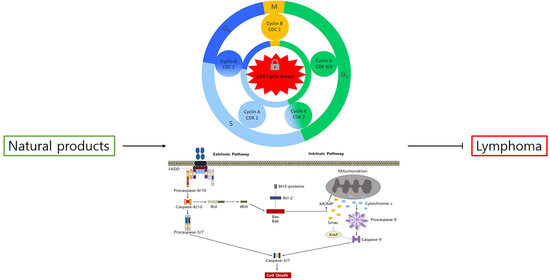
Review of Natural Compounds for the Management and Prevention of Lymphoma
Abstract
1. Lymphoma
2. Cancer System Biology
3. Natural Products and Cancer
4. Methods
5. Natural Compounds and Lymphoma
5.1. Microorganism-Derived Compounds and Lymphoma
5.2. Plant-Derived Compounds and Lymphoma
5.2.1. In Vitro Studies
5.2.2. In Vitro and In Vivo Studies
| System | Compound | Source | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| In vitro | Amorphispironones B | Amorpha fruticosa | L5178Y | IC50 7.6 μM | Induction of cytotoxicity | [33] | |
| In vitro | Arctigenin | BC3 | Glucose(-), 5 μM; 2, 4, 6 h | Induction of apoptosis | ↑ c-caspase-3, -9, c-PARP | [34] | |
| BC3, Ramos | Glucose(-), 1, 5, 10 μM | ↓ MMP, ATP | |||||
| BC3, BCBL1 | Glucose(-), 5, 10 μM; 3, 4, 6 h | ↑ GRP94, CHOP ↓ p-p38, p-ERK1/2, p-p90RSK, GRP78, ATF6α | |||||
| Ramos, DG75 | Glucose(-), 5 μM, 4, 6 h | ↑ GRP94, CHOP ↓ GRP78, ATF6α | |||||
| In vitro | Curcumin | CH12F3 | 3, 6, 9, 10, 20, 30, 40, 50 μM; 4, 24 h | Induction of DNA damage and apoptosis | ↑ γH2AX, PAPR1, PCNA, caspase-3, -9 ↓ Rad51 | [35] | |
| In vitro | Dalbinol | Amorpha fruticosa | L5178Y | IC50 0.2 μM | Induction of cytotoxicity | [33] | |
| Dalpanol | Amorpha fruticosa | L5178Y | IC50 0.7 μM | Induction of cytotoxicity | [33] | ||
| Deguelin | Amorpha fruticosa | L5178Y | IC50 0.2 μM | Induction of cytotoxicity | [33] | ||
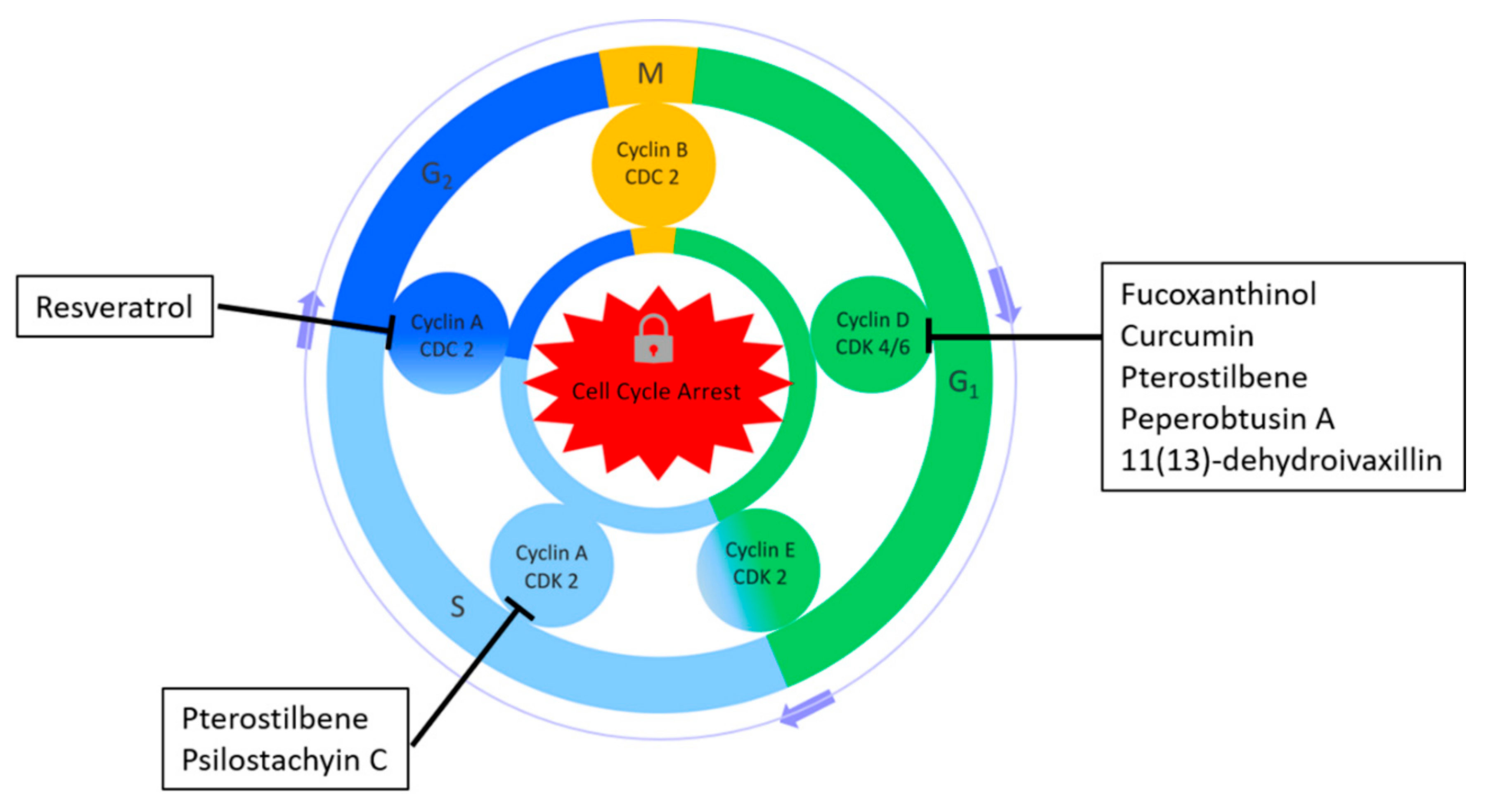
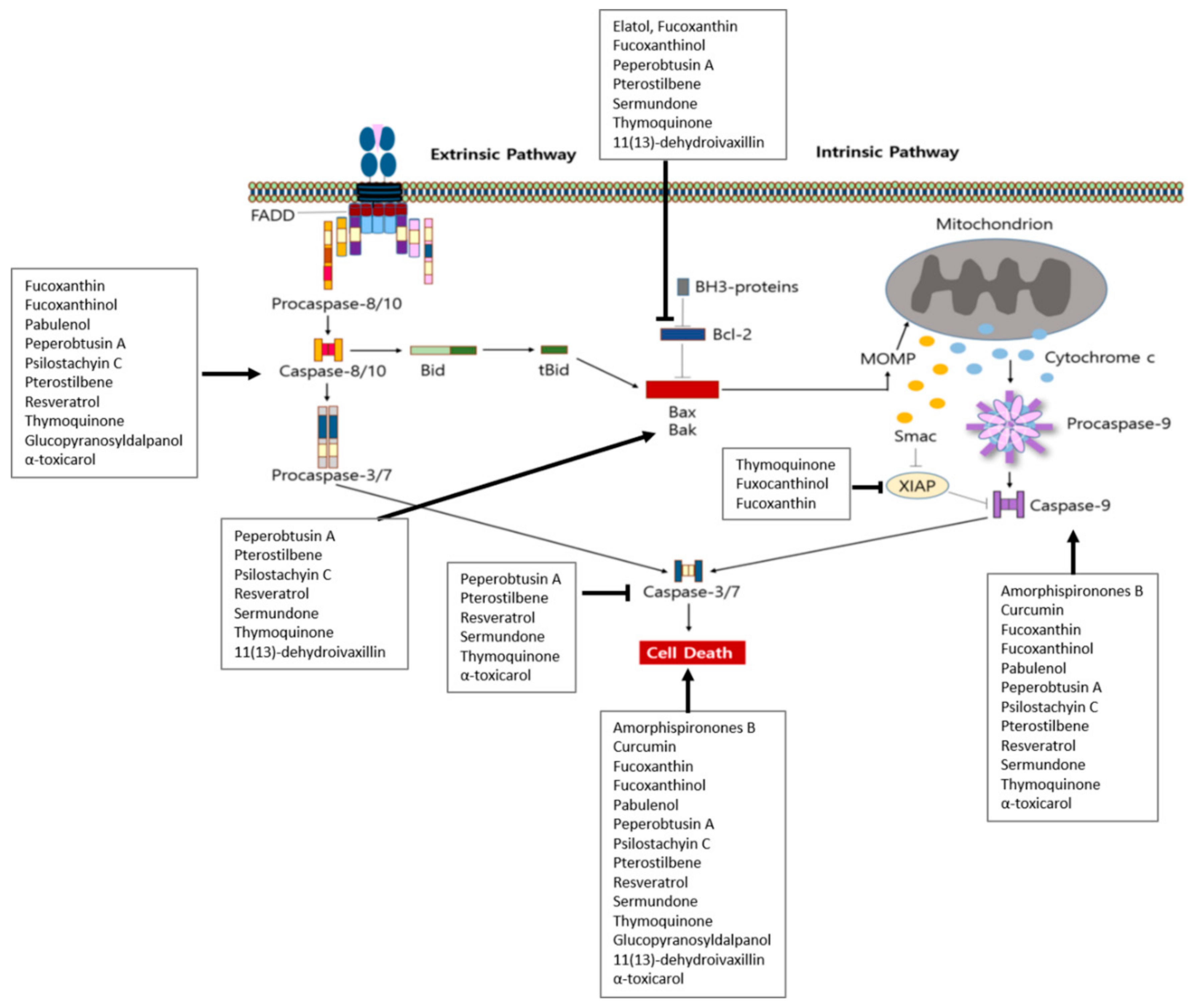
| In vitro | Fucoxanthin | Cladosiphon okamuranus Tokida | Raji, Daudi, B95-8/Ramos | 5 μM; 24 h | Induction of apoptosis | [36] | |
| Daudi, KM-H2, L540 | 2.5 μM; 24 h | Induction of cell cycle arrest | |||||
| In vitro | Fucoxanthinol | Cladosiphon okamuranus Tokida | Raji, Daudi, B95-8/Ramos | 2.5 μM; 24 h | Induction of apoptosis | [36] | |
| Daudi, KM-H2, L540 | 1.25 μM; 24 h | Induction of cell cycle arrest | |||||
| Daudi | 0.63, 1.25, 2.5, 5 μM; 24 h | Induction of apoptosis and cell cycle arrest | ↑ c-PARP, c-caspase-3, -9 ↓ Bcl-2, cIAP-2, XIAP, cyclin D1, cyclin D2, NF-κB-DNA binding | ||||
| In vitro | Fuxocanthinol | Cladosiphon okamuranus Tokida | BCBL-1, TY-1 | 1.3, 2.5, 5 μM; 24 h | Induction of apoptosis | ↑ c-PARP, c-caspase-3, -8, -9 | [53] |
| BCBL-1 | 1.3, 2.5, 5 μM; 24 h | Induction of cell cycle arrest | ↓ Bcl-xL, XIAP, survivin, p-pRb, cyclin D2, CDK4, CDK6, c-Myc, p-IKK β, p-IkB α, IKK α, IKK β, IKK γ, Akt, PDK1, p-cas9, β-catenin, JunB, JunD, NF-κB-DNA binding activity, AP-1 binding | ||||
| In vitro | Heraclenin | Ducrosia anethifolia | L5178Y PAR | IC50 32.73 μM | Inhibition of proliferation | [37] | |
| L5178Y MDR | IC50 46.54 μM | ||||||
| Heraclenol | Ducrosia anethifolia | L5178Y PAR | IC50 52.31 μM | Inhibition of proliferation | |||
| L5178Y MDR | IC50 46.57 μM | ||||||
| In vitro | Hydroxyamorphispironone | Amorpha fruticosa | L5178Y | IC50 1.3 μM | Induction of cytotoxicity | [33] | |
| In vitro | Imperatorin | Ducrosia anethifolia | L5178Y PAR | IC50 36.12 μM | Inhibition of proliferation | [37] | |
| L5178Y MDR | IC50 42.24 μM | ||||||
| Isogospherol | Ducrosia anethifolia | L5178Y PAR | IC50 46.53 μM | Inhibition of proliferation | |||
| L5178Y MDR | IC50 48.75 μM | ||||||
| In vitro | Isotylocrebrine | Citrus tachibana (Makino) T. Tanaka | MT-1 | EC50 48.3 nM; 4 h | Inhibition of proliferation | [38] | |
| MT-2 | EC50 25.4 nM; 4 h | ||||||
| MT-2 | EC50 13.0 nM; 4 h | ||||||
| In vitro | Isotylocrebrine Noxide | Citrus tachibana (Makino) T. Tanaka | MT-1 | EC50 379.5 nM; 4 h | [38] | ||
| MT-2 | EC50 246.7 nM; 4 h | ||||||
| In vitro | Methyl angolensate | Soymida febrifuga | Daudi | 10, 50, 100, 250 μM; 24, 48, 72 h | Inhibition of proliferation Activation of apoptosis ROS formation | ↑ c-PARP, MRE11, RAD50, NBS1, p-ATM, KU70, KU80 ↓ p53, p73 | [39] |
| In vitro | Oxypeucedanin | Ducrosia anethifolia | L5178Y PAR | IC50 25.98 μM | Inhibition of proliferation | [37] | |
| L5178Y MDR | IC50 28.89 μM | ||||||
| Oxypeucedanin methanolate | Ducrosia anethifolia | L5178Y MDR | IC50 35.88 μM | Inhibition of proliferation | |||
