Ad-Dressing Stem Cells: Hydrogels for Encapsulation
Abstract
:1. Introduction
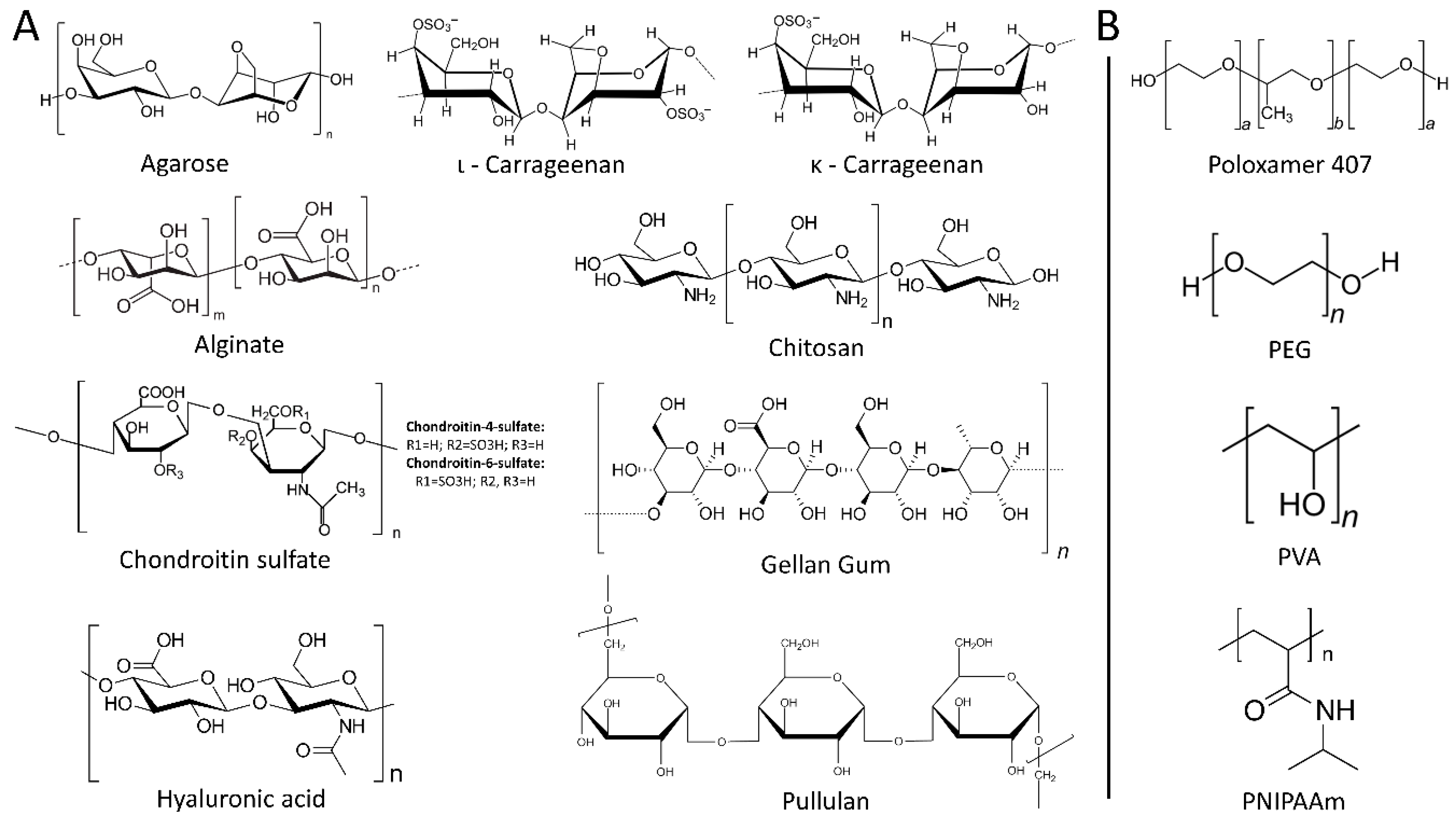
2. Hydrogels
2.1. Natural Hydrogels
2.1.1. Polysaccharides
Agarose
Alginate
Carrageenan
Chitosan
Chondroitin Sulfate (CS)
Gellan Gum
Hyaluronic Acid
Pullulan
2.1.2. Proteins
Collagen
Elastin
Elastin-Like Protein and Hyaluronic Acid
Fibrin
Gelatin
Keratin
Silk Fibroin
2.2. Synthetic Hydrogels
2.2.1. Poloxamer 407
2.2.2. Polyethylene Glycol (PEG)
2.2.3. Poly(N-isopropylacrylamide) (PNIPAAm)
2.2.4. Polyvinyl Alcohol (PVA)
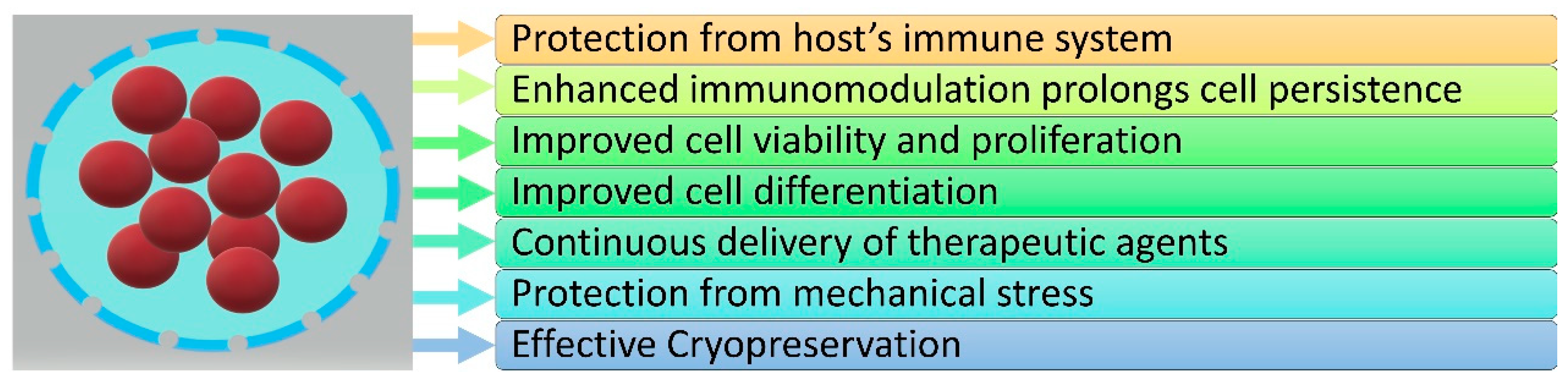
3. The Role of Hydrogels in Stem Cell Encapsulation
3.1. Protection from Host’s Immune System
3.2. Enhanced Immunomodulation Prolongs Cell Persistence
3.3. Improved Cell Viability and Proliferation
3.4. Improved Cell Differentiation
3.5. Continuous Delivery of Therapeutic Agents
3.6. Protection from Mechanical Stress
3.7. Effective Cryopreservation
4. Discussion—Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, X.L.; Zhang, Y.; Li, X.; Fu, Q.L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020. [Google Scholar] [CrossRef] [Green Version]
- Paspaliaris, V.; Kolios, G. Stem cells in Osteoporosis: From Biology to New Therapeutic Approaches. Stem Cells Int. 2019, 2019, 1730978. [Google Scholar] [CrossRef] [Green Version]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef]
- Sumer, H.; Liu, J.; Roh, S. Mesenchymal Stem Cells and Regenerative Medicine. Stem Cells Int. 2018, 2018, 9810972. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.C.; Wang, M.L.; Chen, E.Q.; Tang, H. Stem Cells Transplantation in the Treatment of Patients with Liver Failure. Curr. Stem Cell Res. Ther. 2018, 13, 193–201. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.J.; Kim, Y.A.; Lee, D.H.; Noh, J.K.; Kwon, C.H.; Jung, S.M.; Lee, S.K. Differentiation and major histocompatibility complex antigen expression in human liver-derived stem cells. Transplant. Proc. 2012, 44, 1113–1115. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, M.; Wang, F.; Heng, B.C.; Zhou, J.; Cai, Z.; Liu, H. Understanding the Immunological Mechanisms of Mesenchymal Stem Cells in Allogeneic Transplantation: From the Aspect of Major Histocompatibility Complex Class I. Stem Cells Dev. 2019, 28, 1141–1150. [Google Scholar] [CrossRef]
- Galvez-Martin, P.; Martin, J.M.; Ruiz, A.M.; Clares, B. Encapsulation in Cell Therapy: Methodologies, Materials, and Clinical Applications. Curr. Pharm. Biotechnol. 2017, 18, 365–377. [Google Scholar] [CrossRef]
- Kühtreiber, W.M.; Lanza, R.P.; Chick, W.L. Cell Encapsulation Technology and Therapeutics; Springer Science & Business Media: New York, NY, USA, 1999. [Google Scholar]
- Aeron, G.; Shiwangi, M. Immobilization and microencapsulation. J. Adv. Res. Biotechnol. 2017, 2, 1–4. [Google Scholar] [CrossRef]
- Hashemi, M.; Kalalinia, F. Application of encapsulation technology in stem cell therapy. Life Sci. 2015, 143, 139–146. [Google Scholar] [CrossRef]
- Chang, T.M. Semipermeable microcapsules. Science 1964, 146, 524–525. [Google Scholar] [CrossRef]
