A Review of Process Systems Engineering (PSE) Tools for the Design of Ionic Liquids and Integrated Biorefineries
Abstract
:1. Introduction
2. Applications of PSE in the Development of New and Green Chemicals
2.1. Ionic Liquids
2.2. Potential Applications of Ionic Liquids
2.3. Challenges in the Design of Optimal Ionic Liquids
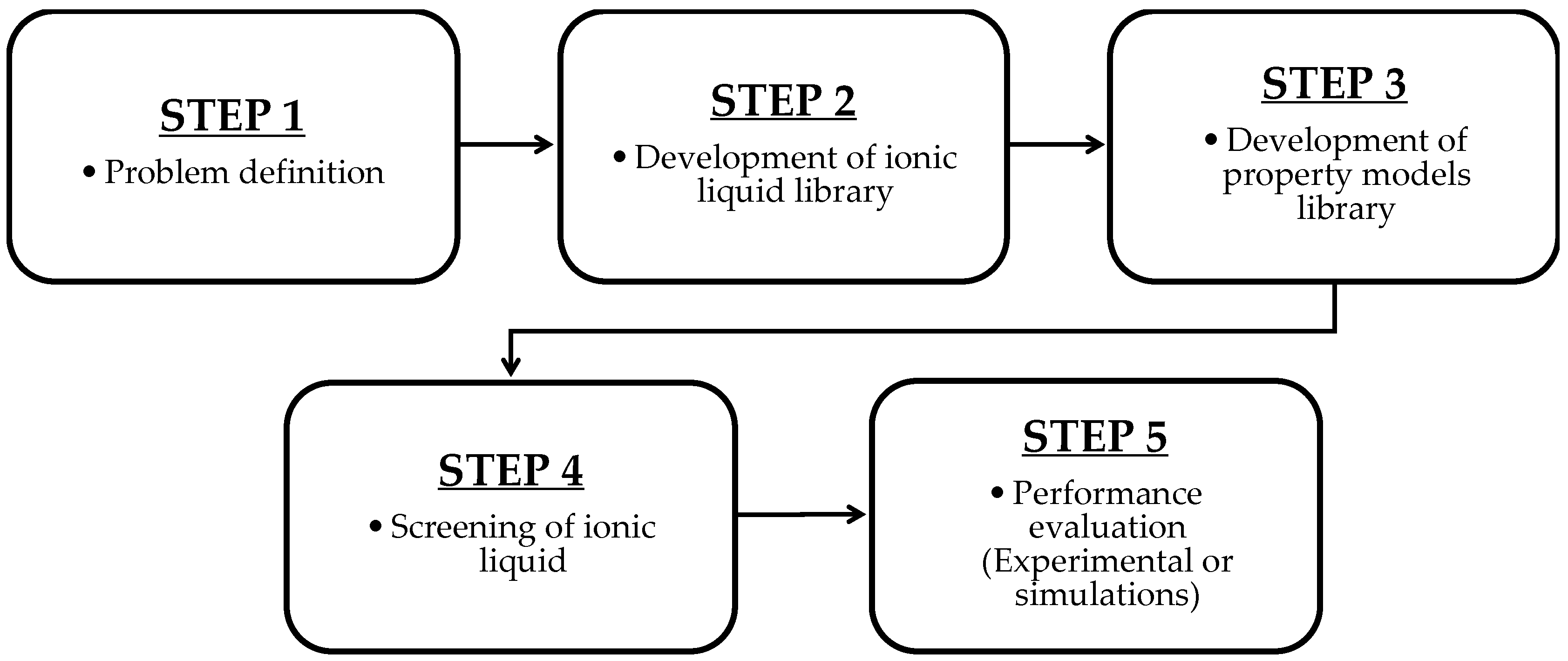
2.4. CAMD for Ionic Liquid Design
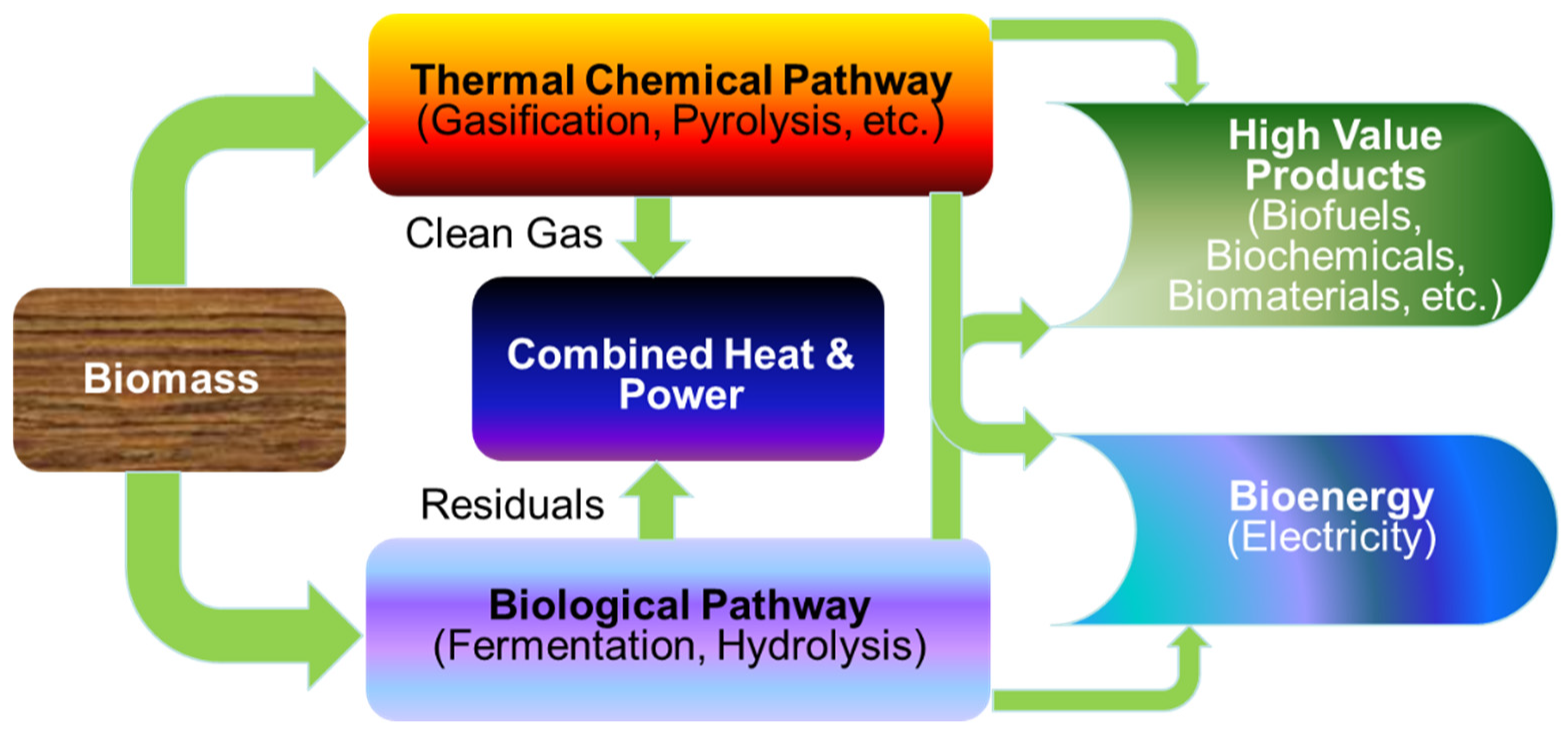
3. Applications of PSE in Integrated Biorefineries
3.1. Introduction to Integrated Biorefineries/Types of Integrated Biorefineries
3.1.1. Physical/Mechanical Processes
3.1.2. Thermochemical Processes
3.1.3. Chemical Processes
3.1.4. Biochemical/Biological Processes
3.2. Synthesis and Design of Integrated Biorefineries
3.2.1. Hierarchical Approaches
3.2.2. Heuristic Searches
3.2.3. Insight-Based Approaches
3.2.4. Algorithmic Approaches
3.2.5. Mathematical Optimization Approaches
3.2.6. Hybrid Methods
3.3. Challenges in Designing Integrated Biorefineries
- Able to minimize energy consumption and potential environmental impact through material and energy integrations between different conversion platforms.
