Design and Fabrication of Partially Foamed Grid Structure Using Additive Manufacturing and Solid State Foaming
Abstract
1. Introduction
2. Experimental Apparatus and Method
2.1. Material and Foaming Process
2.2. Measurements
3. Results and Discussion
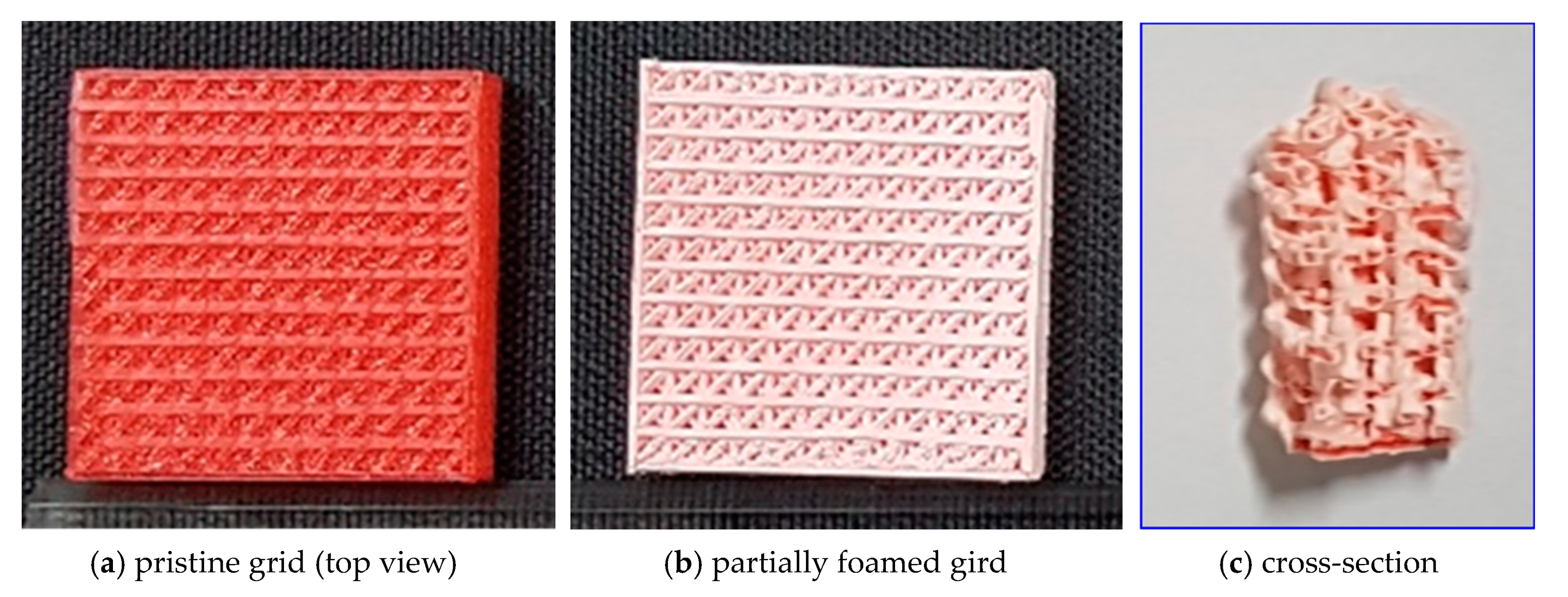
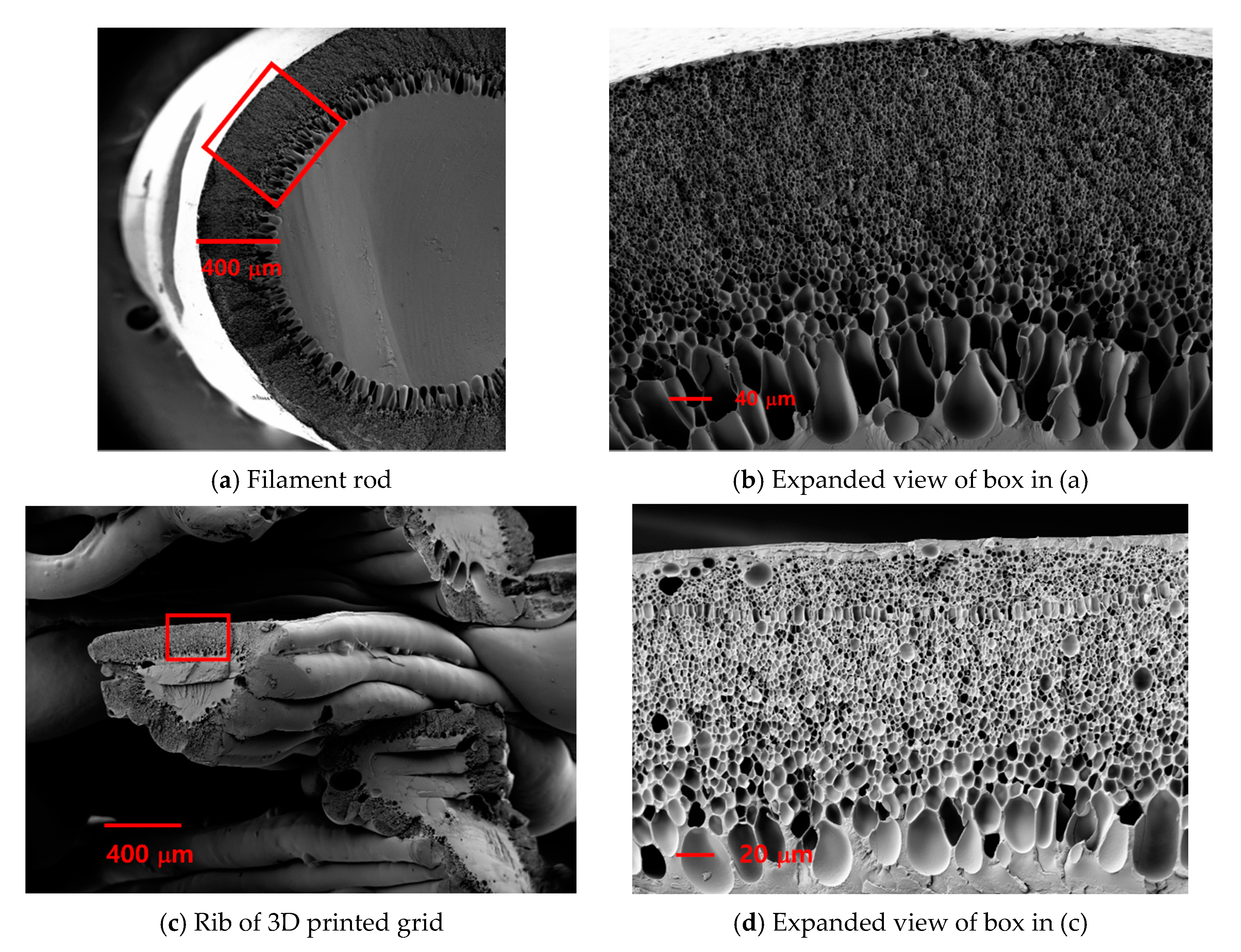
3.1. Sorption Kinetics and Microstructural Characteristics
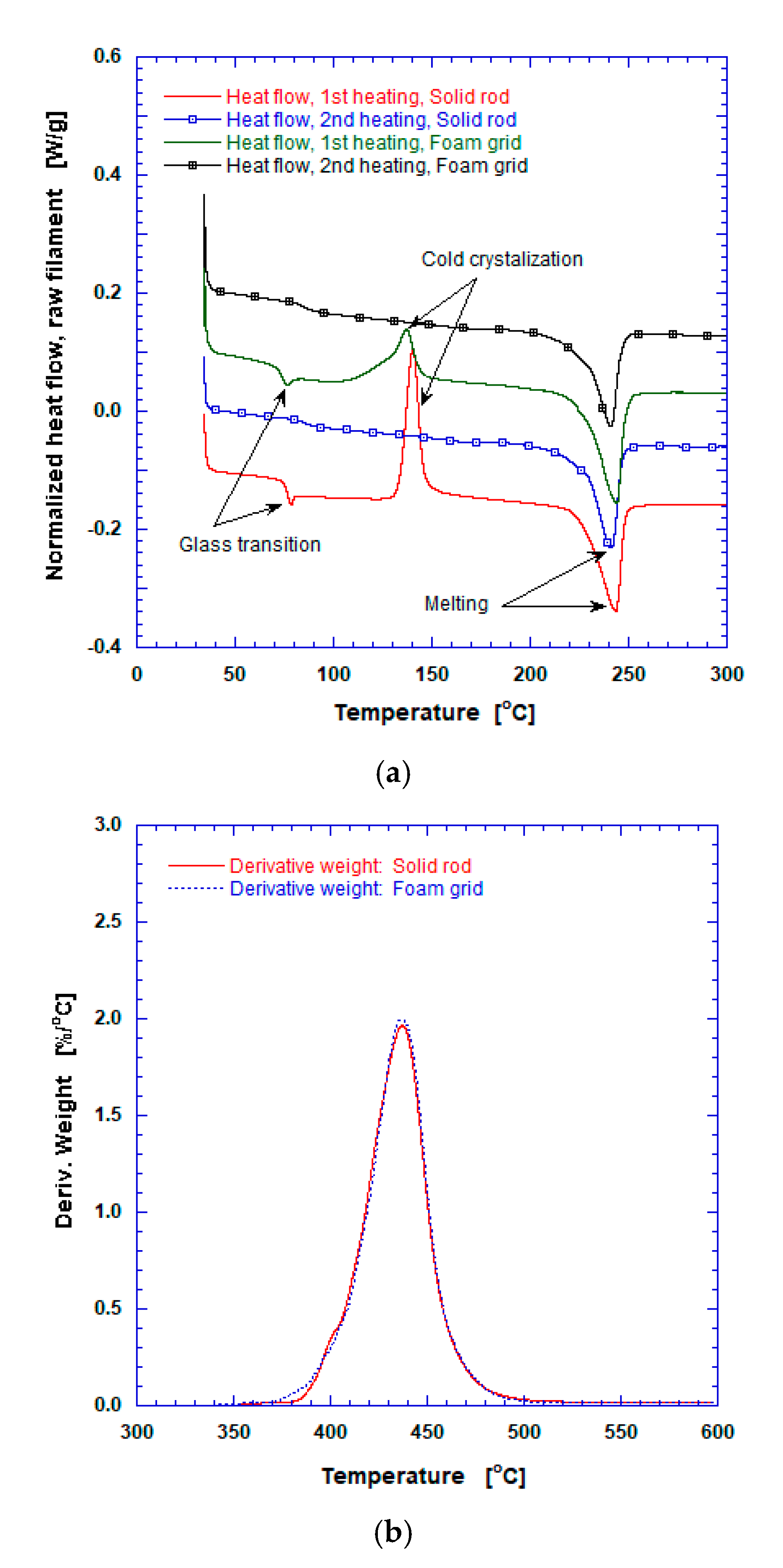
3.2. Calorimetric and Pyrolysis Analyses
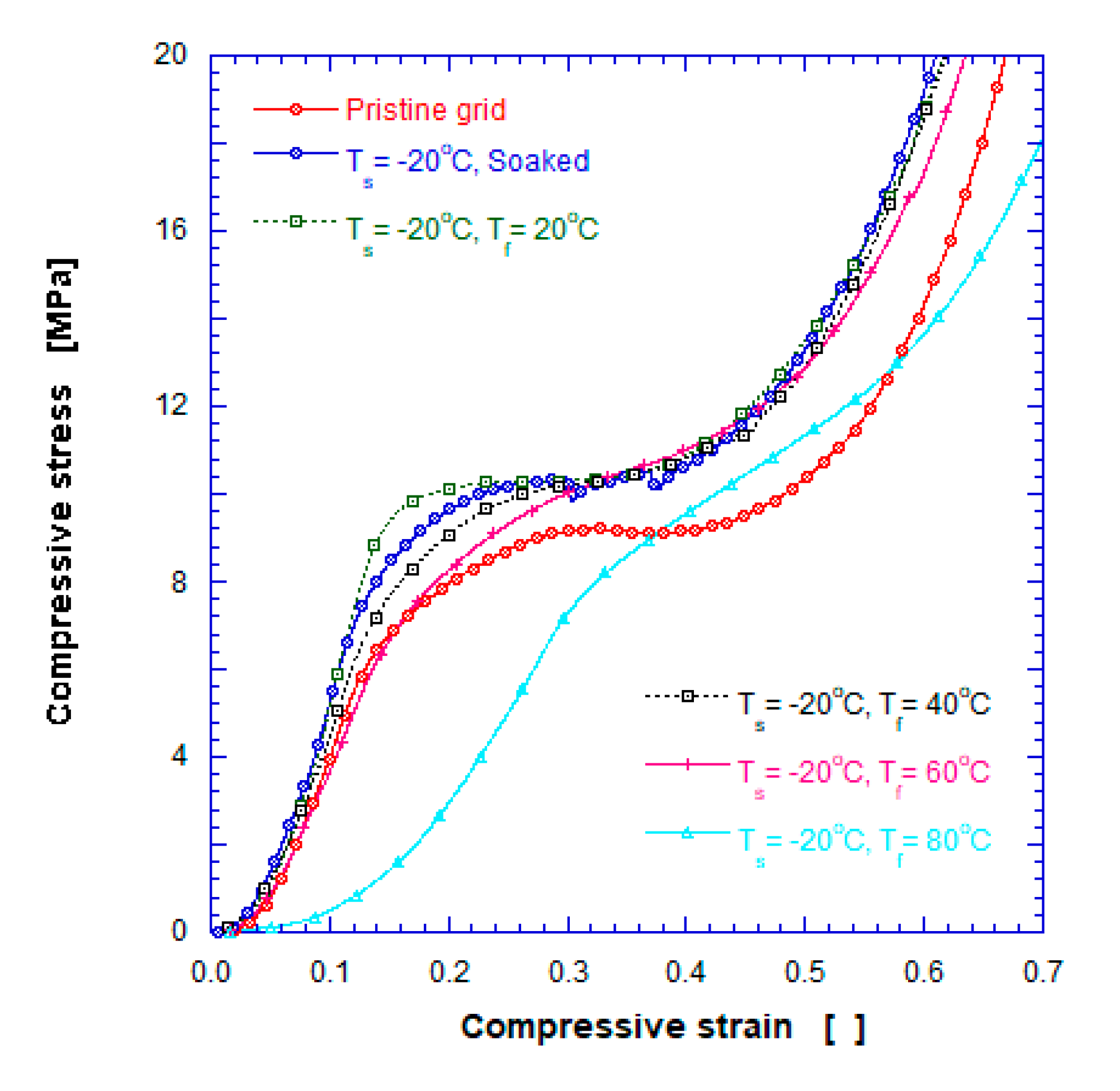
3.3. Mechanical Compressive Stress
3.4. Thermal Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, S.T. Polymeric Foams: Innovations in Process, Technologies and Products; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Maio, E.D.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Martini, J.E.; Waldman, F.A.; Suh, N.P. The Production and analysis of microcellular thermoplastic foams. SPE Tech. Pap. 1982, 28, 674. [Google Scholar]
- Guo, H.; Kumar, V. Some thermodynamic and kinetic low-temperature properties of the PC-CO2 system and morphological characteristics of solid-state PC nanofoams produced with liquid CO2. Polymer 2015, 56, 46–56. [Google Scholar] [CrossRef]