- Kanda, P.; Alarcon, E.I.; Yeuchyk, T.; Parent, S.; De Kemp, R.A.; Variola, F.; Courtman, D.; Stewart, D.J.; Davis, D.R. Deterministic Encapsulation of Human Cardiac Stem Cells in Variable Composition Nanoporous Gel Cocoons To Enhance Therapeutic Repair of Injured Myocardium. ACS Nano 2018, 12, 4338–4350. [Google Scholar] [CrossRef]
- Santos-Vizcaino, E.; Orive, G.; Pedraz, J.L.; Hernandez, R.M. Clinical Applications of Cell Encapsulation Technology. Methods Mol. Biol. 2020, 2100, 473–491. [Google Scholar] [CrossRef]
- Cerqueira, M.T.; Da Silva, L.P.; Santos, T.C.; Pirraco, R.P.; Correlo, V.M.; Reis, R.L.; Marques, A.P. Gellan gum-hyaluronic acid spongy-like hydrogels and cells from adipose tissue synergize promoting neoskin vascularization. ACS Appl. Mater. Interfaces 2014, 6, 19668–19679. [Google Scholar] [CrossRef]
- Steiner, D.; Lingens, L.; Fischer, L.; Kohn, K.; Detsch, R.; Boccaccini, A.R.; Fey, T.; Greil, P.; Weis, C.; Beier, J.P.; et al. Encapsulation of Mesenchymal Stem Cells Improves Vascularization of Alginate-Based Scaffolds. Tissue Eng. Part A 2018, 24, 1320–1331. [Google Scholar] [CrossRef]
- Young, S.A.; Flynn, L.E.; Amsden, B.G. Adipose-Derived Stem Cells in a Resilient In Situ Forming Hydrogel Modulate Macrophage Phenotype. Tissue Eng. Part A 2018, 24, 1784–1797. [Google Scholar] [CrossRef]
- Park, T.Y.; Jeon, E.Y.; Kim, H.J.; Choi, B.H.; Cha, H.J. Prolonged cell persistence with enhanced multipotency and rapid angiogenesis of hypoxia pre-conditioned stem cells encapsulated in marine-inspired adhesive and immiscible liquid micro-droplets. Acta Biomater. 2019, 86, 257–268. [Google Scholar] [CrossRef]
- Leslie, S.K.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Production of osteogenic and angiogenic factors by microencapsulated adipose stem cells varies with culture conditions. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell. Res. Ther. 2019, 10, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pentlavalli, S.; Chambers, P.; Sathy, B.N.; O’Doherty, M.; Chalanqui, M.; Kelly, D.J.; Haut-Donahue, T.; McCarthy, H.O.; Dunne, N.J. Simple Radical Polymerization of Poly(Alginate-Graft-N-Isopropylacrylamide) Injectable Thermoresponsive Hydrogel with the Potential for Localized and Sustained Delivery of Stem Cells and Bioactive Molecules. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Heo, D.N.; Hospodiuk, M.; Ozbolat, I.T. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019, 95, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Arfuso, F.; Sethi, G.; Dharmarajan, A.; Warrier, S. Encapsulated human mesenchymal stem cells (eMSCs) as a novel anti-cancer agent targeting breast cancer stem cells: Development of 3D primed therapeutic MSCs. Int. J. Biochem. Cell Biol. 2019, 110, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Rogan, H.; Ilagan, F.; Yang, F. Comparing Single Cell Versus Pellet Encapsulation of Mesenchymal Stem Cells in Three-Dimensional Hydrogels for Cartilage Regeneration. Tissue Eng. Part A 2019, 25, 1404–1412. [Google Scholar] [CrossRef]
- Naderi-Meshkin, H.; Andreas, K.; Matin, M.M.; Sittinger, M.; Bidkhori, H.R.; Ahmadiankia, N.; Bahrami, A.R.; Ringe, J. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol. Int. 2014, 38, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018, 66, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Shu, X.Z.; Prestwich, G.D. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006, 12, 3405–3416. [Google Scholar] [CrossRef]