- Able to accommodate the varying seasonal patterns on feedstock availability and quality through integration of different biomass conversion platforms.
- Able to depolymerize biomass components to intermediate products that match the requirements of subsequent processing technologies.
- Able to maximize the yield and quality of value-added products.
4. Application of Molecular Design within the Context of Integrated Biorefineries
4.1. Integrated Tools for Ionic Liquid Design within Integrated Biorefineries
4.2. Opportunities for Further Research
4.2.1. Expanding the Optimization Scope/Parameters for Integrated Biorefinery Design
4.2.2. Design of Novel Ionic Liquids
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| [4bmpy][TCM] | 1-butyl-4-methylpyridinium tricyanomethanide |
| [Bim][NTf2] | 1-butyl imidazolium bis(trifluoromethylsulfonyl)imide |
| [BMim][Cl] | 1-butyl-3-methylimidazolium chloride |
| [BMIM][OAc] | 1-butyl-3-methylimidazolium acetate |
| [Bmim]2[CuCl4] | Bis(1-butyl-3-methyl imidazolium) copper tetrachloride salt |
| [Bmim]2[SnCl4] | Bis(1-butyl-3-methyl imidazolium) stannum tetrachloride salt |
| [EMIM][MS] | 1-Ethyl-3-methylimidazolium methyl sulfate |
| [Emim]Ac | 1-Ethyl-3-methylimidazolium acetate |
| [EMIM]Cl | 1-methylimidazolium chloride |
| [NH2p-bim][BF4] | 1-butyl-3-propylamineimidazolium tetrafluoroborate |
| BMIM-BF4 | 1-butyl-3-methylimidazolium hexafluorophosphate |
| C9H14NBF4 | 1-butylpyridinium tetrafluoroborate |
| CO2 | Carbon dioxide |
| EAN | Ethyl ammonium nitrate |
| H2S | Hydrogen sulfide |
| NH3 | Ammonia |
| SO2 | Sulfur dioxide |
Abbreviations
| ANN | Artificial neural networks |
| ASDI | Absorption selectivity desorption index |
| CAILD | Computer-aided ionic liquid design |
| CAMD | Computer-aided molecular design |
| CHP | Combined heat and power |
| COSMO | Conductor-like screening model |
| COSMO-RS | COSMO for real solvents |
| COSMO-SAC | COSMO for segment activity coefficient |
| DES | Deep eutectic solvent |
| DFT | Density functional theory |
| EOS | Equations of state |
| FT | Fischer-Tropsch |
| GBM | Gradient boosted regression |
| GC | Group contribution |
| GC-COSMO | Group contribution-based COSMO |
| IDAC | Infinite dilution activity coefficient |
| IL | Ionic liquid |
| LCA | Life cycle assessment |
| LLE | Liquid-liquid extraction |
| LSSVM | Least-squared support vector machine |
| MC | Monte Carlo simulations |
| MD | Molecular dynamics simulations |
| MILP | Mixed-integer linear program |
| MINLP | Mixed-integer nonlinear program |
| ML | Machine learning |
| PPMV | Parts per million volume |
| PSE | Process systems engineering |
| QM | Quantum mechanics |
| QSAR | Quantitative structure-activity relationship |
| QSPR | Quantitative structure-property relationship |
| RF | Random forests |
| RHST | Rough hard-sphere theory |
| UNIFAC | Universal quasichemical functional-group activity coefficients |
| ZIF | Zeolitic imidazolate framework |
References
- Grossmann, I.E.; Westerberg, A.W. Research challenges in process systems engineering. AIChE J. 2000, 46, 1700–1703. [Google Scholar] [CrossRef]
- Grossmann, I.E. Challenges in the new millennium: Product discovery and design, enterprise and supply chain optimization, global life cycle assessment. Comput. Aided Chem. Eng. 2004, 29, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Kontogeorgis, G.M.; Woodley, J.M. Group Contribution Based Estimation Method for Properties of Ionic Liquids. Ind. Eng. Chem. Res. 2019, 58, 4277–4292. [Google Scholar] [CrossRef]
- Karunanithi, A.T.; Mehrkesh, A. Computer-aided design of tailor-made ionic liquids. AIChE J. 2013, 59, 4627–4640. [Google Scholar] [CrossRef]
- Austin, N.D.; Sahinidis, N.V.; Trahan, D.W. A COSMO-based approach to computer-aided mixture design. Chem. Eng. Sci. 2017, 159, 93–105. [Google Scholar] [CrossRef]
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current Status, Challenges, and Future Direction. Energy Fuels 2006, 20, 1727–1737. [Google Scholar] [CrossRef]
- Sammons, N.E.; Yuan, W.; Eden, M.R.; Aksoy, B.; Cullinan, H.T. Optimal biorefinery product allocation by combining process and economic modeling. Chem. Eng. Res. Des. 2008, 86, 800–808. [Google Scholar] [CrossRef]
- Tay, D.H.S.; Ng, D.K.; Sammons, N.E.; Eden, M.R. Fuzzy Optimization Approach for the Synthesis of a Sustainable Integrated Biorefinery. Ind. Eng. Chem. Res. 2011, 50, 1652–1665. [Google Scholar] [CrossRef]
- Kokossis, A.; Tsakalova, M.; Pyrgakis, K. Design of integrated biorefineries. Comput. Chem. Eng. 2015, 81, 40–56. [Google Scholar] [CrossRef]
- Voll, P. Automated Optimization-Based Synthesis of Distributed Energy Supply Systems; RWTH Aachen University: Aachen, Germany, 2013. [Google Scholar]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imp. Sci. 1914, 1800, 405–422. [Google Scholar]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Eftekhari, A. Supercapacitors utilising ionic liquids. Energy Storage Mater. 2017, 9, 47–69. [Google Scholar] [CrossRef]
- Morton, M.D.; Hamer, C.K. Ionic liquids—The beginning of the end or the end of the beginning? A look at the life of ionic liquids through patent claims. Sep. Purif. Technol. 2018, 196, 3–9. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, B.; Koo, Y.-M.; Macfarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [Green Version]
- Freemantle, M. Chemistry Basf’s Smart Ionic Liquid. Chem. Eng. News Arch. 2003, 81, 9. [Google Scholar] [CrossRef]
- Kazemi, S.; Nor, M.I.M.; Teoh, W.H. Thermodynamic and economic investigation of an ionic liquid as a new proposed geothermal fluid in different organic Rankine cycles for energy production. Energy 2020, 193, 116722. [Google Scholar] [CrossRef]
- Kermani, N.A.; Petrushina, I.; Rokni, M. Evaluation of ionic liquids as replacements for the solid piston in conventional hydrogen reciprocating compressors: A review. Int. J. Hydrogen Energy 2020, 45, 16337–16354. [Google Scholar] [CrossRef]