- Xia, T.; Xi, Z.; Tao Liu, T.; Zhao, L. Solid state foaming of poly(ethylene terephthalate) based on periodical CO2-renewing sorption process. Chem. Eng. Sci. 2017, 168, 124–136. [Google Scholar] [CrossRef]
- Yu, J.; Song, L.; Chen, F.; Fan, P.; Sun, L.; Zhong, M.; Yang, J. Preparation of polymer foams with a gradient of cell size: Further exploring the nucleation effect of porous inorganic materials in polymer foaming. Mater. Today Commun. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Kiran, E. Morphological modifications and formation of morphologically-gradient polymers in dense fluids. In Proceedings of the 5th International Symposium on High Pressure Process Technology and Chemical Engineering, Segovia, Spain, 24–27 June 2007; pp. 1–10. [Google Scholar]
- Forest, C.; Chaumont, P.; Cassagnaua, P.; Swoboda, B.; Sonntag, P. Polymer nano-foams for insulating applications prepared from CO2 foaming. Prog. Polym. Sci. 2015, 39, 1721–1741. [Google Scholar] [CrossRef]
- Park, B.K.; Hwang, D.J.; Kwon, D.E.; Yoon, T.J.; Lee, Y.-W. Fabrication and Characterization of Multiscale PLA Structures Using Integrated Rapid Prototyping and Gas Foaming Technologies. Nanomaterials 2018, 8, 575. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, P.; Li, W. Fabrication of functionally graded porous polymer via supercritical carbon dioxide foaming. Compos. Part B 2011, 42, 318–325. [Google Scholar] [CrossRef]
- Yoon, T.J.; Kong, W.; Kwon, D.E.; Park, B.K.; Lee, Y.-W. Preparation of solid-state micro- and nanocellular acrylonitrile-butadiene-styrene (ABS) foams using sub- and supercritical CO2 as blowing agents. J. Supercrit. Fluids 2017, 124, 30–37. [Google Scholar] [CrossRef]
- Ono, T.; Wu, X.; Horiuchi, S.; Furuya, T.; Yoda, T. Two-step foaming process for production of PMMA nanocellular polymer foams via ultra-high pressure and rapid depressurization. J. Supercrit. Fluids 2020, 165, 104963. [Google Scholar] [CrossRef]
- Martin-de Leon, J.; Bernardo, V.; Rodriguez-Perez, M.A. Key production parameters to obtain transparent nanocellular PMMA. Macromol. Mater. Eng. 2017, 302, 1700343. [Google Scholar] [CrossRef]
- Kakumanu, V.; Sundarram, S.S. Dual pore network polymer foams for biomedical applications via combined solid state foaming and additive manufacturing. Mater. Lett. 2018, 213, 366–369. [Google Scholar] [CrossRef]