- Diniz, I.M.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef] [Green Version]
- Zigon-Branc, S.; Markovic, M.; Van Hoorick, J.; Van Vlierberghe, S.; Dubruel, P.; Zerobin, E.; Baudis, S.; Ovsianikov, A. Impact of Hydrogel Stiffness on Differentiation of Human Adipose-Derived Stem Cell Microspheroids. Tissue Eng. Part A 2019, 25, 1369–1380. [Google Scholar] [CrossRef]
- Dollinger, B.R.; Gupta, M.K.; Martin, J.R.; Duvall, C.L. Reactive Oxygen Species Shielding Hydrogel for the Delivery of Adherent and Nonadherent Therapeutic Cell Types. Tissue Eng. Part A 2017, 23, 1120–1131. [Google Scholar] [CrossRef]
- Fath-Bayati, L.; Ai, J. Assessment of mesenchymal stem cell effect on foreign body response induced by intraperitoneally implanted alginate spheres. J. Biomed. Mater. Res. A 2020, 108, 94–102. [Google Scholar] [CrossRef]
- McCrary, M.R.; Jesson, K.; Wei, Z.Z.; Logun, M.; Lenear, C.; Tan, S.; Gu, X.; Jiang, M.Q.; Karumbaiah, L.; Yu, S.P.; et al. Cortical Transplantation of Brain-Mimetic Glycosaminoglycan Scaffolds and Neural Progenitor Cells Promotes Vascular Regeneration and Functional Recovery after Ischemic Stroke in Mice. Adv. Healthc. Mater. 2020, 9, e1900285. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Li, C.; Guan, Y.; Dang, Y.; Li, X.; Fan, Z.; Shen, J.; Ma, L.; Guan, J. High oxygen preservation hydrogels to augment cell survival under hypoxic condition. Acta Biomater. 2020, 105, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Lalegul-Ulker, O.; Seker, S.; Elcin, A.E.; Elcin, Y.M. Encapsulation of bone marrow-MSCs in PRP-derived fibrin microbeads and preliminary evaluation in a volumetric muscle loss injury rat model: Modular muscle tissue engineering. Artif. Cells Nanomed. Biotechnol. 2019, 47, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Fan, Z.; Xu, Y.; Niu, H.; Xie, X.; Liu, Z.; Guan, J. Thermosensitive and Highly Flexible Hydrogels Capable of Stimulating Cardiac Differentiation of Cardiosphere-Derived Cells under Static and Dynamic Mechanical Training Conditions. ACS Appl. Mater. Interfaces 2016, 8, 15948–15957. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Fan, Z.; Xu, Y.; Lo, W.; Wang, X.; Niu, H.; Li, X.; Xie, X.; Khan, M.; Guan, J. pH-Sensitive and Thermosensitive Hydrogels as Stem-Cell Carriers for Cardiac Therapy. ACS Appl. Mater. Interfaces 2016, 8, 10752–10760. [Google Scholar] [CrossRef]
- Tang, J.; Cui, X.; Caranasos, T.G.; Hensley, M.T.; Vandergriff, A.C.; Hartanto, Y.; Shen, D.; Zhang, H.; Zhang, J.; Cheng, K. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017, 11, 9738–9749. [Google Scholar] [CrossRef]
- Chang, S.; Finklea, F.; Williams, B.; Hammons, H.; Hodge, A.; Scott, S.; Lipke, E. Emulsion-based encapsulation of pluripotent stem cells in hydrogel microspheres for cardiac differentiation. Biotechnol. Prog. 2020, e2986. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lu, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C.; et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 2014, 35, 5679–5688. [Google Scholar] [CrossRef]
