Abstract
Polyphenols, obtained from natural resources, may possess important pharmacological effects. The polyphenolic profiles of the stem extracts of six Ferocactus species (sp.): F. gracilis, F. pottsii, F. herrerae, F. horridus, F. glaucescens, and F. emoryi, were measured using high-performance liquid chromatography (HPLC) with diode-array detection (DAD). Additionally, anticancer, antibacterial, and antifungal activities were examined. Results showed the presence of high to moderate amounts of polyphenols in the extracts (phenolic acids: Protocatechuic acid, 3,4-dihydroxyphenylacetic acid, caffeic acid, and vanillic acid; flavonoids: Rutoside and quercitrin). The highest amounts of 3,4-dihydroxyphenylacetic acid were found in F. glaucescens ((132.09 mg 100 g−1 dry weight (DW)), F. pottsii (75.71 mg 100 g−1 DW), and F. emoryi (69.14 mg 100 g−1 DW) while rutoside content was highest in F. glaucescens (107.66 mg 100 g−1 DW). Maximum antiproliferative activities were observed against HeLa and Jurkat cancer cells, with F. glaucescens, F. emoryi, and F. pottsii showing the highest anticancer activity. Most bacteria were sensitive to Ferocactus sp. stem extracts. Escherichia coli and Staphylococcus aureus were the most sensitive. Excellent antifungal effects were observed against Aspergillus ochraceus and A. niger. However, Penicillium funiculosum, P. ochrochloron, and Candida albicans were relatively resistant. This is the first study reporting novel sources of polyphenols in Ferocactus sp. with anticancer and antimicrobial activities.
Keywords:
Ferocactus; stem extract; polyphenols; anticancer; antibacterial; antifungal; cytotoxicity 1. Introduction
Polyphenols (e.g., phenolic acids, lignins, tannins, and flavonoids) represent a wide group of plant secondary metabolites that play a crucial role in counteracting various types of stresses in plants, apart from contributing to the organoleptic properties of plants and plant-derived food [1,2]. Polyphenols are well known for their beneficial effects on human health, due to their antioxidant, anticancer, cardioprotective, anti-inflammatory, and antimicrobial properties [3,4,5,6,7]. In addition, studies have reported that polyphenols could improve some pathological conditions, such as neurodegenerative diseases, type 2 diabetes, and obesity [5,8,9,10]. The identification and discovery of new sources of phenolic compounds will assist in the development of new treatment options for various human cancers.