- Bian, J.; Peng, F.; Peng, X.-P.; Xiao, X.; Peng, P.; Xu, F.; Sun, R.-C. Effect of [Emim]Ac pretreatment on the structure and enzymatic hydrolysis of sugarcane bagasse cellulose. Carbohydr. Polym. 2014, 100, 211–217. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Ullah, Z.; Nasrullah, A.; Khan, M.I.; Gonfa, G.; Ahmad, P.; Muhammad, N. Kinetics and thermodynamic parameters of ionic liquid pretreated rubber wood biomass. J. Mol. Liq. 2016, 223, 754–762. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, Ł.; Kurc, B.; Skrzypczak, A.; Jesionowski, T. A comparison of protic and aprotic ionic liquids as effective activating agents of kraft lignin. Developing functional MnO2/lignin hybrid materials. J. Mol. Liq. 2018, 261, 456–467. [Google Scholar] [CrossRef]
- Zuo, L.; Ao, X.; Guo, Y. Study on the synthesis of dual-chain ionic liquids and their application in the extraction of flavonoids. J. Chromatogr. A 2020, 1628, 461446. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Cao, X.-J. Extracting keratin from chicken feathers by using a hydrophobic ionic liquid. Process. Biochem. 2012, 47, 896–899. [Google Scholar] [CrossRef]
- Zhu, Z.; Ri, Y.; Jia, H.; Li, X.; Wang, Y.; Wang, Y. Process evaluation on the separation of ethyl acetate and ethanol using extractive distillation with ionic liquid. Sep. Purif. Technol. 2017, 181, 44–52. [Google Scholar] [CrossRef]
- Ayuso, M.; Cañada-Barcala, A.; Larriba, M.; Navarro, P.; Delgado-Mellado, N.; García, J.; Rodríguez, F. Enhanced separation of benzene and cyclohexane by homogeneous extractive distillation using ionic liquids as entrainers. Sep. Purif. Technol. 2020, 240, 116583. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Mandal, M.K. Synthesis and characterization of ionic liquid based mixed matrix membrane for acid gas separation. J. Clean. Prod. 2017, 156, 174–183. [Google Scholar] [CrossRef]
- Lei, Z.; Dai, C.; Song, W. Adsorptive absorption: A preliminary experimental and modeling study on CO2 solubility. Chem. Eng. Sci. 2015, 127, 260–268. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, W.; Xiao, J.; Wei, X.-H. Absorption of Sulfur Dioxide by Tetraglyme–Sodium Salt Ionic Liquid. Mol. 2019, 24, 436. [Google Scholar] [CrossRef] [Green Version]
- Shang, D.; Zhang, S.; Zeng, S.; Jiang, K.; Gao, H.; Dong, H.; Yang, Q.; Zhang, S. Protic ionic liquid [Bim][NTf2] with strong hydrogen bond donating ability for highly efficient ammonia absorption. Green Chem. 2017, 19, 937–945. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, S.; Huo, F.; Shang, D.; He, H.; Bai, L.; Zhang, X.; Li, J. Metal chloride anion-based ionic liquids for efficient separation of NH3. J. Clean. Prod. 2019, 206, 661–669. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Maginn, E.J. Ionic liquids: Innovative fluids for chemical processing. AIChE J. 2001, 47, 2384–2389. [Google Scholar] [CrossRef]
- Fan, Y.; Dong, X.; Zhong, Y.; Li, J.; Miao, J.; Hua, S.; Li, Y.; Cheng, B.; Chen, W. Effects of ionic liquids on the hydrolysis of casein by lumbrokinase. Biochem. Eng. J. 2016, 109, 35–42. [Google Scholar] [CrossRef]
- Francą, J.M.P.; De Castro, C.A.N.; Lopes, M.M.; Nunes, V.M.B. Influence of Thermophysical Properties of Ionic Liquids in Chemical Process Design. J. Chem. Eng. Data 2009, 54, 2569–2575. [Google Scholar]
- Predel, T.; Schlücker, E. Ionic Liquids in Oxygen Compression. Chem. Eng. Technol. 2009, 32, 1183–1188. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mohammadpourfard, M.; Heris, S. Ionic liquids: Promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des. 2020, 160, 264–300. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Svinyarov, I. Ionic liquid-supported solid–liquid extraction of bioactive alkaloids. II. Kinetics, modeling and mechanism of glaucine extraction from Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2013, 103, 279–288. [Google Scholar] [CrossRef]
- Seiler, M.; Jork, C.; Kavarnou, A.; Arlt, W.; Hirsch, R. Separation of azeotropic mixtures using hyperbranched polymers or ionic liquids. AIChE J. 2004, 50, 2439–2454. [Google Scholar] [CrossRef]
- Boli, E.; Voutsas, E. Ionic liquids as entrainers for the separation of azeotropic mixtures: Experimental measurements and COSMO-RS predictions. Chem. Eng. Sci. 2020, 219, 115579. [Google Scholar] [CrossRef]
- Fadeev, A.G.; Meagher, M.M. Opportunities for ionic liquids in recovery of biofuels. Chem. Commun. 2001, 3, 295–296. [Google Scholar] [CrossRef]
- Anthony, J.L.; Maginn, A.E.J.; Brennecke, J.F. Solution Thermodynamics of Imidazolium-Based Ionic Liquids and Water. J. Phys. Chem. B 2001, 105, 10942–10949. [Google Scholar] [CrossRef]
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Gas Solubilities in 1-n-Butyl-3-methylimidazolium Hexafluorophosphate. ACS Symp. Ser. 2002, 818, 260–269. [Google Scholar]
- Xue, Z.; Zhang, Z.; Han, J.; Chen, Y.; Mu, T. Carbon dioxide capture by a dual amino ionic liquid with amino-functionalized imidazolium cation and taurine anion. Int. J. Greenh. Control. 2011, 5, 628–633. [Google Scholar] [CrossRef]
- Chen, H.; He, Y.; Zhu, J.; Alias, H.; Ding, Y.; Nancarrow, P.; Hardacre, C.; Rooney, D.W.; Tan, C. Rheological and heat transfer behaviour of the ionic liquid, [C4mim] [NTf2]. Int. J. Heat Fluid Flow 2008, 29, 149–155. [Google Scholar] [CrossRef]
- Dong, Q.; Muzny, C.D.; Kazakov, A.; Diky, V.; Magee, J.W.; Widegren, J.A.; Chirico, R.D.; Marsh, K.N.; Frenkel, M. ILThermo: A Free-Access Web Database for Thermodynamic Properties of Ionic Liquids. J. Chem. Eng. Data 2007, 52, 1151–1159. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Mulero, A.; Alavianmehr, M.M. Predictive methods and semi-classical Equations of State for pure ionic liquids: A review. J. Chem. Thermodyn. 2019, 130, 47–94. [Google Scholar] [CrossRef]
- Coutinho, J.A.P.; Carvalho, P.J.; Oliveira, N.M. Predictive methods for the estimation of thermophysical properties of ionic liquids. RSC Adv. 2012, 2, 7322–7346. [Google Scholar] [CrossRef]
