 32, 1245–1254. [Google Scholar] [CrossRef]
- Selchan, A.M. The Separation of Low Density Polyethylene Laminates from Paper. Paper Engineering Senior Thesis, Western Michigan University, Kalamazoo, MI, USA, May 1982. [Google Scholar]
- Chen, W.; Shi, S.; Zhang, J.; Chen, M.; Zhou, X. Co-pyrolysis of waste newspaper with high-density polyethylene: Synergistic effect and oil characterization. Energy Convers. Manag. 2016, 112, 41–48. [Google Scholar] [CrossRef]
- Salan, T.; Hakkı Alma, M.; Altuntaş, E. The fuel properties of pyrolytic oils obtained from catalytic pyrolysis of non-recyclable pulper rejects using activated natural mineral. Energy Sources Part A Recovery Util. Environ. Effects 2019, 41, 1460–1473. [Google Scholar] [CrossRef]
- Kannan, P.; Al Shoaibi, A.; Srinivasakannan, C. Optimization of waste plastics gasification process using aspen-plus. Intech 2012. [Google Scholar] [CrossRef] [Green Version]
- Acar, M.C.; Böke, Y.E. Simulation of biomass gasification process using Aspen plus. In Proceedings of the 14th International Combustion Symposium (INCOS2018), Karabük, Turkey, 25–27 April 2018. [Google Scholar]
- Poloni, C.; Pediroda, V. GA coupled with computationally expensive simulations: Tools to improve efficiency. In Genetic Algorithms and Evolution Strategies in Engineering and Computer Science; John Wiley and Sons: Chichester, NH, USA, 1997; pp. 267–288. ISBN 0471977101. [Google Scholar]
- Nola, F.D.; Giardiello, G.; Gimelli, A.; Molteni, A.; Muccillo, M.; Picariello, R.; Tornese, D. Reduction of the experimental effort in engine calibration by using neural networks and 1D engine simulation. Energy Procedia 2018, 148, 344–351. [Google Scholar] [CrossRef]
- Gimelli, A.; Muccillo, M.; Sannino, R. Effects of uncertainties on the stability of the results of an optimal sized modular cogeneration plant. Energy Procedia 2017, 126, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Padovan, L.; Pediroda, V.; Poloni, C. Multi objective robust design optimization of airfoils in transonic field (M.O.R.D.O.). In Proceedings of the International Congress on Evolutionary Methods for Design, Optimization and Control with Applications to Industrial Problems EUROGEN 2003, Barcelona, Spain, 15–17 September 2003. [Google Scholar]
- Brancati, R.; Muccillo, M.; Tufano, F. Crank mechanism friction modeling for control-oriented applications. In IFToMM ITALY 2020. Mechanisms and Machine Science: Advances in Italian Mechanism Science; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Gimelli, A.; Sannino, R. A multi-variable multi-objective methodology for experimental data and thermodynamic analysis validation: An application to micro gas turbines. Appl. Therm. Eng. 2018, 134, 501–512. [Google Scholar] [CrossRef]