Polyphenols are strong antioxidants that have an important role in controlling bacterial diseases. Many polyphenolic compounds have shown antibacterial activities against several gram-positive bacteria, such as Staphylococcus aureus, Bacillus subtilis, and Listeria monocytogenes, and gram-negative bacteria, such as Escherichia coli and Pseudomonas aeruginosa [11,12,13,14,15]. The antifungal properties of polyphenols have also been reported in several studies against plant pathogenic fungi, including Botrytis cinerea and Fusarium oxysporum [16,17], as well as human pathogenic fungi, including Candida albicans and others [13,18].
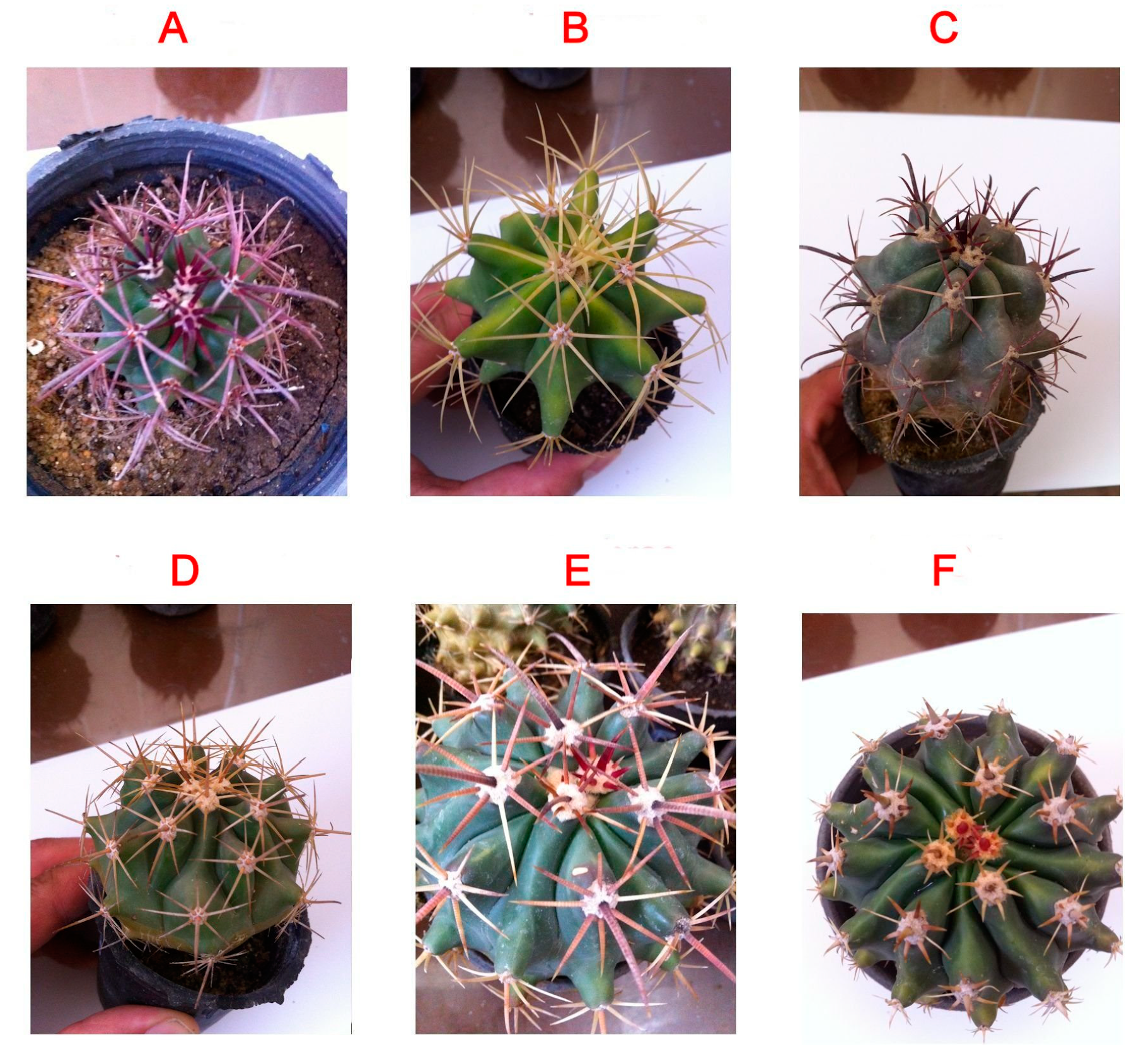
Ferocactus (Cactaceae) is a relatively small genus (30 species) consisting of barrel-shaped cacti with small to large spines and small, colored flowers. The plants are native to Mexico and the United States of America and are typically grown as ornamental plants in warmer regions. The family Cactaceae is known for important economic genera such as Opuntia sp. (which are used as food and medicine) [19] and Mammillaria [15]. However, no study has been performed to investigate the medicinal value of Ferocactus species (sp.), such as F. gracilis, F. pottsii, F. herrerae, F. horridus, F. glaucescens, and F. emoryi (Figure 1). Other Ferocactus sp., such as F. wislizeni, produce fruits that are used as lemons and limes. In addition, the fruits and stems of F. hamatacanthus are used in making cactus candy [2]. The plant extract of F. echidne has been used for the synthesis of silver nanoparticles owing to its strong reducing properties [20]. The polyphenolic profile and biological activities of this genus have not been studied before.
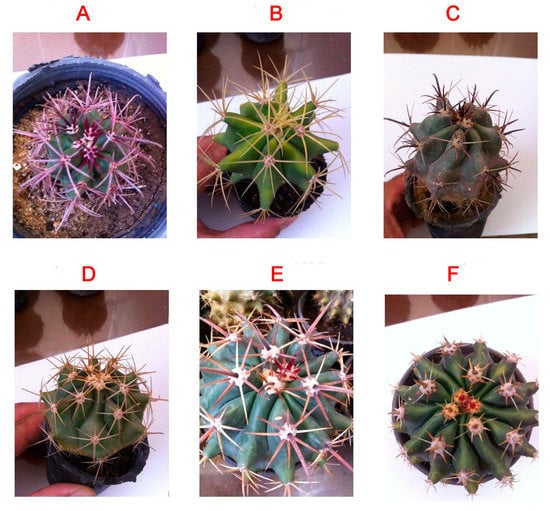
Figure 1.
Morphological appearances of the six Ferocactus sp. (A) F. emoryi, (B) F. glaucescens, (C) F. gracilis, (D) F. pottsii, (E) F. herrerae, (F) F. horridus.