- Torrecilla, J.S.; García, J.; Rojo, E.; Rodríguez, F. Estimation of toxicity of ionic liquids in Leukemia Rat Cell Line and Acetylcholinesterase enzyme by principal component analysis, neural networks and multiple lineal regressions. J. Hazard. Mater. 2009, 164, 182–194. [Google Scholar] [CrossRef]
- Abramenko, N.; Kustov, L.M.; Metelytsia, L.; Kovalishyn, V.; Tetko, I.; Peijnenburg, W. A review of recent advances towards the development of QSAR models for toxicity assessment of ionic liquids. J. Hazard. Mater. 2020, 384, 121429. [Google Scholar] [CrossRef]
- Taghizadehfard, M.; Hosseini, S.M.; Pierantozzi, M.; Alavianmehr, M.M. Predicting the volumetric properties of pure and mixture of amino acid-based ionic liquids. J. Mol. Liq. 2019, 294, 111604. [Google Scholar] [CrossRef]
- Thawarkar, S.; Khupse, N.; Shinde, D.R.; Kumar, A. Understanding the behavior of mixtures of protic-aprotic and protic-protic ionic liquids: Conductivity, viscosity, diffusion coefficient and ionicity. J. Mol. Liq. 2019, 276, 986–994. [Google Scholar] [CrossRef]
- McLeese, S.E.; Eslick, J.C.; Hoffmann, N.J.; Scurto, A.M.; Camarda, K.V. Design of Ionic Liquids via Computational Molecular Design. Comput. Chem. Eng. 2010, 34, 1476–1480. [Google Scholar] [CrossRef]
- Austin, N.D.; Sahinidis, N.V.; Trahan, D.W. Computer-aided molecular design: An introduction and review of tools, applications, and solution techniques. Chem. Eng. Res. Des. 2016, 116, 2–26. [Google Scholar] [CrossRef]
- Chemmangattuvalappil, N.G. Development of solvent design methodologies using computer-aided molecular design tools. Curr. Opin. Chem. Eng. 2020, 27, 51–59. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-like Screening Model for Real Solvents: A New Approach to the Quantitative Calculation of Solvation Phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Lin, S.-T.; Sandler, S.I. A Priori Phase Equilibrium Prediction from a Segment Contribution Solvation Model. Ind. Eng. Chem. Res. 2002, 41, 899–913. [Google Scholar] [CrossRef]
- Farahipour, R.; Mehrkesh, A.; Karunanithi, A.T. A systematic screening methodology towards exploration of ionic liquids for CO2 capture processes. Chem. Eng. Sci. 2016, 145, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Woodley, J.M.; Kontogeorgis, G.; Gani, R. Integrated Ionic Liquid and Process Design involving Hybrid Separation Schemes. Comput.-Aided Chem. Eng. 2018, 44, 1045–1050. [Google Scholar]
- Song, Z.; Zhou, T.; Qi, Z.; Sundmacher, K. Systematic Method for Screening Ionic Liquids as Extraction Solvents Exemplified by an Extractive Desulfurization Process. ACS Sustain. Chem. Eng. 2017, 5, 3382–3389. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, C.; Qi, Z.; Zhou, T.; Sundmacher, K. Computer-aided design of ionic liquids as solvents for extractive desulfurization. AIChE J. 2018, 64, 1013–1025. [Google Scholar] [CrossRef]
- Chao, H.; Song, Z.; Cheng, H.; Chen, L.; Qi, Z. Computer-aided design and process evaluation of ionic liquids for n-hexane-methylcyclopentane extractive distillation. Sep. Purif. Technol. 2018, 196, 157–165. [Google Scholar] [CrossRef]
- Hessel, V.; Li, X.; Chao, H.; Mo, F.; Zhou, T.; Cheng, H.; Chen, L.; Qi, Z. Computer-aided ionic liquid design for alkane/cycloalkane extractive distillation process. Green Energy Environ. 2019, 4, 154–165. [Google Scholar]
- Mai, N.L.; Koo, Y.-M. Computer-Aided Design of Ionic Liquids for High Cellulose Dissolution. ACS Sustain. Chem. Eng. 2016, 4, 541–547. [Google Scholar] [CrossRef]
- Firaha, D.S.; Hollóczki, O.; Kirchner, B. Computer-Aided Design of Ionic Liquids as CO2 Absorbents. Angew. Chem. Int. Ed. 2015, 54, 7805–7809. [Google Scholar] [CrossRef] [PubMed]
- Paduszyński, K.; Królikowski, M.; Zawadzki, M.; Orzeł, P. Computer-Aided Molecular Design of New Task-Specific Ionic Liquids for Extractive Desulfurization of Gasoline. ACS Sustain. Chem. Eng. 2017, 5, 9032–9042. [Google Scholar] [CrossRef]
- Karunanithi, A.T.; Farahipour, R.; Dilmurat, K. Ionic Liquids: Applications by Computational Design. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Wiley: Chichester, UK, 2016; pp. 1–13. [Google Scholar]
- Peng, D.; Zhang, J.; Cheng, H.; Chen, L.; Qi, Z. Computer-aided ionic liquid design for separation processes based on group contribution method and COSMO-SAC model. Chem. Eng. Sci. 2017, 159, 58–68. [Google Scholar] [CrossRef]
- Chen, Y.; Koumaditi, E.; Gani, R.; Kontogeorgis, G.M.; Woodley, J.M. Computer-aided design of ionic liquids for hybrid process schemes. Comput. Chem. Eng. 2019, 130, 106556. [Google Scholar] [CrossRef]
- Venkatraman, V.; Evjen, S.; Lethesh, K.C.; Raj, J.J.; Knuutila, H.K.; Fiksdahl, A. Rapid, comprehensive screening of ionic liquids towards sustainable applications. Sustain. Energy Fuels 2019, 3, 2798–2808. [Google Scholar] [CrossRef]
- Xu, Y.; Hanna, M.A.; Isom, L. “Green” Chemicals from Renewable Agricultural Biomass—A Mini Review. Open Agric. J. 2008, 2, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Kamm, B.; Kamm, M.; Soyez, K. The Green Biorefinery, Concept of Technology. In Proceeding of the first International Symposium on Green Biorefinery, Neuruppin, Germany, October 1997; Society of Ecological Technology and System Analysis: Berlin, Germany, 1998. [Google Scholar]
- Frost, J.W.; Draths, K.M. Biocatalytic Syntheses of Aromatics from D-Glucose: Renewable Microbial Sources of Aromatic Compounds. Annu. Rev. Microbiol. 1995, 49, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Gravitis, J.; Zandersons, J.; Vedernikov, N.; Kruma, I.; Ozols-Kalnins, V. Clustering of bio-products technologies for zero emissions and eco-efficiency. Ind. Crop. Prod. 2004, 20, 169–180. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- NREL. National Renewable Energy Laboratory [Internet]. In Biomass Research.; 2009. Available online: http://www.nrel.gov/biomass/biorefinery.html (accessed on 13 October 2020).