- Park, T.Y.; Oh, J.M.; Cho, J.S.; Sim, S.B.; Lee, J.; Cha, H.J. Stem cell-loaded adhesive immiscible liquid for regeneration of myocardial infarction. J. Control. Release 2020, 321, 602–615. [Google Scholar] [CrossRef]
- Ballios, B.G.; Cooke, M.J.; Donaldson, L.; Coles, B.L.; Morshead, C.M.; van der Kooy, D.; Shoichet, M.S. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Rep. 2015, 4, 1031–1045. [Google Scholar] [CrossRef] [Green Version]
- Scheper, V.; Hoffmann, A.; Gepp, M.M.; Schulz, A.; Hamm, A.; Pannier, C.; Hubka, P.; Lenarz, T.; Schwieger, J. Stem Cell Based Drug Delivery for Protection of Auditory Neurons in a Guinea Pig Model of Cochlear Implantation. Front. Cell. Neurosci. 2019, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.D.; Mendes, S.S.; Leite-Almeida, H.; Gimble, J.M.; Tam, R.Y.; Shoichet, M.S.; Sousa, N.; Silva, N.A.; Salgado, A.J. Combination of a peptide-modified gellan gum hydrogel with cell therapy in a lumbar spinal cord injury animal model. Biomaterials 2016, 105, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Zarei-Kheirabadi, M.; Sadrosadat, H.; Mohammadshirazi, A.; Jaberi, R.; Sorouri, F.; Khayyatan, F.; Kiani, S. Human embryonic stem cell-derived neural stem cells encapsulated in hyaluronic acid promotes regeneration in a contusion spinal cord injured rat. Int. J. Biol. Macromol. 2020, 148, 1118–1129. [Google Scholar] [CrossRef]
- Kumar, D.; Gerges, I.; Tamplenizza, M.; Lenardi, C.; Forsyth, N.R.; Liu, Y. Three-dimensional hypoxic culture of human mesenchymal stem cells encapsulated in a photocurable, biodegradable polymer hydrogel: A potential injectable cellular product for nucleus pulposus regeneration. Acta Biomater. 2014, 10, 3463–3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickers, L.; Thorpe, A.A.; Snuggs, J.; Sammon, C.; Le Maitre, C.L. Mesenchymal stem cell therapies for intervertebral disc degeneration: Consideration of the degenerate niche. JOR Spine 2019, 2, e1055. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo San Jose, L.; Stephens, P.; Song, B.; Barrow, D. Microfluidic Encapsulation Supports Stem Cell Viability, Proliferation, and Neuronal Differentiation. Tissue Eng. Part C Methods 2018, 24, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Fan, L.; Xing, J.; Wang, Q.; Lin, C.; Liu, C.; Deng, X.; Ning, C.; Zhou, L.; Rong, L.; et al. Inhibition of astrocytic differentiation of transplanted neural stem cells by chondroitin sulfate methacrylate hydrogels for the repair of injured spinal cord. Biomater. Sci. 2019, 7, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Aijaz, A.; Teryek, M.; Goedken, M.; Polunas, M.; Olabisi, R.M. Coencapsulation of ISCs and MSCs Enhances Viability and Function of both Cell Types for Improved Wound Healing. Cell. Mol. Bioeng. 2019, 12, 481–493. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Huang, S.; Wen, J.; Jiao, Y.; Su, X.; Shi, G.; Huang, J. PF-127 hydrogel plus sodium ascorbyl phosphate improves Wharton’s jelly mesenchymal stem cell-mediated skin wound healing in mice. Stem Cell Res. Ther. 2020, 11, 143. [Google Scholar] [CrossRef] [Green Version]
- Kaisang, L.; Siyu, W.; Lijun, F.; Daoyan, P.; Xian, C.J.; Jie, S. Adipose-derived stem cells seeded in Pluronic F-127 hydrogel promotes diabetic wound healing. J. Surg. Res. 2017, 217, 63–74. [Google Scholar] [CrossRef]