Experimental data regarding the bioactivity of the stem of Ferocactus sp. is limited. In this study, the polyphenolic profiles of six Ferocactus sp. were evaluated (qualitatively and quantitatively) for the first time using high-performance liquid chromatography with diode-array detection (HPLC-DAD) method. The anticancer, antibacterial, and antifungal effects of stem extracts were also explored.
2. Results
2.1. Chemical Profiles of the Ferocactus Polyphenolic Extracts
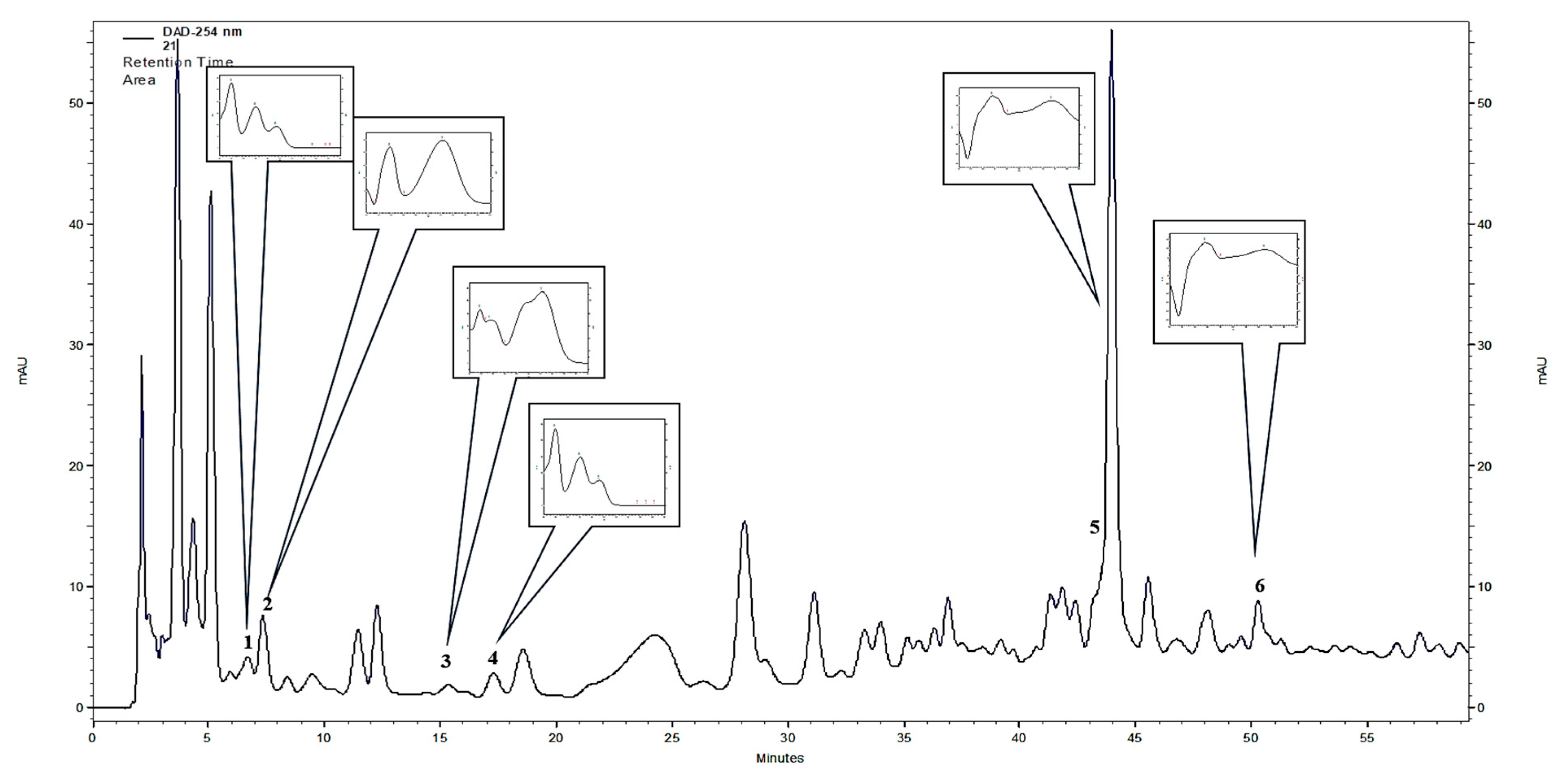
Out of the 21 compounds screened, six polyphenols were identified in the stem extracts of the plants from the Ferocactus sp., using HPLC-DAD. These polyphenols included 4 phenolic acids: Protocatechuic acid, 3,4-dihydroxyphenylacetic acid, caffeic acid, and vanillic acid, and two flavonoids: Rutoside and quercitrin (Table 1 and Figure 2). The two major compounds found in all six plants of the Ferocactus sp. were 3,4-dihydroxyphenylacetic acid and quercitrin. The amounts of 3,4-dihydroxyphenylacetic acid varied from 41.12 to 132.09 mg 100 g−1 dry weight (DW), and the highest amounts were found in F. glaucescens (132.09 ± 15.51 mg 100 g−1 DW), F. pottsii (75.71 ± 7.26 mg 100 g−1 DW), and F. emoryi (69.14 ± 6.7 mg 100 g−1 DW). The quercitrin content varied from 24.08 to 43.18 mg 100 g−1 DW, and the highest amount was detected in F. gracilis. The rutoside content varied from 7.83 to 107.66 mg 100 g−1 DW, and the highest amount was detected in F. glaucescens. The concentrations of protocatechuic acid, caffeic acid, and vanillic acid in the stem extracts were detected in smaller quantities, ranging from 1.53 to 8.59 mg 100 g−1 DW (Table 1). Based on these results, F. glaucescens can be considered as a rich source of polyphenols (Table 1).

Table 1.
Polyphenol compositions of Ferocactus sp. stem extracts (mg 100 g−1 DW ± SD).
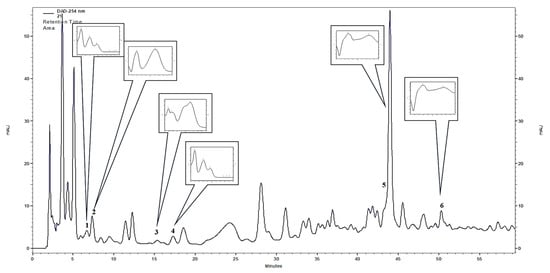
Figure 2.
HPLC-DAD (λ = 254 nm, UV spectra range 200–400 nm) chromatogram of F. glaucescens stem extract (1) protocatechuic acid, (2) 3,4-dihydroxyphenylacetic acid, (3) caffeic acid, (4) vanillic acid, (5) rutoside, (6) quercitrin (HPLC-DAD, high-performance liquid chromatography with diode-array detection).