- Huang, H.-J.; Ramaswamy, S.; Tschirner, U.W.; RamaRao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Spath, P.L.; Dayton, D.C. Preliminary Screening—Technical and Economic Assessment of Synthesis Gas to Fuels and Chemicals with Emphasis on the Potential for Biomass-Derived Syngas; National Renewable Energy Laboratory: Golden, CO, USA, 2003. [Google Scholar]
- Bridgwater, A. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Brownsort, P.A. Biomass Pyrolysis Processess: Performance Parameters and Their Influence on Biochar System Benefits; The University of Edinburgh: Edinburgh, UK, 2009. [Google Scholar]
- Senneca, O. Kinetics of pyrolysis, combustion and gasification of three biomass fuels. Fuel Process. Technol. 2007, 88, 87–97. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biorefineries for biofuel upgrading: A critical review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Romano, R.T.; Zhang, R. Co-digestion of onion juice and wastewater sludge using an anaerobic mixed biofilm reactor. Bioresour. Technol. 2008, 99, 631–637. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; van Hooijdonk, G.; Faaij, A.P. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Nishida, N.; Stephanopoulos, G.; Westerberg, A.W. A review of process synthesis. AIChE J. 1981, 27, 321–351. [Google Scholar] [CrossRef]
- Douglas, J.M. Process synthesis for waste minimization. Ind. Eng. Chem. Res. 1992, 31, 238–243. [Google Scholar] [CrossRef]
- Kokossis, A.C.; Yang, A. On the use of systems technologies and a systematic approach for the synthesis and the design of future biorefineries. Comput. Chem. Eng. 2010, 34, 1397–1405. [Google Scholar] [CrossRef] [Green Version]
- Stephanopoulos, G.; Reklaitis, G.V. Process systems engineering: From Solvay to modern bio- and nanotechnology. Chem. Eng. Sci. 2011, 66, 4272–4306. [Google Scholar] [CrossRef]
- Ng, D.K.; Ng, R.T.L. Applications of process system engineering in palm-based biomass processing industry. Curr. Opin. Chem. Eng. 2013, 2, 448–454. [Google Scholar] [CrossRef]
- Douglas, J.M. A hierarchical decision procedure for process synthesis. AIChE J. 1985, 31, 353–362. [Google Scholar] [CrossRef]
- Li, X.; Kraslawski, A. Conceptual process synthesis: Past and current trends. Chem. Eng. Process. Process. Intensif. 2004, 43, 583–594. [Google Scholar] [CrossRef]
- Ng, D.; Pham, V.; Jiménez-Gutiérrez, A.; Spriggs, H. A Hierarchical Approach to the Synthesis and Analysis of Integrated Biorefineries. In Design Energy and the Environment, Proceedings of the Seventh International Conference on the Foundations of Computer-Aided Process Design; CRC Press: Boca Raton, FL, USA, 2009; pp. 425–432. [Google Scholar]
- Conde-Mejía, C.; Jiménez-Gutiérrez, A.; El-Halwagi, M.M. Application of a Hierarchical Approach for the Synthesis of Biorefineries. In Process Design Strategies for Biomass Conversion Systems; Wiley: Chichester, UK, 2015; pp. 39–61. [Google Scholar]
- Tey, S.-Y.; Wong, S.S.; Lam, J.A.; Ong, N.Q.; Foo, D.C.; Ng, D.K. Extended hierarchical decomposition approach for the synthesis of biorefinery processes. Chem. Eng. Res. Des. 2020, 166, 40–54. [Google Scholar] [CrossRef]
- Stephanopoulos, G.; Westerberg, A.W. Studies in process synthesis—II. Chem. Eng. Sci. 1976, 31, 195–204. [Google Scholar] [CrossRef]
- Frangopoulos, C.A.; von Spakovsky, M.R.; Sciubba, E. A brief review of methods for the design and synthesis optimization of energy systems. Int. J. Appl. Thermodyn. 2002, 4, 151–160. [Google Scholar]
- Kasivisvanathan, H.; Tan, R.R.; Ng, D.K.; Aziz, M.K.A.; Foo, D.C. Heuristic framework for the debottlenecking of a palm oil-based integrated biorefinery. Chem. Eng. Res. Des. 2014, 92, 2071–2082. [Google Scholar] [CrossRef]
- Patel, B. A Thermodynamic Targeting Approach for the Synthesis of Sustainable Biorefineries. Comput.-Aided Chem. Eng. 2015, 37, 1283–1288. [Google Scholar]
- Oppenheim, A.V. Study Guide for Discrete-Time Signal Processing; Prentice-Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Benjamin, M.F.D.; Cayamanda, C.D.; Tan, R.R.; Razon, L.F. P-graph approach to criticality analysis in integrated bioenergy systems. Clean Technol. Environ. Policy 2017, 19, 1841–1854. [Google Scholar] [CrossRef]
- Lam, H.L.; Klemeš, J.J.; Varbanov, P.S.; Kravanja, Z. P-Graph Synthesis of Open-Structure Biomass Networks. Ind. Eng. Chem. Res. 2013, 52, 172–180. [Google Scholar] [CrossRef]
- Yeo, J.Y.J.; How, B.S.; Teng, S.Y.; Leong, W.D.; Ng, W.P.; Lim, C.H.; Ngan, S.L.; Sunarso, J.; Lam, H.L. Synthesis of Sustainable Circular Economy in Palm Oil Industry Using Graph-Theoretic Method. Sustainability 2020, 12, 8081. [Google Scholar] [CrossRef]
- Grossmann, I.E. Review of Nonlinear Mixed-Integer and Disjunctive Programming Techniques. Optim. Eng. 2002, 3, 227–252. [Google Scholar] [CrossRef]
- Bao, B.; Ng, D.K.; Tay, D.H.; Jiménez-Gutiérrez, A.; El-Halwagi, M.M. A shortcut method for the preliminary synthesis of process-technology pathways: An optimization approach and application for the conceptual design of integrated biorefineries. Comput. Chem. Eng. 2011, 35, 1374–1383. [Google Scholar] [CrossRef]
- Pham, V.; El-Halwagi, M. Process synthesis and optimization of biorefinery configurations. AIChE J. 2011, 58, 1212–1221. [Google Scholar] [CrossRef] [Green Version]
- Ng, R.T.L.; Ng, D.K. Systematic Approach for Synthesis of Integrated Palm Oil Processing Complex. Part 1: Single Owner. Ind. Eng. Chem. Res. 2013, 52, 10206–10220. [Google Scholar] [CrossRef]
- Yuan, Z.; Eden, M.R. Superstructure optimization of integrated fast pyrolysis-gasification for production of liquid fuels and propylene. AIChE J. 2016, 62, 3155–3176. [Google Scholar] [CrossRef]
- Rizwan, M.; Almansoori, A.; Elkamel, A. An overview on synthesis and design of microalgal biorefinery configurations by employing superstructure-based optimization approach. Energy Syst. 2019, 10, 941–966. [Google Scholar] [CrossRef]