- Al-Jaibaji, O.; Swioklo, S.; Gijbels, K.; Vaes, B.; Figueiredo, F.C.; Connon, C.J. Alginate encapsulated multipotent adult progenitor cells promote corneal stromal cell activation via release of soluble factors. PLoS ONE 2018, 13, e0202118. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.R.; Quiros, M.; Han, W.M.; O’Leary, M.N.; Cox, G.N.; Nusrat, A.; Garcia, A.J. IFN-gamma-tethered hydrogels enhance mesenchymal stem cell-based immunomodulation and promote tissue repair. Biomaterials 2019, 220, 119403. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasturk, O.; Kaplan, D.L. Cell armor for protection against environmental stress: Advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater. 2019, 95, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bae, C.; Kook, Y.M.; Koh, W.G.; Lee, K.; Park, M.H. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res. Ther. 2019, 10, 51. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Soleimani Rad, J. Chemical gelling of hydrogels-based biological macromolecules for tissue engineering: Photo- and enzymatic-crosslinking methods. Int. J. Biol. Macromol. 2019, 139, 760–772. [Google Scholar] [CrossRef]
- Kamperman, T.; Karperien, M.; Le Gac, S.; Leijten, J. Single-Cell Microgels: Technology, Challenges, and Applications. Trends Biotechnol. 2018, 36, 850–865. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Del. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Lee, D.; Cho, S.; Park, H.S.; Kwon, I. Ocular Drug Delivery through pHEMA-Hydrogel Contact Lenses Co-Loaded with Lipophilic Vitamins. Sci. Rep. 2016, 6, 34194. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Uludag, H.; De Vos, P.; Tresco, P.A. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 2000, 42, 29–64. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and Its Derivatives as Supports for Enzyme Immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Dang, S.M.; Gerecht-Nir, S.; Chen, J.; Itskovitz-Eldor, J.; Zandstra, P.W. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells 2004, 22, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Batorsky, A.; Liao, J.; Lund, A.W.; Plopper, G.E.; Stegemann, J.P. Encapsulation of adult human mesenchymal stem cells within collagen-agarose microenvironments. Biotechnol. Bioeng. 2005, 92, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hashimoto, I.; Kawakami, K. Production of cell-enclosing hollow-core agarose microcapsules via jetting in water-immiscible liquid paraffin and formation of embryoid body-like spherical tissues from mouse ES cells enclosed within these microcapsules. Biotechnol. Bioeng. 2008, 99, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Babace, A.; Haase, K.; Stewart, D.J.; Godin, M. Strategies for controlling egress of therapeutic cells from hydrogel microcapsules. J. Tissue Eng. Regen. Med. 2019, 13, 612–624. [Google Scholar] [CrossRef]
- Sarker, B.; Zehnder, T.; Rath, S.N.; Horch, R.E.; Kneser, U.; Detsch, R.; Boccaccini, A.R. Oxidized Alginate-Gelatin Hydrogel: A Favorable Matrix for Growth and Osteogenic Differentiation of Adipose-Derived Stem Cells in 3D. ACS Biomater. Sci. Eng. 2017, 3, 1730–1737. [Google Scholar] [CrossRef]