2.2. Anticancer Activities of the Ferocactus Polyphenolic Extracts
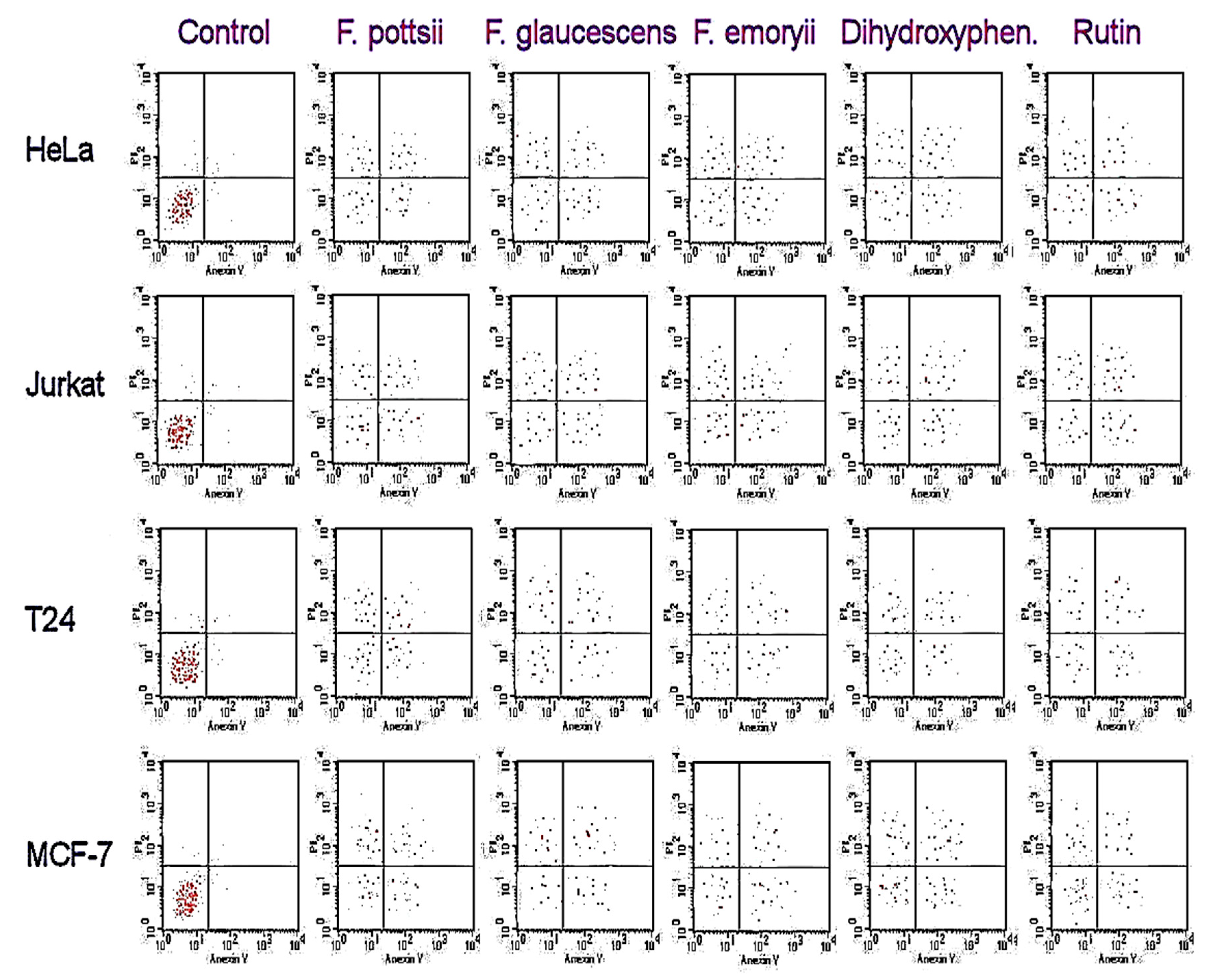
The stem extracts of the six plants from the Ferocactus sp. showed antiproliferative activities against human cancer cells, as shown in Table 2. The highest antiproliferative activities were observed against HeLa and Jurkat cancer cells. The highest anticancer activity was found in the extracts of F. glaucescens, F. emoryi, and F. pottsii. The anticancer activities of polyphenols were comparable in the F. glaucescens, F. emoryi, and F. pottsii extracts. The antiproliferative effects of 3,4-dihydroxy-phenylacetic acid and rutoside against Human Colorectal Adenocarcinoma Cell Line (HT-29) did not show any significant difference compared to vinblastine sulfate. After 48 h of treatment with different extracts, the apoptotic assay showed high accumulation of necrosis in the early and late apoptotic cells when compared to the control (Figure 3). Treatment with 2- 3,4-dihydroxyphenylacetic acid and rutoside showed similar accumulation of necrotic cells, as seen in treatment with the stem extracts of F. glaucescens, F. emoryi, and F. pottsii.

Table 2.
In vitro antiproliferative activity inhibitory concentration (IC50 (µg/mL)) of Ferocactus sp. stem extracts (mg mL−1) and the main identified compounds on different cancer cell lines. Values are presented as means of three replicates. Different letters within a column indicate significant differences at p ≤ 0.05.
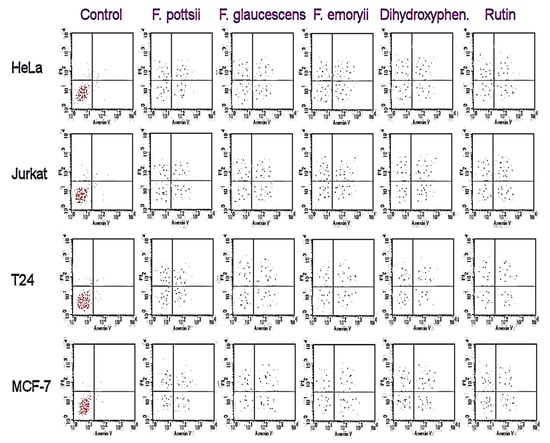
Figure 3.
Apoptotic cell population (IC50) using flow cytometry.
2.3. Antibacterial Activities of the Ferocactus Polyphenolic Extracts
The stem extracts of the different Ferocactus sp. showed remarkable antibacterial activities against Pseudomonas aeruginosa, Bacillus cereus, Listeria monocytogenes, Escherichia coli, Mariniluteicoccus flavus, and Staphylococcus aureus, as shown in Table 3. The highest antibacterial activities were observed in the stem extracts of F. glaucescens, F. emoryi, and F. pottsii. Polyphenol standards of 3,4-dihydroxyphenylacetic acid, rutoside, and quercitrin showed comparable or higher activities than those of the extracts. Most bacteria were sensitive to different Ferocactus sp. stem extracts; especially, E. coli and S. aureus were found to be most sensitive, as demonstrated by low minimum inhibitor concentration (MIC) values.

Table 3.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of Ferocactus sp. stem extracts (mg mL−1) and the main identified compounds. Values are presented as mean of three replicates.