- Aruldoss, M.; Lakshmi, T.M.; Venkatesan, V.P. A survey on multi criteria decision making methods and its applications. Am. J. Inf. Syst. 2013, 1, 31–43. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef] [Green Version]
- Gimelli, A.; Luongo, A. 2.3 MW biomass steam power plant: Experimental and thermodynamic analysis. In Proceedings of the International Conference on Renewable Energies and Power Quality (ICREPQ’12), Santiago De Compostela, Spain, 28–30 March 2012. [Google Scholar] [CrossRef]
- Gimelli, A.; Luongo, A.; Amoresano, A. A gasification system for the disposal of industrial waste and energy generation: Experimentation and thermodynamic analysis. In Proceedings of the International Conference on Renewable Energies and Power Quality (ICREPQ’12), Santiago De Compostela, Spain, 28–30 March 2012. [Google Scholar] [CrossRef]
- Gimelli, A.; Luongo, A. Thermodynamic and experimental analysis of a biomass steam power plant: Critical issues and their possible solutions with CCGT systems. Energy Procedia 2014, 45, 227–236. [Google Scholar] [CrossRef] [Green Version]


| Proximate Analysis | Mass % | Ultimate Analysis | Mass % |
|---|---|---|---|
| Moisture | 20 | Carbon | 85.76 |
| Volatile fraction | 95.30 | Hydrogen | 13.86 |
| Fixed carbon | 4.50 | Nitrogen | 0.12 |
| Ash (inert) | 0.20 | Chlorine | 0 |
| Sulfur | 0.06 | ||
| Oxygen | 0 | ||
| Ash (inert) | 0.20 |
| SYNGAS COMPOSITION | ||
|---|---|---|
| Chemical Species | Experimental Value | |
| Carbon dioxide | 7.8% v/v | |
| Nitrogen | 70.1% v/v | |
| Water | - | |
| Methane | 11.7% v/v | |
| Propene | 0.99% v/v | |
| Propane | 0.19% v/v | |
| 1,3 Cyclopentadiene | 0.06% v/v | |
| 1,3 Butadiene | 0.11% v/v | |
| Oxygen | 0.8% v/v | |
| Carbon monoxide | 7.9% v/v | |
| Hydrogen | 0.4% v/v | |
| Sulfur dioxide | ||
| Name | Unit of Measurement | Parameter Range |
|---|---|---|
| Reactor temperature | [K] | 500–900 |
| mass flow rate | [kg/s] | 0.002–0.2 |
| Air mass flow rate | [kg/s] | 0.08–0.3 |
| Mass Fraction, kg/1 kg of Pulper | |
|---|---|
| ETHYL-01 | 0.8415 |
| ASH | 0.0015 |
| N2 | 0.0012 |
| S | 0.0006 |
| C | 0.1375 |
| H2 | 0.0177 |
| Decision Variables | Constrains | ||
|---|---|---|---|
| Name | Unit of Measurement | Range | |
| [K] | 500–900 | [-] | |
| [-] | 0.1–1 | ||
| [kg/s] | 0.002–0.2 | 1 < λ < 14 | |
| [kg/s] | 0.08–3 | ||
| Species | Objective Functions |
|---|---|
| Mass Fraction[kg/1 kg of, Figure 4] | Mass Fraction[kg/1 kg of, Figure 4] | Mass Fraction[kg/1 kg of, Figure 4] | [kg/s] | [kg/s] | [K] | |
|---|---|---|---|---|---|---|