| L5178Y PAR | IC50 33.23 μM | ||||||
| Pabulenol | Ducrosia anethifolia | L5178Y MDR | IC50 30.47 μM | Inhibition of proliferation | |||
| L5178Y PAR | IC50 29.28 μM | ||||||
| In vitro | Peperobtusin A | Peperomia tetraphylla | U937 | 25, 50, 75, 100 μM; 1, 3, 6, 24 | Induction of cell cycle arrest and apoptosis | ↑ ROS, Bax, c-caspase-8, -9, -3, p-p38 ↓ MMP, Bcl-2, Bid, caspase-3, p38 | [40] |
| In vitro | Psilostachyin C | Ambrosia spp. | BW5147 | 10 μg/mL; 24 h | Induction of apoptosis, necrosis Cell arrest in S phage Inhibition of cell viability, cell proliferation | ↓ SOD, CAT, Px | [41] |
| In vitro | Resveratrol | Various plants | Ramos | 20, 50, 70, 100 μM; 1, 3, 6, 10, 24 h | Induction of antiproliferative and proapoptotic activity | ↑ c-caspase-3, c-PARP, NOXA, PUMA, p-ATM, p-BRCA1, γ-H2AX, Rad 50, Mre 11, p-p95, DNA-PKcs, KU80 ↓ TCL-1. Myc, Bach2 | [42] |
| In vitro | Resveratrol | Various plants | SNT-8, SNK-10, SNT-16 | 25 μM; 0.5, 1, 3, 6, 12, 24, 48 h | Induction of cell cycle arrest | ↓ Cyclin A2 | [43] |
| Induction of DNA damage response and apoptosis Inhibition of proliferation | ↑ pATM, γ-H2A.X., p-Chk2, p-p53, Bax, Bad, c-caspase-9, -3 ↓ Mcl-1, survivin, p-AKT, p-Stat3 | ||||||
| In vitro | Rotenone | Amorpha fruticosa | L5178Y | IC50 0.3 μM | Induction of cytotoxicity | [33] | |
| rot-2′-enonic acid | Amorpha fruticosa | L5178Y | IC50 0.6 μM | Induction of cytotoxicity | [33] | ||
| In vitro | Schweinfurthin | Macaranga alnifolia Baker | WSU-DLCL2 | 100 nM; 24 h | Inhibition of proliferation | ↑ p-EIF2a ↓ mTOR, AKT | [44] |
| In vitro | Sermundone | Amorpha fruticosa | L5178Y | IC50 0.2 μM | Induction of cytotoxicity | [33] | |
| In vitro | Thymoquinone | Nigella sativa | BC-1 | 10, 25 μM; 24 h | Induction of apoptosis Increase ROS generation Loss of MMP | ↑ Bax, c-caspase-3, -9, c-PARP, DR5 ↓ Bcl-2, p-AKT, p-FOXO1, p-GSK3, p-Bad | [46] |
| BC-3 | Induction of apoptosis, ROS generation | ↑ Bax, c-caspase-3, -9, c-PARP ↓ Bcl-2, p-AKT, p-FOXO1, p-GSK3, p-Bad | |||||
| BCBL-1 | Induction of apoptosis, ROS generation | ↓ p-AKT, p-FOXO1, p-GSK3, p-Bad | |||||
| HBL-6 | Induction of apoptosis | ||||||
| In vitro | Thymoquinone | Nigella sativa Linn. | ABC-DLBCL (HBL-1, RIVA) | 5, 10 mM; 24 h | Induction of ROS and apoptosis Inhibition of cell viability | ↑ c-caspase-9, -3, PARP, Bax ↓ NF-κB, IκBa, Bcl-2, Bcl-Xl, XIAP, Survivin, translocation of p65 subunit of NF-κB, p-p65 | [45] |
| In vitro | Tylophorine N-oxide | Citrus tachibana (Makino) T. Tanaka | MT-1 | EC50 1590.0 nM; 4 h | Inhibition of proliferation | [38] | |
| MT-2 | EC50 1490.0 nM; 4 h | ||||||
| In vitro | Tylophorinine N-oxide | Citrus tachibana (Makino) T. Tanaka | MT-1 | (1)EC50 28.8 nM; 4 h | Inhibition of proliferation | [38] | |
| MT-2 | (2)EC50 4.8 nM; 4 h | ||||||
| In vitro | 3-demethyl-14b-hydroxyisotylocrebrine | Citrus tachibana (Makino) T. Tanaka | MT-1 | (1)EC50 2.8 nM; 4 h | Inhibition of proliferation | [38] | |
| MT-2 | (2)EC50 2.6 nM; 4 h | ||||||
| In vitro | 3, 3′, 4-tri-O-methylellagic acid | Combretum dolichopetalum | L5179Y | IC50 29.0 µM | Induction of cytotoxicity | [31] | |
| In vitro | 4-Deoxyraputindole C | Raputia praetermissa | Raji | 20, 40, 60, 80, 100 μM; 6, 12, 24 h | Induction of cell death | ↑ mitochondrial superoxide ↓ MMP, cathepsin B/L | [47] |
| In vitro | 6a,12a- dehydrodeguelin | Amorpha fruticosa | L5178Y | IC50 10.2 μM | Induction of cytotoxicity | [33] | |
| 6′-O-β-D-Glucopyranosyldalpanol | Amorpha fruticosa | L5178Y | IC50 1.7 μM | Induction of cytotoxicity | [33] | ||
| In vitro | 14-hydroxytylophorine N-oxide | Citrus tachibana (Makino) T. Tanaka | MT-1 | EC50 69.8 nM; 4 h | Inhibition of proliferation | [38] | |
| MT-2 | EC50 26.8 nM; 4 h | ||||||
| In vitro | α-toxicarol | Amorpha fruticosa | L5178Y | IC50 0.2 μM | Induction of cytotoxicity | [33] | |
| In vitro | β-Asarone | Raji | 100, 200, 400 μM; 72 h | Induction of apoptosis | ↑ c-caspase-9, -3, c-PARP ↓ procaspase-9, -3, PARP | [48] | |
| 100 μM | Induction of anticancer effects | ↓ NF-κB/p65, p-NF-κB/p65, NF-κB/p65 nuclear translocation | |||||