- Jelle, B.P. Traditional, state-of-the-art and future thermal building insulation materials and solutions—Properties, requirements and possibilities. Energy Build. 2011, 43, 2549–2563. [Google Scholar] [CrossRef]
- Choi, B.; Yeo, I.; Lee, J.; Kang, W.K.; Song, T.H. Pillar-supported vacuum insulation panel with multi-layered filler material. Int. J. Heat Mass Transf. 2016, 102, 902–910. [Google Scholar] [CrossRef]
- Kwon, J.S.; Jang, C.H.; Jung, H.; Song, T.H. Vacuum maintenance in vacuum insulation panels exemplified with a staggered beam VIP. Energy Build. 2010, 42, 590–597. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, C.-J.; Lee, Y.W. Characterization of Gradient Foam Using Additive Manufacturing and Gas Foaming Technologies. In Proceedings of the 4th International Conference on Manufacturing Technologies (ICMT 2020), Seattle, DC, USA, 17–20 January 2020; Abstract MC20-321-A. Available online: http://icmt.org/icmt2020.html (accessed on 16 October 2020).
- Nofar, M.; Ameli, A.; Park, C.B. Development of polylactide bead foams with double crystal melting peaks. Polymer 2015, 69, 83–94. [Google Scholar] [CrossRef]
- ISO (the International Organization for Standardization). ISO 22007-2:2015, Plastics—Determination of thermal conductivity and thermal diffusivity—Part 2: Transient plane heat source (hot disc) method. 2015. [Google Scholar]