- Fasahati, P.; Wu, W.; Maravelias, C.T. Process synthesis and economic analysis of cyanobacteria biorefineries: A superstructure-based approach. Appl. Energy 2019, 253, 113625. [Google Scholar] [CrossRef]
- Dickson, R.; Liu, J.J. Optimization of seaweed-based biorefinery with zero carbon emissions potential. Comput.-Aided Chem. Eng. 2019, 46, 247–252. [Google Scholar]
- Ramapriya, G.M.; Won, W.; Maravelias, C.T. A superstructure optimization approach for process synthesis under complex reaction networks. Chem. Eng. Res. Des. 2018, 137, 589–608. [Google Scholar] [CrossRef]
- Ng, R.T.L.; Tay, D.H.S.; Ng, D.K. Simultaneous Process Synthesis, Heat and Power Integration in a Sustainable Integrated Biorefinery. Energy Fuels 2012, 26, 7316–7330. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Cheng, C.K. Biorefinery for the Production of Biodiesel, Hydrogen and Synthesis Gas Integrated with CHP from Oil Palm in Malaysia. Chem. Prod. Process Model. 2018, 11, 305–314. [Google Scholar] [CrossRef]
- Pyrgakis, K.; Kokossis, A.C. Total Site Analysis as a Synthesis Model to Select, Optimize and Integrate Processess in Multiple-Product Biorefineries. Chem. Eng. Trans. 2016, 52, 913–918. [Google Scholar]
- Kasivisvanathan, H.; Ng, R.T.; Tay, D.H.; Ng, D.K. Fuzzy optimisation for retrofitting a palm oil mill into a sustainable palm oil-based integrated biorefinery. Chem. Eng. J. 2012, 200, 694–709. [Google Scholar]
- Wan, Y.K.; Sadhukhan, J.; Ng, K.S.; Ng, D.K. Techno-economic evaluations for feasibility of sago-based biorefinery, Part 1: Alternative energy systems. Chem. Eng. Res. Des. 2016, 107, 263–279. [Google Scholar] [CrossRef]
- Wan, Y.K.; Sadhukhan, J.; Ng, D.K. Techno-economic evaluations for feasibility of sago-based biorefinery, Part 2: Integrated bioethanol production and energy systems. Chem. Eng. Res. Des. 2016, 107, 102–116. [Google Scholar] [CrossRef]
- Mongkhonsiri, G.; Charoensuppanimit, P.; Anantpinijwatna, A.; Gani, R.; Assabumrungrat, S. Process development of sustainable biorefinery system integrated into the existing pulping process. J. Clean. Prod. 2020, 255, 120278. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Mustafa, M.A.; Misailidis, N.; Mateos-Salvador, F.; Du, C.; Campbell, G.M. Value analysis tool for feasibility studies of biorefineries integrated with value added production. Chem. Eng. Sci. 2008, 63, 503–519. [Google Scholar] [CrossRef] [Green Version]
- Ponce-Ortega, J.M.; Pham, V.; El-Halwagi, M.M.; El-Baz, A.A. A Disjunctive Programming Formulation for the Optimal Design of Biorefinery Configurations. Ind. Eng. Chem. Res. 2012, 51, 3381–3400. [Google Scholar] [CrossRef]
- Tay, D.H.S.; Ng, R.T.; Ng, D.K. Modular Optimization Approach for Process Synthesis and Integration of an Integrated Biorefinery. Comput. Aided Chem. Eng. 2012, 31, 1045–1049. [Google Scholar]
- Ng, R.T.L.; Hassim, M.H.; Ng, D.K. Process synthesis and optimization of a sustainable integrated biorefinery via fuzzy optimization. AIChE J. 2013, 59, 4212–4227. [Google Scholar] [CrossRef]
- Kasivisvanathan, H.; Ubando, A.T.; Ng, D.K.; Tan, R.R. Robust Optimization for Process Synthesis and Design of Multifunctional Energy Systems with Uncertainties. Ind. Eng. Chem. Res. 2014, 53, 3196–3209. [Google Scholar] [CrossRef]
- Kelloway, A.; Daoutidis, P. Process Synthesis of Biorefineries: Optimization of Biomass Conversion to Fuels and Chemicals. Ind. Eng. Chem. Res. 2014, 53, 5261–5273. [Google Scholar] [CrossRef]
- Kasivisvanathan, H.; Ng, D.K.; Poplewski, G.; Tan, R.R. Flexibility Optimization for a Palm Oil-Based Integrated Biorefinery with Demand Uncertainties. Ind. Eng. Chem. Res. 2016, 55, 4035–4044. [Google Scholar] [CrossRef]
- Albarelli, J.Q.; Onorati, S.; Caliandro, P.; Peduzzi, E.; Meireles, M.A.A.; Marechal, F.; Ensinas, A.V. Multi-objective optimization of a sugarcane biorefinery for integrated ethanol and methanol production. Energy 2017, 138, 1281–1290. [Google Scholar] [CrossRef]
- Sy, C.L.; Ubando, A.T.; Aviso, K.B.; Tan, R.R. Multi-objective target oriented robust optimization for the design of an integrated biorefinery. J. Clean. Prod. 2018, 170, 496–509. [Google Scholar] [CrossRef]
- Martin, M.; Grossmann, I.E. On the Systematic Synthesis of Sustainable Biorefineries. Ind. Eng. Chem. Res. 2013, 52, 3044–3064. [Google Scholar] [CrossRef] [Green Version]
- Caballero, J.A.; Odjo, A.; Grossmann, I.E. Flowsheet optimization with complex cost and size functions using process simulators. AIChE J. 2007, 53, 2351–2366. [Google Scholar] [CrossRef]
- Ng, D.K.; Foo, D.C.; Tan, R.R. Automated Targeting Technique for Single-Impurity Resource Conservation Networks. Part 1: Direct Reuse/Recycle. Ind. Eng. Chem. Res. 2009, 48, 7637–7646. [Google Scholar] [CrossRef]
- Ng, D.K.; Foo, D.C.; Tan, R.R. Automated Targeting Technique for Single-Impurity Resource Conservation Networks. Part 2: Single-Pass and Partitioning Waste-Interception Systems. Ind. Eng. Chem. Res. 2009, 48, 7647–7661. [Google Scholar] [CrossRef]
- Ng, D.K.; Foo, D.C.; Tan, R.R.; Pau, C.H.; Tan, Y.L. Automated targeting for conventional and bilateral property-based resource conservation network. Chem. Eng. J. 2009, 149, 87–101. [Google Scholar] [CrossRef]
- Ng, D.K. Automated targeting for the synthesis of an integrated biorefinery. Chem. Eng. J. 2010, 162, 67–74. [Google Scholar] [CrossRef]
- Tay, D.H.S.; Ng, D.K.S. Automated Targeting for the Synthesis of an Integrated Biorefinery. J. Clean. Prod. 2012, 34, 38–48. [Google Scholar] [CrossRef]
- Shabbir, Z.; Tay, D.H.S.; Ng, D.K. A hybrid optimisation model for the synthesis of sustainable gasification-based integrated biorefinery. Chem. Eng. Res. Des. 2012, 90, 1568–1581. [Google Scholar] [CrossRef]
- Koukios, E.; Koullas, D.; Koukios, I.D.; Avgerinos, E. Critical parameters for optimal biomass refineries: The case of biohydrogen. Clean Technol. Environ. Policy 2010, 12, 147–151. [Google Scholar] [CrossRef]