| In vitro | β-Phenethyl isothiocyanate (PEITC) | Raji | 10 μM; 3 h | Reduction in mitochondrial respiration rate Increase in cellular H2O2 levels Rapid depletion of cellular and mitochondrial glutathione | ↓ NDUFS3 | [49] | |
| In vitro | (þ)-Oxypeucedanin hydrate | Ducrosia anethifolia | L5178Y MDR | IC50 41.96 μM | Inhibition of proliferation | [37] | |
| L5178Y PAR | IC50 60.58 μM | ||||||
| In vitro | Compound 6 | Tabernaemontana elegans Stapf | L5178Y | IC50 11.38 μM; 24 h | Induction of cytotoxicity | [50] | |
| Compound 8 | L5178Y | IC50 63.91 μM; 24 h | |||||
| Compound 9 | L5178Y | IC50 35.56 μM; 24 h | |||||
| Compound 10 | L5178Y | IC50 29.21 μM; 24 h | |||||
| Compound 15 | L5178Y | IC50 34.28 μM; 24 h | |||||
| Compound 16 | L5178Y | IC50 20.77 μM; 24 h | |||||
| Compound 23 | L5178Y | IC50 33.30 μM; 24 h | |||||
| In vitro and in vivo | Chelerythrine | Chelidonium majus. L. | BALB/c (H2d) mice | 1.25, 2.5 mg/kg; 34 d | Increase in survival duration Inhibition of Dalton’s Lymphoma cell growth | [51] | |
| TANK | 2.5 mg/kg | ↑ NKG2D ↓ NKG2A | |||||
| In vitro and in vivo | Elatol | SU-DHL-6, OCI-Ly3, RIVA | 500 nM, 1, 10 μM; 24, 48, 72, 96 h | Induction of apoptosis | [52] | ||
| SU-DHL-6, OCI-Ly3 | 5 μM; 16 h | ↓ cyclinD3, MYC, MCL1, PIM2 | |||||
| 1, 10 μM; 4, 16, 24 h | Inhibition of protein synthesis | ||||||
| OCI-Ly3 | 5 μM; 16 h | ↓ BCL-2, survivin | |||||
| SCID mice(engrafted with SU-DHL-6) | 20 mg/kg; 20 days | Inhibition of tumor growth | |||||
| SCID mice(OCI-Ly3 xenograft) | 40 mg/kg; 30 days↑ | ||||||
| In vitro and in vivo | Fucoxanthin | Cladosiphon okamuranus Tokida | BCBL-1, TY-1 | 2.5, 5, 10 μM; 24 h | Induction of apoptosis | ↑ c-PARP, c-caspase-3. -8, -9 | [53] |
| BCBL-1 | 2.5, 5, 10 μM; 24 h | Induction of cell cycle arrest | ↓ Bcl-xL, XIAP, p-pRb p-IKK β, p-IkB α, IKK α, IKK β, IKK γ, Akt, PDK1, p-caspase-9, β-catenin, JunB, JunD | ||||
| SCID mice (BCBL-1 Xenograft) | 150 mg/kg; 56 days | Inhibition of tumor growth | |||||
| In vitro and in vivo | Pterostilbene | Jeko-1,Granta-519, Mino, Z-138 | 10, 20, 40, 60, 80 μM; 24, 48, 72 h | Induction of cytotoxicity | [54] | ||
| Jeko-1, Granta-519 | 20, 40, 80 μM; 48 h | Induction of cell cycle arrest and apoptosis | ↑ c-caspase-3, -8, -9, Bax ↓ CDK4, CDK6, cyclinD1, MMP, Bcl-2, Bcl-xL, p-PI3K, p-Akt, p-mTOR, p-p70S6K | ||||
| NOD/SCID mice (JeKo-1 Xenograft) | 50 mg/kg; 15 days | Inhibition of tumor growth | ↓ p-mTOR | ||||
| In vitro and in vivo | Resveratrol | Various plants | EL4 | 5, 10, 25, 50, 100 μM; 6, 12, 24 h | Induction of apoptosis | ↑ AhR, Fas, FasL, Sirt1, Bax, Bid, cytochrome-c, SIRT1 c-caspase-8, -3, -9, c-PARP ↓ p-IκBα, NF-κB | [55] |
| NOD/SCID/γcnull mice/EL4 | 10, 50, 100 mg/kg; 38 days | Suppression of tumor growth Increase in survival time | |||||
| In vitro and in vivo | 11(13)-dehydroivaxillin | Carpesium genus | Daudi, Namalwa, SU-DHL-4, SU-DHL-2 | 5, 7, 10 μM; 24 h | Induction of apoptosis | [56] | |
| SU-DHL-2, NAMALWA | ↑ c-PARP, c-caspase-3 | ||||||
| Daudi, NAMALWA, SU-DHL-2 | 5, 10 μM; 6 h | ↓ cyclin D1, Bcl-2, IκBα, | |||||
| Daudi, SU-DHL-2 | 10 μM; 4 h | ↓ p-IκBα, p-p65 | |||||
| 5, 7 μM; 24 h | ↓ IKKα/IKKβ, c-MYC, cyclinD1, NF-κB | ||||||
| B-NSG mice(Daudi, SU-DHL-2 xenograft) | 50 mg/kg; 10 days | Inhibition of tumor growth | ↓ IKKα/IKKβ, PCNA |
5.3. Animal-Derived Compounds and Lymphoma
6. Discussion
Limitation of This Study
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mugnaini, E.N.; Ghosh, N. Lymphoma. Prim. Care 2016, 43, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Ambinder, R.F. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J. Clin. 2018, 68, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. Non-Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin. Proc. 2015, 90, 1152–1163. [Google Scholar] [CrossRef]
- Mondello, P.; Mian, M. Frontline treatment of diffuse large B-cell lymphoma: Beyond R-CHOP. Hematol. Oncol. 2019, 37, 333–344. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar]
- Werner, H.M.; Mills, G.B.; Ram, P.T. Cancer Systems Biology: A peek into the future of patient care? Nat. Rev. Clin. Oncol. 2014, 11, 167–176. [Google Scholar] [CrossRef]