- Gustafsson, S.E. Transient plane source techniques for thermal conductivity and thermal diffusivity measurements of solid materials. Rev. Sci. Instrum. 1991, 62, 797–804. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, B.K.; Kim, C.-J.; Kwon, D.E.; Lee, Y.-W. Design and Fabrication of Partially Foamed Grid Structure Using Additive Manufacturing and Solid State Foaming. Processes 2020, 8, 1594. https://doi.org/10.3390/pr8121594
Park BK, Kim C-J, Kwon DE, Lee Y-W. Design and Fabrication of Partially Foamed Grid Structure Using Additive Manufacturing and Solid State Foaming. Processes. 2020; 8(12):1594. https://doi.org/10.3390/pr8121594
Chicago/Turabian StylePark, Byung Kyu, Charn-Jung Kim, Dong Eui Kwon, and Youn-Woo Lee. 2020. "Design and Fabrication of Partially Foamed Grid Structure Using Additive Manufacturing and Solid State Foaming" Processes 8, no. 12: 1594. https://doi.org/10.3390/pr8121594
APA StylePark, B. K., Kim, C.-J., Kwon, D. E., & Lee, Y.-W. (2020). Design and Fabrication of Partially Foamed Grid Structure Using Additive Manufacturing and Solid State Foaming. Processes, 8(12), 1594. https://doi.org/10.3390/pr8121594