- Fernando, A.L.; Duarte, M.P.; Almeida, J.; Boléo, S.; Mendes, B. Environmental impact assessment of energy crops cultivation in Europe. Biofuels Bioprod. Biorefining 2010, 4, 594–604. [Google Scholar] [CrossRef]
- Andiappan, V.; Ko, A.S.Y.; Lau, V.W.S.; Ng, L.Y.; Ng, R.T.L.; Chemmangattuvalappil, N.G.; Ng, D.K. Synthesis of sustainable integrated biorefinery via reaction pathway synthesis: Economic, incremental enviromental burden and energy assessment with multiobjective optimization. AIChE J. 2015, 61, 132–146. [Google Scholar] [CrossRef]
- Tey, T.O.; Chen, S.; Cheong, Z.X.; Choong, A.S.X.; Ng, L.Y.; Chemmangattuvalappil, N.G. Synthesis of a sustainable integrated biorefinery to produce value-added chemicals from palm-based biomass via mathematical optimisation. Sustain. Prod. Consum. 2020, 26, 288–315. [Google Scholar] [CrossRef]
- Filho, J.F.S.D.C.; Romano, P.N.; De Almeida, J.M.A.R.; Sousa-Aguiar, E.F. Critical catalytic routes: From the conventional bioethanol production model toward the integrated biorefinery concept. Curr. Opin. Green Sustain. Chem. 2019, 20, 33–38. [Google Scholar] [CrossRef]
- Tang, M.C.; Chin, M.W.S.; Lim, K.M.; Mun, Y.S.; Ng, R.T.L.; Tay, D.H.S.; Ng, D.K. Systematic approach for conceptual design of an integrated biorefinery with uncertainties. Clean Technol. Environ. Policy 2013, 15, 783–799. [Google Scholar] [CrossRef]
- Tay, D.H.S.; Ng, D.K.; Tan, R.R. Robust optimization approach for synthesis of integrated biorefineries with supply and demand uncertainties. Environ. Prog. Sustain. Energy 2013, 32, 384–389. [Google Scholar] [CrossRef]
- Cheali, P.; Quaglia, A.; Gernaey, K.V.; Sin, G. Effect of Market Price Uncertainties on the Design of Optimal Biorefinery Systems—A Systematic Approach. Ind. Eng. Chem. Res. 2014, 53, 6021–6032. [Google Scholar] [CrossRef]
- Giuliano, A.; Poletto, M.; Barletta, D. Process optimization of a multi-product biorefinery: The effect of biomass seasonality. Chem. Eng. Res. Des. 2016, 107, 236–252. [Google Scholar] [CrossRef]
- Čuček, L.; Martín, M.; Grossmann, I.E.; Kravanja, Z. Multi-period synthesis of optimally integrated biomass and bioenergy supply network. Comput. Chem. Eng. 2014, 66, 57–70. [Google Scholar] [CrossRef]
- Awudu, I.; Zhang, J. Uncertainties and sustainability concepts in biofuel supply chain management: A review. Renew. Sustain. Energy Rev. 2012, 16, 1359–1368. [Google Scholar] [CrossRef]
- Shabani, N.; Akhtari, S.; Sowlati, T. Value chain optimization of forest biomass for bioenergy production: A review. Renew. Sustain. Energy Rev. 2013, 23, 299–311. [Google Scholar] [CrossRef]
- Cambero, C.; Sowlati, T. Assessment and optimization of forest biomass supply chains from economic, social and environmental perspectives—A review of literature. Renew. Sustain. Energy Rev. 2014, 36, 62–73. [Google Scholar] [CrossRef]
- De Meyer, A.; Cattrysse, D.; Rasinmäki, J.; Van Orshoven, J. Methods to optimise the design and management of biomass-for-bioenergy supply chains: A review. Renew. Sustain. Energy Rev. 2014, 31, 657–670. [Google Scholar] [CrossRef] [Green Version]
- Atashbar, N.Z.; Labadie, N.; Prins, C. Modelling and optimisation of biomass supply chains: A review. Int. J. Prod. Res. 2018, 56, 3482–3506. [Google Scholar] [CrossRef]
- Mirkouei, A.; Haapala, K.R.; Sessions, J.; Murthy, G.S. A review and future directions in techno-economic modeling and optimization of upstream forest biomass to bio-oil supply chains. Renew. Sustain. Energy Rev. 2017, 67, 15–35. [Google Scholar] [CrossRef]
- Stuart, P.R.; El-Halwagi, M.M. Integrated Biorefineries: Design, Analysis, and Optimization, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; p. 873. [Google Scholar]
- Lo, S.L.Y.; How, B.S.; Leong, W.D.; Teng, S.Y.; Rhamdhani, M.A.; Sunarso, J. Techno-economic analysis for biomass supply chain: A state-of-the-art review. Renew. Sustain. Energy Rev. 2020, 135, 110164. [Google Scholar] [CrossRef]
- Ng, R.T.L.; Ng, D.K.; Tan, R.R. Systematic Approach for Synthesis of Integrated Palm Oil Processing Complex. Part 2: Multiple Owners. Ind. Eng. Chem. Res. 2013, 52, 10221–10235. [Google Scholar] [CrossRef]
- Ng, R.T.L.; Ng, D.K.; Tan, R.R.; El-Halwagi, M.M. Disjunctive fuzzy optimisation for planning and synthesis of bioenergy-based industrial symbiosis system. J. Environ. Chem. Eng. 2014, 2, 652–664. [Google Scholar] [CrossRef]
- Barla, F.; Nikolakopoulos, A.; Kokossis, A.C. Design of Circular Economy Plants—The Case of the Textile Waste Biorefinery. Comput. Aided Chem. Eng. 2017, 1933–1938. [Google Scholar]
- Lichtenthaler, F.W.; Mondel, S. Perspectives in the use of low molecular weight carbohydrates as organic raw materials. Pure Appl. Chem. 1997, 69, 1853–1866. [Google Scholar] [CrossRef] [Green Version]
- Wilpiszewska, K.; Spychaj, T. Chemical modification of starch with hexamethylene diisocyanate derivatives. Carbohydr. Polym. 2007, 70, 334–340. [Google Scholar] [CrossRef]
- Wyman, C.E. Potential Synergies and Challenges in Refining Cellulosic Biomass to Fuels, Chemicals, and Power. Biotechnol. Prog. 2003, 19, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Mao, Z.; Li, Y.; Wan, C.; Wang, T.; Zhang, L.; Zhang, L. Liquefaction of crop residues for polyol production. Bioresource 2006, 1, 248–256. [Google Scholar] [CrossRef]
- Elliott, D.C. Biomass, Chemicals from. In Encyclopedia of Energy; Elsevier B.V.: Amsterdam, The Netherlands, 2004; pp. 163–174. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass—Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S Department of Energy: Washington, DC, USA, 2004.
- Holladay, J.; Bozell, J.; White, J.; Johnson, D. Top Value-Added Chemicals from Biomass—Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; U.S. Department of Energy: Washington, DC, USA, 2007.