- Du, W.; Elemento, O. Cancer systems biology: Embracing complexity to develop better anticancer therapeutic strategies. Oncogene 2015, 34, 3215–3225. [Google Scholar] [CrossRef]
- Jang, Y.G.; Go, R.E.; Hwang, K.A.; Choi, K.C. Resveratrol inhibits DHT-induced progression of prostate cancer cell line through interfering with the AR and CXCR4 pathway. J. Steroid Biochem. Mol. Biol. 2019, 192, 105406. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.; Hathout, R.M.; Sammour, O.A. Attempts to enhance the anti-cancer activity of curcumin as a magical oncological agent using transdermal delivery. Adv. Tradit. Med. 2020, 1–15. [Google Scholar] [CrossRef]
- Lim, H.J.; Park, M.N.; Kim, C.; Kang, B.; Song, H.S.; Lee, H.; Kim, S.H.; Shim, B.S.; Kim, B. MiR-657/ATF2 Signaling Pathway Has a Critical Role in Spatholobus suberectus Dunn Extract-Induced Apoptosis in U266 and U937 Cells. Cancers 2019, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Xiong, L.; Peng, C. (-)-Sativan Inhibits Tumor Development and Regulates miR-200c/PD-L1 in Triple Negative Breast Cancer Cells. Front. Pharm. 2020, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Zhu, H.; Meng, C.W.; Ren, Y.R.; Dai, O.; Xiong, L. New Isoflavanes from Spatholobus suberectus and Their Cytotoxicity against Human Breast Cancer Cell Lines. Molecules 2019, 24, 3218. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yi, S.S.; Lee, H.K.; Heo, T.H.; Park, S.K.; Jun, H.S.; Song, K.D.; Kim, S.J. Antiproliferative Effect of Vine Stem Extract from Spatholobus Suberectus Dunn on Rat C6 Glioma Cells Through Regulation of ROS, Mitochondrial Depolarization, and P21 Protein Expression. Nutr. Cancer 2018, 70, 605–619. [Google Scholar] [CrossRef]
- Kim, C.; Song, H.S.; Park, H.; Kim, B. Activation of ER Stress-Dependent miR-216b Has a Critical Role in Salviamiltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells. Int. J. Mol. Sci. 2018, 19, 1240. [Google Scholar] [CrossRef]
- Cha, J.A.; Song, H.-S.; Kang, B.; Park, M.N.; Park, K.S.; Kim, S.-H.; Shim, B.-S.; Kim, B. miR-211 Plays a Critical Role in Cnidium officinale Makino Extract-Induced, ROS/ER Stress-Mediated Apoptosis in U937 and U266 Cells. Int. J. Mol. Sci. 2018, 19, 865. [Google Scholar] [CrossRef]
- Teixeira, T.R.; Santos, G.S.D.; Armstrong, L.; Colepicolo, P.; Debonsi, H.M. Antitumor Potential of Seaweed Derived-Endophytic Fungi. Antibiotics 2019, 8, 205. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Bae, S.Y.; Liao, L.; Park, S.H.; Kim, W.K.; Shin, J.; Lee, S.K. Antitumor Activity of Asperphenin A, a Lipopeptidyl Benzophenone from Marine-Derived Aspergillus sp. Fungus, by Inhibiting Tubulin Polymerization in Colon Cancer Cells. Mar. Drugs 2020, 18, 110. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.H.; Li, Z.L.; Sun, Y.J.; Hua, H.M.; Liu, T.; Bai, J. Terpenoids from the Marine-Derived Fungus Aspergillus fumigatus YK-7. Molecules 2015, 21, 31. [Google Scholar] [CrossRef]
- Bao, J.; Wang, J.; Zhang, X.Y.; Nong, X.H.; Qi, S.H. New Furanone Derivatives and Alkaloids from the Co-Culture of Marine-Derived Fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 2017, 14, e1600327. [Google Scholar] [CrossRef]
- Hammerschmidt, L.; Aly, A.H.; Abdel-Aziz, M.; Müller, W.E.; Lin, W.; Daletos, G.; Proksch, P. Cytotoxic acyl amides from the soil fungus Gymnascella dankaliensis. Bioorganic Med. Chem. 2015, 23, 712–719. [Google Scholar] [CrossRef]
- Özkaya, F.C.; Ebrahim, W.; El-Neketi, M.; Tansel Tanrıkul, T.; Kalscheuer, R.; Müller, W.E.G.; Guo, Z.; Zou, K.; Liu, Z.; Proksch, P. Induction of new metabolites from sponge-associated fungus Aspergillus carneus by OSMAC approach. Fitoterapia 2018, 131, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Hartmann, R.; Plenker, M.; Mándi, A.; Kurtán, T.; Özkaya, F.C.; Müller, W.E.G.; Kassack, M.U.; Hamacher, A.; Lin, W.; et al. Brominated Azaphilones from the Sponge-Associated Fungus Penicillium canescens Strain 4.14.6a. J. Nat. Prod. 2019, 82, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Brötz-Oesterhelt, H.; Müller, W.E.G.; Wray, V.; Proksch, P. Bioactive polyketides and alkaloids from Penicillium citrinum, a fungal endophyte isolated from Ocimum tenuiflorum. Fitoterapia 2013, 91, 100–106. [Google Scholar] [CrossRef]