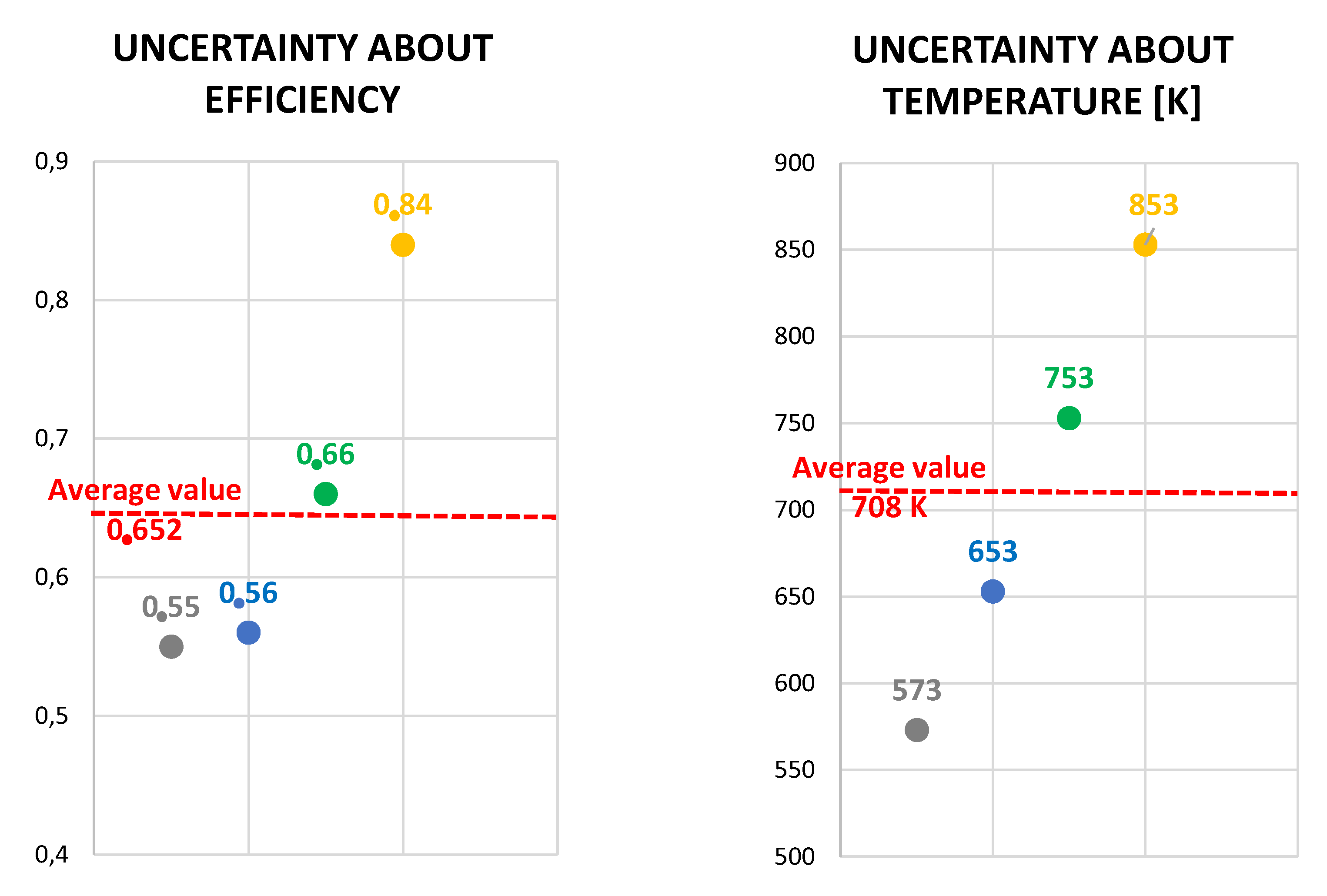
| CR.1 | 0.152 | 0.0196 | 0.825 | 0.748 | 0.043 | 853.15 |
| CR.2 | 0.157 | 0.0202 | 0.820 | 2.670 | 0.110 | 573.15 |
| CR.3 | 0.0547 | 0.0070 | 0.953 | 2.70 | 0.120 | 653.15 |
| CR.4 | 0.1477 | 0.0190 | 0.830 | 1.91 | 0.090 | 753.15 |
| CR.1 volume fraction, m3/1 m3 of Syngas | [CO] | |||||
| 1.45 × 10−4 | 5.60 × 10−6 | 1.23 × 10−12 | 0.001484 | 0.2742 | 0.2362 | |
| ] | ] | |||||
| 1.15 × 10−4 | 0.006835 | 0.479 | 0 | 2.02 × 10−8 | ||
| CR.2 volume fraction, m3/1 m3 of Syngas | ] | ] | ] | ] | ] | [CO] |
| 5.32 × 10−12 | 2.29 × 10−11 | 1.32 × 10−28 | 7.60 × 10−23 | 0.1179 | 5.08 × 10−4 | |
| ] | ] | ] | ] | ] | ||
| 0.139 | 0.0106 | 0.708 | 0 | 4.34 × 10−5 | ||
| CR.3 volume fraction, m3/1 m3 of Syngas | ] | ] | ] | ] | ] | [CO] |
| 1.25 × 10−12 | 5.86 × 10−11 | 4.59 × 10−26 | 2.38 × 10−20 | 0.11131 | 4.42 × 10−3 | |
| ] | ] | ] | ] | ] | ||
| 0.137 | 0.0317 | 0.694 | 6.98 × 10−34 | 4.20 × 10−5 | ||
| CR.4 volume fraction, m3/1 m3 of Syngas | ] | ] | ] | ] | ] | [CO] |
| 1.40 × 10−10 | 5.86 × 10−10 | 1.65 × 10−22 | 2.04 × 10−16 | 0.1323 | 4.98 × 10−2 | |
| ] | ] | ] | ] | ] | ||
| 0.117 | 0.0649 | 0.6272 | 4.12 × 10−30 | 4.37 × 10−5 |
| LHV | ||
| [MJ/kg] | ||
| 50 | ||
| 10.05 | ||
| 45.8 | ||
| 46.3 | ||
| 120 | ||
| 43.55 | ||
| 41.79 |
| Biomass Mass Flow Rate | 2498 kg/h |
|---|---|
| Gross electric power | 2688 kW |
| Electric power for auxiliary | 320 kW |
| Net electric power | 2368 kW |
| Electric efficiency | 23% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gimelli, A.; Muccillo, M.; Sannino, R.; Braccio, G.; Capone, V.; Cornacchia, G.; Manganiello, M.; Mongiello, C.; Sharma, V.K. Experimental and Numerical Analysis of a Low Environmental Impact Pyro-Gasification System for the Energetic Valorization of Waste through a Biomass Steam Power Plant. Processes 2021, 9, 35. https://doi.org/10.3390/pr9010035
Gimelli A, Muccillo M, Sannino R, Braccio G, Capone V, Cornacchia G, Manganiello M, Mongiello C, Sharma VK. Experimental and Numerical Analysis of a Low Environmental Impact Pyro-Gasification System for the Energetic Valorization of Waste through a Biomass Steam Power Plant. Processes. 2021; 9(1):35. https://doi.org/10.3390/pr9010035
Chicago/Turabian StyleGimelli, Alfredo, Massimiliano Muccillo, Raniero Sannino, Giacobbe Braccio, Vincenzo Capone, Giacinto Cornacchia, Matteo Manganiello, Carmine Mongiello, and Vinod Kumar Sharma. 2021. "Experimental and Numerical Analysis of a Low Environmental Impact Pyro-Gasification System for the Energetic Valorization of Waste through a Biomass Steam Power Plant" Processes 9, no. 1: 35. https://doi.org/10.3390/pr9010035