- Rezaei, S.; Shakibaie, M.; Kabir-Salmani, M.; Soltani Moghaddam, M.; Rezvani, M.; Shahali, M.; Naseri, M. Improving the Growth Rate of Human Adipose-Derived Mesenchymal Stem Cells in Alginate/Gelatin Versus Alginate Hydrogels. Iran. J. Biotechnol. 2016, 14, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popa, E.; Reis, R.; Gomes, M. Chondrogenic phenotype of different cells encapsulated in kappa-carrageenan hydrogels for cartilage regeneration strategies. Biotechnol. Appl. Biochem. 2012, 59, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, P.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. J. Bioact. Compat. Polym. 2011, 26, 493–507. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.; Jaiswal, M.K.; Peak, C.W.; Carrow, J.K.; Gentry, J.; Dolatshahi-Pirouz, A.; Gaharwar, A.K. Injectable shear-thinning nanoengineered hydrogels for stem cell delivery. Nanoscale 2016, 8, 12362–12372. [Google Scholar] [CrossRef] [Green Version]
- Kas, H.S. Chitosan: Properties, preparations and application to microparticulate systems. J. Microencapsul. 1997, 14, 689–711. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Alblas, J.; de Wijn, J.R.; Hennink, W.E.; Verbout, A.J.; Dhert, W.J. Hydrogels as extracellular matrices for skeletal tissue engineering: State-of-the-art and novel application in organ printing. Tissue Eng. 2007, 13, 1905–1925. [Google Scholar] [CrossRef]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Ma, Y.; Mao, J.; Zhang, Z.; Tan, H. Cytocompatible in situ forming chitosan/hyaluronan hydrogels via a metal-free click chemistry for soft tissue engineering. Acta Biomater. 2015, 20, 60–68. [Google Scholar] [CrossRef]
- Baeurle, S.A.; Kiselev, M.G.; Makarova, E.S.; Nogovitsin, E.A. Effect of the counterion behavior on the frictional-compressive properties of chondroitin sulfate solutions. Polymer 2009, 50, 1805–1813. [Google Scholar] [CrossRef]
- Foot, M.; Mulholland, M. Classification of chondroitin sulfate A, chondroitin sulfate C, glucosamine hydrochloride and glucosamine 6 sulfate using chemometric techniques. J. Pharm. Biomed. Anal. 2005, 38, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Karumbaiah, L.; Enam, S.F.; Brown, A.C.; Saxena, T.; Betancur, M.I.; Barker, T.H.; Bellamkonda, R.V. Chondroitin Sulfate Glycosaminoglycan Hydrogels Create Endogenous Niches for Neural Stem Cells. Bioconjug. Chem. 2015, 26, 2336–2349. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Veeder, G.T.; Mirrasoul, P.J.; Kaneko, T.; Cottrell, I.W. Agar-like polysaccharide produced by a pseudomonas species: Production and basic properties. Appl. Environ. Microbiol. 1982, 43, 1086–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansson, P.E.; Lindberg, B.; Sandford, P.A. Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohydr. Res. 1983, 124, 135–139. [Google Scholar] [CrossRef]
- Koivisto, J.T.; Joki, T.; Parraga, J.E.; Paakkonen, R.; Yla-Outinen, L.; Salonen, L.; Jonkkari, I.; Peltola, M.; Ihalainen, T.O.; Narkilahti, S.; et al. Bioamine-crosslinked gellan gum hydrogel for neural tissue engineering. Biomed. Mater. 2017, 12, 025014. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Morgelin, M.; Heinegard, D.; Engel, J.; Paulsson, M. The cartilage proteoglycan aggregate: Assembly through combined protein-carbohydrate and protein-protein interactions. Biophys. Chem. 1994, 50, 113–128. [Google Scholar] [CrossRef]