- El-Neketi, M.; Ebrahim, W.; Lin, W.; Gedara, S.; Badria, F.; Saad, H.E.; Lai, D.; Proksch, P. Alkaloids and polyketides from Penicillium citrinum, an endophyte isolated from the Moroccan plant Ceratonia siliqua. J. Nat. Prod. 2013, 76, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kurtán, T.; Yun Wang, C.; Han Lin, W.; Orfali, R.; Müller, W.E.; Daletos, G.; Proksch, P. Cladosporinone, a new viriditoxin derivative from the hypersaline lake derived fungus Cladosporium cladosporioides. J. Antibiot. 2016, 69, 702–706. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.M.; Harwoko, H.; Müller, W.E.G.; Hamacher, A.; Kassack, M.U.; Fouad, M.A.; Kamel, M.S.; Lin, W.; Ebrahim, W.; Liu, Z.; et al. Cyclic heptapeptides from the soil-derived fungus Clonostachys rosea. Bioorganic Med. Chem. 2019, 27, 3954–3959. [Google Scholar] [CrossRef]
- Yu, X.; Müller, W.E.; Guo, Z.; Lin, W.; Zou, K.; Liu, Z.; Proksch, P. Indole alkaloids from the coprophilous fungus Aphanoascus fulvescens. Fitoterapia 2019, 136, 104168. [Google Scholar]
- Umeokoli, B.O.; Ebrahim, W.; El-Neketi, M.; Müller, W.E.G.; Kalscheuer, R.; Lin, W.; Liu, Z.; Proksch, P. A new depsidone derivative from mangrove sediment derived fungus Lasiodiplodia theobromae. Nat. Prod. Res. 2019, 33, 2215–2222. [Google Scholar] [CrossRef]
- Uzor, P.F.; Ebrahim, W.; Osadebe, P.O.; Nwodo, J.N.; Okoye, F.B.; Müller, W.E.; Lin, W.; Liu, Z.; Proksch, P. Metabolites from Combretum dolichopetalum and its associated endophytic fungus Nigrospora oryzae--Evidence for a metabolic partnership. Fitoterapia 2015, 105, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Niveshika; Verma, E.; Maurya, S.K.; Mishra, R.; Mishra, A.K. The Combined Use of in Silico, in Vitro, and in Vivo Analyses to Assess Anti-cancerous Potential of a Bioactive Compound from Cyanobacterium Nostoc sp. MGL001. Front. Pharm. 2017, 8, 873. [Google Scholar] [CrossRef]
- Muharini, R.; Díaz, A.; Ebrahim, W.; Mándi, A.; Kurtán, T.; Rehberg, N.; Kalscheuer, R.; Hartmann, R.; Orfali, R.S.; Lin, W.; et al. Antibacterial and Cytotoxic Phenolic Metabolites from the Fruits of Amorpha fruticosa. J. Nat. Prod. 2017, 80, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Shigemi, Z.; Hara, N.; Moriguchi, M.; Ikeda, M.; Watanabe, T.; Fujimuro, M. Arctigenin induces the apoptosis of primary effusion lymphoma cells under conditions of glucose deprivation. Int. J. Oncol. 2018, 52, 505–517. [Google Scholar] [CrossRef]
- Zhao, Q.; Guan, J.; Qin, Y.; Ren, P.; Zhang, Z.; Lv, J.; Sun, S.; Zhang, C.; Mao, W. Curcumin sensitizes lymphoma cells to DNA damage agents through regulating Rad51-dependent homologous recombination. Biomed. Pharm. 2018, 97, 115–119. [Google Scholar] [CrossRef]
- Tafuku, S.; Ishikawa, C.; Yasumoto, T.; Mori, N. Anti-neoplastic effects of fucoxanthin and its deacetylated product, fucoxanthinol, on Burkitt’s and Hodgkin’s lymphoma cells. Oncol. Rep. 2012, 28, 1512–1518. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Nové, M.; Spengler, G.; Kúsz, N.; Hohmann, J.; Csupor, D. Antiproliferative and cytotoxic activities of furocoumarins of Ducrosia anethifolia. Pharm. Biol. 2018, 56, 658–664. [Google Scholar] [CrossRef]
- Nakano, D.; Ishitsuka, K.; Ikeda, M.; Tsuchihashi, R.; Okawa, M.; Okabe, H.; Tamura, K.; Kinjo, J. Screening of promising chemotherapeutic candidates from plants against human adult T-cell leukemia/lymphoma (IV): Phenanthroindolizidine alkaloids from Tylophora tanakae leaves. J. Nat. Med. 2015, 69, 397–401. [Google Scholar] [CrossRef]
- Chiruvella, K.K.; Raghavan, S.C. A natural compound, methyl angolensate, induces mitochondrial pathway of apoptosis in Daudi cells. Investig. New Drugs 2011, 29, 583–592. [Google Scholar] [CrossRef]
- Shi, L.; Qin, H.; Jin, X.; Yang, X.; Lu, X.; Wang, H.; Wang, R.; Yu, D.; Feng, B. The natural phenolic peperobtusin A induces apoptosis of lymphoma U937 cells via the Caspase dependent and p38 MAPK signaling pathways. Biomed. Pharm. 2018, 102, 772–781. [Google Scholar] [CrossRef]