2.4. Antifungal Activities of the Ferocactus Polyphenolic Extracts
Ferocactus stem extracts showed good antifungal properties against the selected fungi, as shown in Table 4. The MIC and minimum fungicidal concentration (MFC) values were generally low for all the Ferocactus sp. Excellent antifungal effects were observed against Aspergillus ochraceus and A. niger. However, Penicillium funiculosum, P. ochrochloron, and Candida albicans were relatively more resistant. The activities of the extracts matched those of the commercial reagent ketoconazole (KTZ). The antifungal activities of phenolic standards, 3,4-dihydroxyphenylacetic acid, rutoside, and quercitrin, were comparable to those of F. glaucescens, F. emoryi, and F. pottsii extracts.

Table 4.
Minimum inhibitory (MIC) and minimum fungicidal concentration (MFC) of Ferocactus sp. stem extracts (mg mL−1) and the identified compounds. Values are presented as mean of three replicates.
3. Discussion
The qualitative and quantitative HPLC-DAD analyses of the stem extracts of six Ferocactus sp., F. gracilis, F. pottsii, F. herrerae, F. horridus, F. glaucescens, and F. emoryi, indicated the presence of six polyphenolic compounds, namely protocatechuic acid, 3,4-dihydroxyphenylacetic acid, caffeic acid, vanillic acid, rutoside, and quercitrin. The highest concentrations of detected polyphenols were confirmed in F. glaucescens (Table 1). The major polyphenols found in high concentrations in all the studied Ferocactus sp. were 3,4-dihydroxyphenylacetic acid, rutoside, and quercitrin. Protocatechuic acid, caffeic acid, and vanillic acid were detected in smaller quantities, ranging from 1.53 to 8.59 mg 100 g−1 DW (Table 1). The highest concentration of 3,4-dihydroxyphenylacetic acid was found in F. glaucescens (132.09 mg 100 g−1 DW) and this value was several times higher than that found in the other species (Table 1). The abundant availability of 3,4-dihydroxyphenylacetic acid in a natural plant source is not common. Dihydroxyphenylacetic acid has been reported to be found in much lower concentrations in Eucalyptus globulus bark [21]. On the other hand, quercitrin is not as rare as 3,4-dihydroxyphenylacetic acid. It is commonly found in vegetables and fruits [22]. Similarly, rutoside is common in foods and has important therapeutic potential [23].
The stem extracts of different Ferocactus sp. showed obvious antiproliferative effects against various cancer cells, especially against HeLa and Jurkat cancer cells. The extracts of F. glaucescens, F. emoryi, and F. pottsii showed highest antiproliferative effects. This could be attributed to the abundant presence of specific bioactive polyphenol compounds, such as 3,4-dihydroxyphenylacetic acid, rutoside, quercitrin, and protocatechuic acid in these extracts. The 3,4-Dihydroxyphenylacetic acid was found to have apoptotic effect on human colon adenocarcinoma cells [24]. The extracts of F. gracilis, F. herrerae, and F. horridus showed moderate antiproliferative activities against most cancer cells. Previous investigations on other genera of Cactaceae, such as the famous genus Opuntia sp., revealed antiproliferative activities of the plant juice against HT-29 cells [25]. Cell cycle arrest in the apoptotic assay at the G1, G2/M, and S was reported. These effects were attributed to the phytochemical composition (betacyanins, isorhamnetin derivatives, and ferulic acid) of these plants. In Cereus peruvianus Mill (Cactaceae), antiproliferative activity was observed, owing to a high composition of unsaturated fatty acids [26].
The apoptotic activity of 3,4-dihydroxybenzoic acid (protocatechuic acid) has been reported in human gastric carcinoma cells [27] by the induction of JNK/p38 activity in protocatechuic acid (PCA)-responsive cell lines. Rutoside (3,3′,4′,5,7-pentahydroxyflavone-3-rhamnoglucoside) is a flavonol, commonly found in plants, and has cytoprotective, antioxidant, and anticarcinogenic activities against several cancer cell types [23]. Rutoside induces G2/M cell cycle arrest and activates apoptosis in human neuroblastoma cancer cells [28]. In another study, rutoside acted against cancer cells through antioxidant mechanism [29]. Quercitrin has shown strong anticancer activities owing to apoptosis-inducing effects [30]. Similar to earlier studies, accumulation of necrotic cells in the cell cycle was observed in this study.
Antibacterial effects were observed in the stem extracts of the six Ferocactus sp. The highest antibacterial activities were observed in the stem extracts of F. glaucescens, F. emoryi, and F. pottsii. Further, polyphenol standards of 3,4-dihydroxyphenylacetic acid, rutoside, and quercitrin showed comparable or higher values than those observed in the extracts, thus implying that these polyphenols were responsible for the antibacterial effects. Rutoside has been implicated in antibacterial activities against B. cereus and Salmonella enteritidis [31] and S. aureus [32]. Quercitrin and other flavonoids have also shown antibacterial activities against several bacteria [33]. Polyphenols, in general, are known for their antibacterial activities [34]. Furthermore, Ferocactus stem extracts showed good antifungal properties. Excellent antifungal effects were observed against Aspergillus ochraceus and A. niger. However, the antifungal activities were lower against Penicillium funiculosum, P. ochrochloron, and Candida albicans. Several reports have indicated that rutoside, quercitrin, protocatechuic acid, and vanillic acid have antifungal activities [3,4,35,36].