- Nettles, D.L.; Vail, T.P.; Morgan, M.T.; Grinstaff, M.W.; Setton, L.A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann. Biomed. Eng. 2004, 32, 391–397. [Google Scholar] [CrossRef]
- Chung, C.; Mesa, J.; Randolph, M.A.; Yaremchuk, M.; Burdick, J.A. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J. Biomed. Mater. Res. A 2006, 77, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, S.H.; Kim, B.S.; Cho, Y.S.; Park, Y. Development and Evaluation of Hyaluronic Acid-Based Hybrid Bio-Ink for Tissue Regeneration. Tissue Eng. Regen. Med. 2018, 15, 761–769. [Google Scholar] [CrossRef] [PubMed]
- San Juan, A.; Hlawaty, H.; Chaubet, F.; Letourneur, D.; Feldman, L.J. Cationized pullulan 3D matrices as new materials for gene transfer. J. Biomed. Mater. Res. A 2007, 82, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Leathers, T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2003, 62, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Maier, V.; Maier, S.S.; Butnaru, M.; Danu, M.; Ibanescu, C.; Pinteala, M.; Popa, M. Biomacromolecular-based ionic-covalent hydrogels for cell encapsulation: The atelocollagen—Oxidized polysaccharides couples. Carbohydr. Polym. 2017, 169, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014, 802, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Kuo, K.C.; Lin, R.Z.; Tien, H.W.; Wu, P.Y.; Li, Y.C.; Melero-Martin, J.M.; Chen, Y.C. Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater. 2015, 27, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Rosenbloom, J. Elastin: Relation of protein and gene structure to disease. Lab. Investig. 1984, 51, 605–623. [Google Scholar]
- Ozsvar, J.; Mithieux, S.M.; Wang, R.; Weiss, A.S. Elastin-based biomaterials and mesenchymal stem cells. Biomater. Sci. 2015, 3, 800–809. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Hou, C.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013, 34, 413–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, P.J.; Bryant, S.J.; Anseth, K.S. Tailoring the degradation of hydrogels formed from multivinyl poly(ethylene glycol) and poly(vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules 2003, 4, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhu, D.; Paul, A.; Cai, L.; Enejder, A.; Yang, F.; Heilshorn, S.C. Covalently Adaptable Elastin-Like Protein–Hyaluronic Acid (ELP–HA) Hybrid Hydrogels with Secondary Thermoresponsive Crosslinking for Injectable Stem Cell Delivery. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Tzouanas, S.N.; Ekenseair, A.K.; Kasper, F.K.; Mikos, A.G. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. J. Biomed. Mater. Res. A 2014, 102, 1222–1230. [Google Scholar] [CrossRef] [Green Version]
- Aparnathi, M.K.; Patel, J.S. Biodegradable Gelatin Methacrylate Gel as a Potential Scaffold for Bone Tissue Engineering of Canine Adipose-Derived Stem Cells. J. Stem Cells 2016, 11, 111–119. [Google Scholar]