- Martino, R.; Beer, M.F.; Elso, O.; Donadel, O.; Sülsen, V.; Anesini, C. Sesquiterpene lactones from Ambrosia spp. are active against a murine lymphoma cell line by inducing apoptosis and cell cycle arrest. Toxicol. In Vitro 2015, 29, 1529–1536. [Google Scholar] [CrossRef]
- Jara, P.; Spies, J.; Cárcamo, C.; Arancibia, Y.; Vargas, G.; Martin, C.; Salas, M.; Otth, C.; Zambrano, A. The Effect of Resveratrol on Cell Viability in the Burkitt’s Lymphoma Cell Line Ramos. Molecules 2017, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, C.; Zhou, J.; Cao, S.; Xu, C.; Tang, F.; Zhi, X.; Chen, B.; Wang, S.; Yin, L. Resveratrol inhibits Extranodal NK/T cell lymphoma through activation of DNA damage response pathway. J. Exp. Clin. Cancer Res. 2017, 36, 133. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zheng, W.; Hata Sugi, N.; Agarwala, K.L.; Xu, Q.; Wang, Z.; Tendyke, K.; Lee, W.; Parent, L.; Li, W.; et al. Small molecule schweinfurthins selectively inhibit cancer cell proliferation and mTOR/AKT signaling by interfering with trans-Golgi-network trafficking. Cancer Biol. Ther. 2015, 16, 589–601. [Google Scholar] [CrossRef]
- Hussain, A.R.; Uddin, S.; Ahmed, M.; Al-Dayel, F.; Bavi, P.P.; Al-Kuraya, K.S. Phosphorylated IκBα predicts poor prognosis in activated B-cell lymphoma and its inhibition with thymoquinone induces apoptosis via ROS release. PLoS ONE 2013, 8, e60540. [Google Scholar] [CrossRef]
- Hussain, A.R.; Ahmed, M.; Ahmed, S.; Manogaran, P.; Platanias, L.C.; Alvi, S.N.; Al-Kuraya, K.S.; Uddin, S. Thymoquinone suppresses growth and induces apoptosis via generation of reactive oxygen species in primary effusion lymphoma. Free Radic. Biol. Med. 2011, 50, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Vital, W.D.; Torquato, H.F.V.; Jesus, L.O.P.; Judice, W.A.S.; Silva, M.; Rodrigues, T.; Justo, G.Z.; Veiga, T.A.M.; Paredes-Gamero, E.J. 4-Deoxyraputindole C induces cell death and cell cycle arrest in tumor cell lines. J. Cell. Biochem. 2019, 120, 9608–9623. [Google Scholar] [CrossRef]
- Lv, L.N.; Wang, X.C.; Tao, L.J.; Li, H.W.; Li, S.Y.; Zheng, F.M. β-Asarone increases doxorubicin sensitivity by suppressing NF-κB signaling and abolishes doxorubicin-induced enrichment of stem-like population by destabilizing Bmi1. Cancer Cell Int. 2019, 19, 153. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Hu, Y.; Huang, P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by β-phenethyl isothiocyanate: Mechanisms for anti-leukemia activity. Antioxid Redox Signal. 2011, 15, 2911–2921. [Google Scholar] [CrossRef]
- Paterna, A.; Kincses, A.; Spengler, G.; Mulhovo, S.; Molnár, J.; Ferreira, M.U. Dregamine and tabernaemontanine derivatives as ABCB1 modulators on resistant cancer cells. Eur. J. Med. Chem. 2017, 128, 247–257. [Google Scholar] [CrossRef]
- Kumar, S.; Tomar, M.S.; Acharya, A. Chelerythrine delayed tumor growth and increased survival duration of Dalton’s lymphoma bearing BALB/c H(2d) mice by activation of NK cells in vivo. J. Cancer Res. Ther. 2015, 11, 904–910. [Google Scholar] [CrossRef]
- Peters, T.L.; Tillotson, J.; Yeomans, A.M.; Wilmore, S.; Lemm, E.; Jiménez-Romero, C.; Amador, L.A.; Li, L.; Amin, A.D.; Pongtornpipat, P.; et al. Target-Based Screening against eIF4A1 Reveals the Marine Natural Product Elatol as a Novel Inhibitor of Translation Initiation with In Vivo Antitumor Activity. Clin. Cancer Res. 2018, 24, 4256–4270. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishikawa, C.; Katano, H.; Yasumoto, T.; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011, 300, 225–234. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, Y.; Chen, G.; Xie, Y.; Xu, Z.; Chang, S.; Hu, L.; Li, B.; Bu, W.; Wang, Y.; et al. Targeting the PI3K/Akt/mTOR signaling pathway by pterostilbene attenuates mantle cell lymphoma progression. Acta Biochim. Biophys. Sin. 2018, 50, 782–792. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.P.; Hegde, V.L.; Guan, H.; Hofseth, L.