4. Materials and Methods
4.1. Chemicals
The following standards were used for the qualification and quantification of phenolic acid: Benzoic acid and its derivatives (3,4-dihydroxyphenylacetic acid, ellagic acid, gallic acid, gentisic acid, p-hydroxybenzoic acid, protocatechuic acid, salicylic acid, syringic acid, and vanillic acid), cinnamic acid and its derivatives (caffeic acid, o-coumaric acid, m-coumaric acid, p-coumaric acid, ferulic acid, hydrocaffeic acid, isoferulic acid, and sinapic acid), and depsides (chlorogenic acid, neochlorogenic acid, and rosmarinic acid). To quantify flavonoids, aglycone (kaempferol, luteolin, myricetin, quercetin, and rhamnetin) and glycoside (apigetrin, cynaroside, hyperoside, isoquercetin, quercitrin, robinin, rutoside, trifolin, and vitexin) standards were used. To quantify the catechins derivatives, epicatechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, and catechin were used. All the chemicals were acquired from Sigma-Aldrich, Darmstadt, Germany.
4.2. Preparation of Polyphenolic Extracts
The stems of Ferocactus sp. (F. gracilis H.E.Gates, F. pottsii (Salm–Dyck) Backeb, F. herrerae J.G.Ortega, F. horridus Britton and Rose, F. glaucescens (DC) Britton and Rose, F. emoryi Engelm, Orcutt) were sampled from commercial nurseries in Alexandria, Egypt, and identified by Hosam Elansary. Voucher specimens were deposited at Alexandria (Hosam 0001020–1027). The stem samples were dried by lyophilization (Labconco, USA) and then powdered. Three replicates of the dried samples (0.5 g DW each) were put in 15 mL tubes and subjected to extraction with 10 mL methanol (Chempur, Poland) by sonication (2 × 30 min at 30 °C) in an ultrasonic bath (Sonic-2, POLSONIC, ultrasonic power 2 × 100 W, 40 kHz, water bath dimensions 150 × 135 × 100 mm). The extracts were filtered using Whatman paper and left in crystallizers to evaporate methanol at room temperature (25 °C). The dry residue was dissolved in 1 mL methanol (Merck, HPLC grade purity) [37]. Obtained extracts were filtered through sterilized syringe filters (0.22 μm, Millex®GP, Millipore, Burlington, Mississippi, USA) prior to HPLC analyses. The samples were stored for future bioassays (−80 °C). For bioassays, methanol was totally removed by evaporation using a rotary evaporator. Analytical/HPLC grade chemicals were used (Sigma Aldrich, Germany) for the bioassays. The bacterial and fungal cultures were obtained from the Faculty of Agriculture, Alexandria, Egypt.
4.3. HPLC Analysis of Phenolic Compounds
Analyses of the polyphenolic content in the stem extracts of Ferocactus sp. were performed by the HPLC method, [37,38] using the Merck-Hitachi liquid chromatograph (LaChrom Elite) with a DAD detector L-2455. A Purospher RP-18e (250 × 4 mm, 5 μm, Merck, Darmstadt, Germany) column was used and the temperature was set at 25 °C. The mobile phase consisted of A, methanol; B, methanol: 0.5% acetic acid 1:4 (v/v). The gradient was as follows: 100% B for 0–20 min, 100–80% B for 20–35 min, 80–60% B for 35–55 min, 60–0% B for 55–70 min, 0% B for 70–75 min, 0–100% B for 75–80 min, 100% B for 80–90 min, with a flow rate (1 mL min−1). The injection volume was 20 µL and the compounds of interest were detected at 254 nm. The applied HPLC method was previously validated by our group [37,38]. The parameters tested were as follows: Accuracy, precision at three levels of standard substance concentrations in solution (50%, 100%, and 150%), linearity, limit of detection (LOD), and limit of quantification (LOQ) [37,38]. Identification of compounds was performed either by comparison with UV spectra and retention times (tR) of reference substances or using co-chromatography. The compounds were quantified using the calibration curve method [37,38,39,40]. Data for detected compounds was as follows: Protocatechuic acid, tR = 6.63, λmax = 220,260,294, LOD = 0.024 (mg/mL), LOQ = 0.072 (mg/mL), y = 1357.761x − 2.599, R2 = 0.999; 3,4-dihydroxyphenylacetic acid, tR = 7.32, λmax = 218,280, LOD = 0.019 (mg/mL), LOQ = 0.058 (mg/mL), y = 65.047x − 1.219, R2 = 0.999; caffeic acid, tR = 15.27, λmax = 218,235,323, LOD = 0.029 (mg/mL), LOQ = 0.087 (mg/mL), y = 598.118 − 1.456, R2 = 0.999; vanillic acid, tR = 17.66, λmax = 219,260,292, LOD = 0.025 (mg/mL), LOQ = 0.065 (mg/mL), y = 1276.874x − 1.682, R2 = 0.990; rutoside, tR = 44.63, λmax = 256,355, LOD = 0.011 (mg/mL), LOQ = 0.041 (mg/mL), y = 594.207x − 0.665, R2 = 0.999; and quercitrin, tR = 50.41, λmax = 256,349, LOD = 0.014 (mg/mL), LOQ = 0.032 (mg/mL), y = 579.112x − 14.468, R2 = 0.998.
4.4. Cell Cultures and Treatments
Cell cultures of breast adenocarcinoma (MCF-7), cervical adenocarcinoma (HeLa), T-cell lymphoblast like (Jurkat), colon adenocarcinoma (HT-29), HEK-293 (human normal cells), and urinary bladder carcinoma (T24) were purchased from American Type Culture Collection (ATCC).
4.5. MTT Assay
Cytotoxic activities of stem extracts were tested on MCF-7, HeLa, Jurkat, HT-29, and T24, in addition to HEK-293 (human normal cells), using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method [3,17]. This colorimetric method measured the reduction of MTT, a yellow tetrazolium salt to purple formazan by the action of mitochondrial dehydrogenase enzyme present in the living cells [41].