- Skibar, W.; Grogan, G.; McDonald, J.; Pitts, M. UK Expertise for Exploitation of Biomass-Based Platform Chemicals; The FROPTOP Group: Runcorn, UK, 2009. [Google Scholar]
- Achenie, L.E.K.; Gani, R.; Venkatasubramanian, V. Computer Aided Molecular Design: Theory and Practice, 1st ed.; Elsevier, B.V.: Amsterdam, The Netherlands, 2003; p. 404. [Google Scholar]
- Venkatasubramanian, V.; Chan, K.; Caruthers, J.M. Computer-aided molecular design using genetic algorithms. Comput. Chem. Eng. 1994, 18, 833–844. [Google Scholar] [CrossRef]
- Samudra, A.P.; Sahinidis, N.V. Optimization-based framework for computer-aided molecular design. AIChE J. 2013, 59, 3686–3701. [Google Scholar] [CrossRef]
- Hechinger, M.; Voll, A.; Marquardt, W. Towards an integrated design of biofuels and their production pathways. Comput. Chem. Eng. 2010, 34, 1909–1918. [Google Scholar] [CrossRef]
- Ng, L.Y.; Andiappan, V.; Chemmangattuvalappil, N.G.; Ng, D.K. Novel Methodology for the Synthesis of Optimal Biochemicals in Integrated Biorefineries via Inverse Design Techniques. Ind. Eng. Chem. Res. 2015, 54, 5722–5735. [Google Scholar] [CrossRef]
- Ng, L.Y.; Andiappan, V.; Chemmangattuvalappil, N.G.; Ng, D.K. A systematic methodology for optimal mixture design in an integrated biorefinery. Comput. Chem. Eng. 2015, 81, 288–309. [Google Scholar] [CrossRef]
- Bertran, M.-O.; Frauzem, R.; Sanchez-Arcilla, A.-S.; Zhang, L.; Woodley, J.M.; Gani, R. A generic methodology for processing route synthesis and design based on superstructure optimization. Comput. Chem. Eng. 2017, 106, 892–910. [Google Scholar] [CrossRef]
- Chaávez-Islas, L.M.; Vasquez-Medrano, R.; Flores-Tlacuahuac, A. Optimal molecular design of ionic liquids for high-purity bioethanol production. Ind. Eng. Chem. Res. 2011, 50, 5153–5168. [Google Scholar]
- Chong, F.K.; Foo, D.C.; Eljack, F.; Atilhan, M.; Chemmangattuvalappil, N.G. A systematic approach to design task-specific ionic liquids and their optimal operating conditions. Mol. Syst. Des. Eng. 2016, 1, 109–121. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Zhao, Y.; Gani, R.; Zhang, X.; Zhang, S. Ionic Liquid Design and Process Simulation for Decarbonization of Shale Gas. Ind. Eng. Chem. Res. 2016, 55, 5931–5944. [Google Scholar] [CrossRef] [Green Version]
- Chong, F.K.; Andiappan, V.; Ng, D.K.; Foo, D.C.; Eljack, F.; Atilhan, M.; Chemmangattuvalappil, N.G. Design of Ionic Liquid as Carbon Capture Solvent for a Bioenergy System: Integration of Bioenergy and Carbon Capture Systems. ACS Sustain. Chem. Eng. 2017, 5, 5241–5252. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, T.; Zhang, X.; Zhang, S.; Liang, X.; Gani, R.; Kontogeorgis, G.M. Application of COSMO-RS and UNIFAC for ionic liquids based gas separation. Chem. Eng. Sci. 2018, 192, 816–828. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Kontogeorgis, G.M.; Woodley, J.M. Ionic-Liquid-Based Bioisoprene Recovery Process Design. Ind. Eng. Chem. Res. 2020, 59, 7355–7366. [Google Scholar] [CrossRef]
- Wang, J.; Song, Z.; Cheng, H.; Chen, L.; Deng, L.; Qi, Z. Computer-Aided Design of Ionic Liquids as Absorbent for Gas Separation Exemplified by CO2 Capture Cases. ACS Sustain. Chem. Eng. 2018, 6, 12025–12035. [Google Scholar] [CrossRef]
- Ramadhan, N.J.; Wan, Y.K.; Ng, R.T.; Ng, D.K.; Hassim, M.H.; Aviso, K.B.; Tan, R.R. Life cycle optimisation (LCO) of product systems with consideration of occupational fatalities. Process. Saf. Environ. Prot. 2014, 92, 390–405. [Google Scholar] [CrossRef]
- Cooley, M. Human-centred Systems. In Designing Human-centred Technology: A Cross-disciplinary Project in Computer-Aided Manufacturing, 1st ed.; Rosenbrock, H., Ed.; Springer: London, UK, 1989; pp. 133–143. [Google Scholar]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem. Int. Engl. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, X.; Liu, B.; Zhu, J. Separation of azeotropic mixtures (ethanol and water) enhanced by deep eutectic solvents. Fluid Phase Equilibria 2017, 448, 128–134. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Haghbakhsh, R.; Duarte, A.R.C.; Raeissi, S. A simple model for the viscosities of deep eutectic solvents. Fluid Phase Equilibria 2020, 521, 112662. [Google Scholar] [CrossRef]
| Types of Ionic Liquids | Applications |
|---|---|
| 1-methylimidazolium chloride | Biphasic acid scavenging [18] |
| 1-butylpyridinium tetrafluoroborate | Geothermal fluid in organic Rankine cycle [19] |
| 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | Hydrogen compressor [20] |
| 1-Ethyl-3-methylimidazolium acetate | Sugarcane bagasse pre-treatment [21] |
| 1-butyl-3-methylimidazolium chloride and 1-butyl-3-methylimidazolium acetate | Rubber woods pre-treatment [22] |
| 1-butylimidazolium hydrogen sulfate (VI) | Kraft lignin activator [23] |
| 1,3-dibutyl-2-methylimidazoliumbromide | Flavonoids extraction [24] |
| 1-hydroxyethyl-3-methylimidazoliumbis(trifluoromethanesulfonyl)amide | Keratin extraction [25] |
| 1-Ethyl-3-methylimidazolium methylsulfate | Ethyl acetate and ethanol azeotropic distillation [26] |
| 1-butyl-4-methylpyridinium tricyanomethanide | Cyclohexane and benzene azeotropic distillation [27] |
| 1-ethyl-3-methylimmidazolium-ethylsulphate | Carbon dioxide and hydrogen sulfide separation [28] |
| 1-butyl-3-propylamineimidazolium tetrafluoroborate | Carbon dioxide capture [29] |
| 1-butyl-3-methylimidazolium hexafluorophosphate | Carbon dioxide capture [30] |
| Tetraglyme-sodium salt ionic liquids | Sulfur dioxide separation [31] |
| 1-butyl imidazolium bis(trifluoromethylsulfonyl)imide | Ammonia separation [32] |
| Bis(1-butyl-3-methyl imidazolium) copper tetrachloride salt and bis(1-butyl-3-methyl imidazolium) stannum tetrachloride salt | Ammonia separation [33] |
| Platform | Focus | Main Products |
|---|---|---|
| Sugar | Fermentation of sugars obtained via extraction of biomass feedstocks | Ethanol and other building block chemicals |
| Thermochemical syngas | Gasification of biomass feedstocks | Gaseous and liquid fuels |
| Biogas | Decomposition of biomass feedstocks | Cooking gas |
| Carbon-rich chains | Transesterification of vegetable oil or animal fat | Biodiesel (fatty acid methyl esters) |
| Plant products | Selective breeding and genetic engineering of biological plant | Plant strains that can be used as feedstock for further conversion into chemicals and compounds that are difficult to obtain from plant naturally |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemmangattuvalappil, N.G.; Ng, D.K.S.; Ng, L.Y.; Ooi, J.; Chong, J.W.; Eden, M.R. A Review of Process Systems Engineering (PSE) Tools for the Design of Ionic Liquids and Integrated Biorefineries. Processes 2020, 8, 1678. https://doi.org/10.3390/pr8121678
Chemmangattuvalappil NG, Ng DKS, Ng LY, Ooi J, Chong JW, Eden MR. A Review of Process Systems Engineering (PSE) Tools for the Design of Ionic Liquids and Integrated Biorefineries. Processes. 2020; 8(12):1678. https://doi.org/10.3390/pr8121678
Chicago/Turabian StyleChemmangattuvalappil, Nishanth G., Denny K. S. Ng, Lik Yin Ng, Jecksin Ooi, Jia Wen Chong, and Mario R. Eden. 2020. "A Review of Process Systems Engineering (PSE) Tools for the Design of Ionic Liquids and Integrated Biorefineries" Processes 8, no. 12: 1678. https://doi.org/10.3390/pr8121678
APA StyleChemmangattuvalappil, N. G., Ng, D. K. S., Ng, L. Y., Ooi, J., Chong, J. W., & Eden, M. R. (2020). A Review of Process Systems Engineering (PSE) Tools for the Design of Ionic Liquids and Integrated Biorefineries. Processes, 8(12), 1678. https://doi.org/10.3390/pr8121678