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol (trans-3,5,4′-trihydroxystilbene) suppresses EL4 tumor growth by induction of apoptosis involving reciprocal regulation of SIRT1 and NF-κB. Mol. Nutr. Food Res. 2011, 55, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, H.; Jin, H.; Jin, J.; Yu, M.; Ma, C.; Tong, Y.; Zhou, L.; Lei, H.; Xu, H.; et al. Identification of 11(13)-dehydroivaxillin as a potent therapeutic agent against non-Hodgkin’s lymphoma. Cell Death Dis. 2017, 8, e3050. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Zayed, M.F.; Sayed, H.M. Ingenines A and B, Two New Alkaloids from the Indonesian Sponge Acanthostrongylophora ingens. Drug Res. 2015, 65, 361–365. [Google Scholar] [CrossRef][Green Version]
- Mokhlesi, A.; Hartmann, R.; Kurtán, T.; Weber, H.; Lin, W.; Chaidir, C.; Müller, W.E.G.; Daletos, G.; Proksch, P. New 2-Methoxy Acetylenic Acids and Pyrazole Alkaloids from the Marine Sponge Cinachyrella sp. Mar. Drugs 2017, 15, 356. [Google Scholar] [CrossRef]
- Emam, H.; Zhao, Q.L.; Furusawa, Y.; Refaat, A.; Ahmed, K.; Kadowaki, M.; Kondo, T. Apoptotic cell death by the novel natural compound, cinobufotalin. Chem. Biol. Interact. 2012, 199, 154–160. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Rast, S.; Hauschild, J.; Otte, K.; Alsdorf, W.H.; Madanchi, R.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Dierlamm, J.; et al. Frondoside A induces AIF-associated caspase-independent apoptosis in Burkitt lymphoma cells. Leuk. Lymphoma 2017, 58, 2905–2915. [Google Scholar] [CrossRef]
- Aiello, A.; Fattorusso, E.; Imperatore, C.; Menna, M.; Müller, W.E. Iodocionin, a cytotoxic iodinated metabolite from the Mediterranean ascidian Ciona edwardsii. Mar. Drugs 2010, 8, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Müller, W.E.G.; Lin, W.; Proksch, P. New Acyclic Cytotoxic Jasplakinolide Derivative from the Marine Sponge Jaspis splendens. Mar. Drugs 2019, 17, 100. [Google Scholar] [CrossRef]
- Pan, Z.; Qu, C.; Chen, Y.; Chen, X.; Liu, X.; Hao, W.; Xu, W.; Ye, L.; Lu, P.; Li, D.; et al. Bufotalin induces cell cycle arrest and cell apoptosis in human malignant melanoma A375 cells. Oncol. Rep. 2019, 41, 2409–2417. [Google Scholar] [CrossRef]
- Lin, S.; Lv, J.; Peng, P.; Cai, C.; Deng, J.; Deng, H.; Li, X.; Tang, X. Bufadienolides induce p53-mediated apoptosis in esophageal squamous cell carcinoma cells in vitro and in vivo. Oncol. Lett. 2018, 15, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Matasar, M.J.; Zelenetz, A.D. Overview of lymphoma diagnosis and management. Radiol. Clin. North Am. 2008, 46, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Seda, V.; Mraz, M. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur. J. Haematol. 2015, 94, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Puri, K.D.; Di Paolo, J.A.; Gold, M.R. B-cell receptor signaling inhibitors for treatment of autoimmune inflammatory diseases and B-cell malignancies. Int. Rev. Immunol. 2013, 32, 397–427. [Google Scholar] [CrossRef]
| Compound | Source | Cell Line/Animal Model | Dose; Duration | Efficacy | Reference |
|---|---|---|---|---|---|
| Alismol | Aspergillus fumigatus YK-7 | U937 | IC50 67.1 μM; 3 days | Inhibition of proliferation | [20] |
| aluminiumneohydroxyaspergillin | Co-culture of Aspergillus sclerotiorum and Penicillium | U937 | IC50 4.2 μM; 48 h | Induction of cytotoxicity | [21] |
| Aranorosin | Gymnascella dankaliensis | L5178Y | IC50 0.58 μM | Induction of cytotoxicity | [22] |
| Aranorosin-2-methylether | Gymnascella dankaliensis | L5178Y | IC50 0.44 μM | Induction of cytotoxicity | [22] |
| Asteltoxin E | Aspergillus carneus | L5178Y | IC50 0.2 μM | Induction of cytotoxicity | [23] |
| Bromophilone B | Penicillium canescens | L5178Y | IC50 8.9 μM | Induction of cytotoxicity | [24] |
| Citriquinochroman | Penicillium citrinum, var | L5178Y | IC50 6.1 μM | Induction of cytotoxicity | [26] |
| Cladosporinone | Cladosporium Cladosporioides (Fresen.) G.A. de Vries | L5187Y | IC50 0.88 μM | Induction of cytotoxicity | [27] |
| Cyclo-(Gly-D-Leu-D-allo-Ile-L-Val-L-Val-D-Trp-β-Ala) | Clonostachys rosea | L5178Y | IC50 4.1 μM | Induction of cytotoxicity | [28] |