The percentage inhibition of antiproliferative activity (IAA) was calculated in triplicates:
where and are Abs.570 nm of control and sample, respectively.
4.6. Apoptotic Assay
The inhibitory concentration IC30 and IC50 values were determined in the apoptotic cell population using a flow cytometry (FAC Scan, Becton Dickinson, Iowa, USA) [3,17,42].
4.7. Antibacterial Activity
Antibacterial activity of the stem extracts against B. cereus (ATCC 14579), L. monocytogenes (clinical isolate), E. coli (ATCC 35210), M. flavus (ATCC 10240), S. aureus (ATCC 6538), and P. aeruginosa (ATCC 27853) were investigated using the microdilution method [18,43,44,45]. The optical density was determined at a wavelength of 655 nm. The positive and negative controls used were streptomycin (0.01–10 mg/mL) and dimethyl sulfoxide (DMSO, 1%), respectively.
4.8. Antifungal Activity
Antifungal activity of the stem extracts against economically important fungi, including C. albicans (ATCC 12066), A. flavus (ATCC 9643), P. ochrochloron (ATCC 48663), A. ochraceus (ATCC 12066), A. niger (ATCC 6275), and P. funiculosum (ATCC 56755), was determined using the microdilution method [18,42,43]. The positive and negative controls used were ketoconazole (1–3500 µg/mL) and DMSO (1%), respectively.
4.9. Statistical Analyses
The least significant difference (LSD) was computed using the SPSS software (version 22.0). Experiments were repeated twice. The standard deviation (SD) of means of three replicates was used.
5. Conclusions
To our knowledge, this is the first report that explored the presence of polyphenols in the stem extracts of six Ferocactus sp., and investigated their respective bioactivities as anticancer, antibacterial, and antifungal raw materials. Six polyphenols were identified (phenolic acids: Protocatechuic acid, 3,4-dihydroxyphenylacetic acid, caffeic acid, and vanillic acid and flavonoids: Rutoside and quercitrin). The major compounds found in all the six species were 3,4-dihydroxyphenylacetic acid and quercitrin. Rutoside was present in highest concentration in F. gracilis. The stem extracts of Ferocactus sp. showed antiproliferative activities against human cancer cell lines, with the highest antiproliferative effects observed against Hela and Jurkat cell lines. The apoptotic assay revealed accumulation of necrotic cells in the early and late stages. The highest antiproliferative activities were found in the stem extracts of F. glaucescens, F. emoryi, and F. pottsii. It was observed that, among the tested bacteria, E. coli and S. aureus were the most sensitive to Ferocactus sp. stem extracts, as demonstrated by low MIC values. Ferocactus sp. stem extracts showed good antifungal properties against selected fungi. Excellent antifungal effects were reported against A. ochraceus and A. niger. In summary, Ferocactus sp. stem extracts could be utilized as a novel source of polyphenols and may be recommended as valuable sources of antimicrobial and anticancer from natural materials. Further investigations should be conducted to evaluate the activity of these extracts against other pathogens. The phytochemical analysis conducted in this study was a partial analysis of the selected compounds in the extract. For fingerprinting purposes, a more sophisticated analysis should be used.
Author Contributions
Conceptualization, H.O.E., A.S., H.E., and A.A.B. Data curation, H.O.E., A.S., and F.A.A.-M. Formal analysis, H.O.E., A.S., M.K.-S., and H.E. Funding acquisition, A.S., F.A.A.-M., and A.A.B. Investigation, H.O.E., A.S., M.K.-S., F.A.A.-M., and A.A.B. Methodology, H.O.E., M.K.-S., and A.A.B. Project administration, H.E. Resources, H.E. Visualization, A.S. Writing—original draft, H.O.E., A.S., and F.A.A.-M. Writing—review and editing, H.O.E. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by King Saud University through Researchers Supporting Project number (RSP-2019/118).
Acknowledgments
The authors extend their appreciation to King Saud University, Researchers Supporting Project for funding this work through research group (RSP-2019/118).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and Nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Elansary, H.O. Tree bark phenols regulate the physiological and biochemical performance of Gladiolus flowers. Processes 2020, 8, 71. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; El-Abedin, T.K.A.Z.; Mattar, M.A.; Ekiert, H. Phenolic compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have antioxidant and anticancer activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol profile and pharmaceutical potential of Quercus spp. bark extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Schluesener, H. Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls. Ageing Res. Rev. 2012, 11, 329–345. [Google Scholar] [CrossRef]
- Di Mauro, D.M.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, G.M.; Centonze, G.; Maggiore, R.; D’Antona, N. Polyphenolic fraction from olive mill wastewater: Scale-up and in vitro studies for ophthalmic nutraceutical applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef]