| Gymnastatin A | Gymnascella dankaliensis | L5178Y | IC50 0.64 μM | Induction of cytotoxicity | [22] |
| Gymnastatin B | IC50 5.80 μM | [22] | |||
| Helvolic acid | Aspergillus fumigatus YK-7 | U937 | IC50 57.5 μM; 3 days | Inhibition of proliferation | [20] |
| Isopropylchetominine | Aspergillus carneus | L5178Y | IC50 0.4 μM | Induction of cytotoxicity | [23] |
| methyl 8-hydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate | Penicillium citrinum var. | L5178Y | IC50 0.78, 1.0 μg/mL | Induction of cytotoxicity | [25] |
| Okaramine A | Aphanoascus fulvescens (Cooke) Apinis | L5178Y | IC50 4.0 μM | Induction of cytotoxicity | [29] |
| Okaramine C | IC50 12.8 μM | ||||
| Okaramine G | IC50 13.8 μM | ||||
| Okaramine H | IC50 14.7 μM | ||||
| Pyripyropene E | Aspergillus fumigatus YK-7 | U937 | IC50 4.2 μM; 3 days | Inhibition of proliferation | [20] |
| Sterigmatocystin | Aspergillus carneus | L5178Y | IC50 0.3 μM | Induction of cytotoxicity | [23] |
| Verticillin D | Clonostachys rosea | L5178Y | IC50 0.1 μM | Induction of cytotoxicity | [28] |
| Viriditoxin | Cladosporium | L5187Y | IC50 0.1 μM | Inhibition of proliferation | [27] |
| 1H-Dibenzo (b, e) (1, 4) dioxepin- 11- one,3, 8- dihydroxy- 4-(methoxymethyl)-1,6-dimethyl | Lasiodiplodia theobromae | L5178Y | IC50 7.3 μM | Induction of cytotoxicity | [30] |
| 4-Dehydroxy-altersolanol A | Nigrospora oryzae | L5178Y | IC50 9.4 µM | Induction of cytotoxicity | [31] |
| 5-methyl alternariol ether | Penicillium citrinum var. | L5178Y | IC50 0.78, 1.0 μg/mL | Induction of cytotoxicity | [25] |
| 9-Ethyliminomethyl-12-(morpholin-4-ylmethoxy)-5,8,13,16-tetraaza -hexacene-2,3-dicarboxylic acid | cyanobacterium Nostoc sp. | DLA | IC50 372.4 ng/mL; 24 h | Induction of cytotoxicity | [32] |
| β-5,8,11-trihydroxybergamot-9-ene | Aspergillus fumigatus YK-7 | U937 | IC50 84.9 μM; 3 days | Inhibition of proliferation | [20] |
| Compound | Source | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Annomontine | Acanthostrongylophoraingens | L5178Y | ED50 7.8 μg/mL | Induction of cytotoxicity | [57] | |
| Cinachylenic Acid A, B, C, D | Cinachyrella sp. | L5178Y | IC50 0.3 μM | Induction of cytotoxicity | [58] | |
| Cinobufotalin | Toad | U937 | 0.5, 1 μM; 6, 12, 24 h | Decrease in cell viability and MMP Rapid release of cytosolic superoxide anion, increase in intracellular [Ca2+] | ↑ Fas, c-caspase-3, -8 ↓ Pro-caspase-2, -3, -8, -9, cytosolic Bid, cytosolic Bax | [59] |
| Frondoside A | Cucumaria frondosa | CA46, Namalwa, Ramos, BL-2 | 0.3, 0.6 μM; 48 h | Induction of cell cycle arrest | [60] | |
| CA46 | 0.3 μM; 48 h | Inhibition of prosurvival autophagy | ↑ LC3B-I/II, SQSTM1/p62 | |||
| 0.3, 0.6 μM; 48 h | Induction of apoptosis | ↑ c-PARP ↓ Survivin, Bcl-2 | ||||
| CA46, BL-2, Ramos | 0.3, 0.6 μM ;48 h | Induction of apoptosis | ↑ Cyt C, AIF, HtrA2/Omi | |||
| Ingenine B | Acanthostrongylophoraingens | L5178Y | ED50 9.1 μg/mL | Induction of cytotoxicity | [57] | |
| Iodocionin | Ciona edwardsii | L5178Y | 0.1, 0.3, 1, 3, 10 μg/mL; 72 h | Inhibition of cell proliferation | [61] | |
| (+)-Jasplakinolide. (+)-Jasplakinolide Z5, (+)-Jasplakinolide V | Jaspis splendens | L5178Y | IC50 < 100 nM | Induction of cytotoxicity | [62] | |
| (+)-Jasplakinolide Z6 | IC50 3.2 μM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Park, M.N.; Noh, S.; Kang, S.Y.; Kim, B. Review of Natural Compounds for the Management and Prevention of Lymphoma. Processes 2020, 8, 1164. https://doi.org/10.3390/pr8091164
Cho Y, Park MN, Noh S, Kang SY, Kim B. Review of Natural Compounds for the Management and Prevention of Lymphoma. Processes. 2020; 8(9):1164. https://doi.org/10.3390/pr8091164
Chicago/Turabian StyleCho, Yongmin, Moon Nyeo Park, Seungjin Noh, Seog Young Kang, and Bonglee Kim. 2020. "Review of Natural Compounds for the Management and Prevention of Lymphoma" Processes 8, no. 9: 1164. https://doi.org/10.3390/pr8091164
APA StyleCho, Y., Park, M. N., Noh, S., Kang, S. Y., & Kim, B. (2020). Review of Natural Compounds for the Management and Prevention of Lymphoma. Processes, 8(9), 1164. https://doi.org/10.3390/pr8091164