- Acquaviva, R.; Genovese, C.; Amodeo, A.; Tomasello, B.; Malfa, G.; Sorrenti, V.; Tempera, G.; Addamo, A.P.; Ragusa, S.; Rosa, T.; et al. Biological activities of Teucrium flavum L., Teucrium fruticans L., and Teucrium siculum rafin crude extracts. Plant Biosyst. 2018, 152, 720–727. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Choi, H.Y.; Yang, G.M.; Kim, K.; Saha, S.K.; Cho, S.G. The Anti-Cancer Effect of polyphenols against breast cancer and cancer stem cells: Molecular mechanisms. Nutrients 2016, 8, 581. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A. In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat. Prod. Res. 2015, 29, 2149–2154. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Mahmoud, E.A. Egyptian herbal tea infusions’ antioxidants and their antiproliferative and cytotoxic activities against cancer cells. Nat. Prod. Res. 2015, 29, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Salem, M.Z.M.; Ashmawy, N.A.; Yessoufou, K.; El-Settawy, A.A.A. In vitro antibacterial, antifungal and antioxidant activities of Eucalyptus spp. leaf extracts related to phenolic composition. Nat. Prod. Res. 2017, 31, 2927–2930. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Mahmoud, E.A. Basil cultivar chemotyping still favored over genotyping using core barcodes and possible resources of antioxidants. Essent. Oil Res. 2015, 27, 82–87. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Klimek-Szczykutowicz, M.; Jafernik, K.; Ekiert, H.; Mahmoud, E.A.; Barakat, A.A.; El-Ansary, D.O. Mammillaria Species—polyphenols studies and anti-cancer, anti-oxidant, and anti-bacterial activities. Molecules 2019, 25, 131. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Joya, J.A.; Pastrana-Castro, L.; Nieto-Oropeza, D.; Ventura-Sobrevilla, J.; Rojas-Molina, R.; Aguilar, C.N. The physicochemical, antifungal and antioxidant properties of a mixed polyphenol based bioactive film. Heliyon 2018, 4, e00942. [Google Scholar] [CrossRef]
- Yessoufou, K.; Elansary, H.O.; Mahmoud, E.A.; Skalicka-Wozniak, K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind. Crop Prod. 2015, 74, 752–758. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of traditional medicinal plants in Alexandria. Evid. Based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef]
- Brinker, F. Prickly pear as food and medicine. J. Diet. Suppl. 2009, 6, 362–376. [Google Scholar] [CrossRef]
- Shah, A.T.; Din, M.I.; Bashir, S.; Qadir, M.A.; Rashid, F. Green synthesis and characterization of silver nanoparticles using Ferocactus echidne extract as a reducing agent. Anal. Lett. 2015, 48, 1180–1189. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography–mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef] [PubMed]
- Nishimuro, H.; Ohnishi, H.; Sato, M.; Ohnishi-Kameyama, M.; Matsunaga, I.; Naito, S.; Ippoushi, K.; Oike, H.; Nagata, T.; Akasaka, H.; et al. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients 2015, 7, 2345–2358. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Jordão, N.; da Costa Pereira Soares, N.; deMesquita, J.; Monteiro, M.; Teodoro, A. Pharmacokinetic, antiproliferative and apoptotic effects of phenolic acids in human colon adenocarcinoma cells using in vitro and in silico approaches. Molecules 2018, 23, 2569. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Poejo, J.; Matias, A.A.; Bronze, M.R.; Duarte, C.M.M. Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29). Food Res. Int. 2013, 54, 892–901. [Google Scholar] [CrossRef]
- Jacomini, D.; Sinzker, R.C.; Mangolin, C.A.; Grande, P.A.; Nocchi, S.R.; Nakamura, C.V.; de Oliveira, A.J.B.; Gonçalves, R.A.C. Lipid profile and antiproliferative activity of callus cultures of Cereus peruvianus Mill. Ind. Crop Prod. 2015, 69, 408–414. [Google Scholar] [CrossRef]
- Lin, H.H.; Chen, J.H.; Huang, C.C.; Wang, C.J. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. Int. J. Cancer 2007, 120, 2306–2316. [Google Scholar] [CrossRef]
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci. World J. 2013, 2013, 269165. [Google Scholar] [CrossRef]
- Saleh, A.; ElFayoumi, H.M.; Youns, M.; Barakat, W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 165–175. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Arima, H.; Ashida, H.; Danno, G.-I. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) models. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.M.; Carraro, E.; Auler, M.E.; Khalil, N.M. Quercetin and rutin as potential agents antifungal against Cryptococcus spp. Braz. J. Biol. 2016, 76, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. J. ISRN Pharmacol. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Sulkowska-Ziaja, K.; Maslanka, A.; Szewczyk, A.; Muszynska, B. Physiologically active compounds in four species of Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Luczkiewicz, M.; Ekiert, H. Studies on the accumulation of phenolic acids and flavonoids in different in vitro culture systems of Schisandra chinensis (Turcz.) Baill. using a DAD-HPLC method. Phytochem. Lett. 2017, 20, 462–469. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Kubica, P.; Banaszczak, P.; Wojtanowska-Krośniak, A.; Krośniak, M.; Marzec-Wróblewska, U.; Badura, A.; Zagrodzki, P.; Bucinski, A.; et al. Comparative analysis of different groups of phenolic compounds in fruit and leaf extracts of Aronia sp.: A. melanocarpa, A. arbutifolia, and A. ×prunifolia and their antioxidant activities. Eur. Food Res. Technol. 2017, 243, 1645–1657. [Google Scholar] [CrossRef]
- Elansary, H.O.; El-Ansary, D.O.; Al-Mana, F.A. 5-aminolevulinic acid and soil fertility enhance the resistance of rosemary to Alternaria dauci and Rhizoctonia solani and modulate plant biochemistry. Plants-Basel 2019, 8, 585. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Tomasello, B.; Giardina, R.C.; Dattilo, S.; Mazzei, V.; Sinatra, F.; Caruso, M.; D’Antona, N.; Renis, M. Sugar and mineral enriched fraction from olive mill wastewater for promising cosmeceutical application: Characterization, in vitro and in vivo studies. Food Funct. 2017, 8, 4713–4722. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E.A.; Skaicka-Wozniak, K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crop Prod. 2016, 92, 50–56. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K. In vitro antioxidant, antifungal and antibacterial activities of five international Calibrachoa cultivars. Nat. Prod Res. 2016, 30, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Yessoufou, K.; Mahmoud, E.A.; Skalicka-Wozniak, K. In vitro antioxidant and antimicrobial effects of Ceratostigma plumbaginoides. Nat. Prod. Commun. 2016, 11, 1455–1458. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).