- Sung, B.; Krieger, J.; Yu, B.; Kim, M.H. Colloidal gelatin microgels with tunable elasticity support the viability and differentiation of mesenchymal stem cells under pro-inflammatory conditions. J. Biomed. Mater. Res. A 2018, 106, 2753–2761. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Barati, D.; Kader, S.; Pajoum Shariati, S.R.; Moeinzadeh, S.; Sawyer, R.H.; Jabbari, E. Synthesis and Characterization of Photo-Cross-Linkable Keratin Hydrogels for Stem Cell Encapsulation. Biomacromolecules 2017, 18, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Singh, N. Silk fibroin-alginate based beads for human mesenchymal stem cell differentiation in 3D. Biomater. Sci. 2019, 7, 4687–4697. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, I.R. Artificial skin. I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.A.; Marks, W.H.; Bhatia, S.K. Hydrogel encapsulation to improve cell viability during syringe needle flow. J. Long. Term Eff. Med. Implants 2014, 24, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, O.K.; Lee, J.; Noh, J.; Lee, S.; Park, A.; Rim, M.A.; Reis, R.L.; Khang, G. Evaluation of double network hydrogel of poloxamer-heparin/gellan gum for bone marrow stem cells delivery carrier. Colloids Surf. B. Biointerfaces 2019, 181, 879–889. [Google Scholar] [CrossRef]
- Harris, J.M. Introduction to biomedical and biotechnical applications of polyethylene glycol. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 1997, 38, 520–521. [Google Scholar]
- Choe, G.; Park, J.; Park, H.; Lee, J.Y. Hydrogel Biomaterials for Stem Cell Microencapsulation. Polymers 2018, 10, 997. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Truong, V.X.; Thissen, H.; Frith, J.E.; Forsythe, J.S. Microfluidic Encapsulation of Human Mesenchymal Stem Cells for Articular Cartilage Tissue Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 8589–8601. [Google Scholar] [CrossRef]
- Hunckler, M.D.; Medina, J.D.; Coronel, M.M.; Weaver, J.D.; Stabler, C.L.; Garcia, A.J. Linkage Groups within Thiol-Ene Photoclickable PEG Hydrogels Control In Vivo Stability. Adv. Healthc. Mater. 2019, 8, e1900371. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Acuna, R.; Quiros, M.; Huang, S.; Siuda, D.; Spence, J.R.; Nusrat, A.; Garcia, A.J. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat. Protoc. 2018, 13, 2102–2119. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Ekerdt, B.L.; Fuentes, C.M.; Lei, Y.; Adil, M.M.; Ramasubramanian, A.; Segalman, R.A.; Schaffer, D.V. Thermoreversible Hyaluronic Acid-PNIPAAm Hydrogel Systems for 3D Stem Cell Culture. Adv. Healthc. Mater. 2018, 7, e1800225. [Google Scholar] [CrossRef]
- de Vos, P.; Lazarjani, H.A.; Poncelet, D.; Faas, M.M. Polymers in cell encapsulation from an enveloped cell perspective. Adv. Drug Deliv. Rev. 2014, 67–68, 15–34. [Google Scholar] [CrossRef]
- Vrana, N.E.; O’Grady, A.; Kay, E.; Cahill, P.A.; McGuinness, G.B. Cell encapsulation within PVA-based hydrogels via freeze-thawing: A one-step scaffold formation and cell storage technique. J. Tissue Eng. Regen. Med. 2009, 3, 567–572. [Google Scholar] [CrossRef]
- Qi, Z.; Shen, Y.; Yanai, G.; Yang, K.; Shirouzu, Y.; Hiura, A.; Sumi, S. The in vivo performance of polyvinyl alcohol macro-encapsulated islets. Biomaterials 2010, 31, 4026–4031. [Google Scholar] [CrossRef] [Green Version]
- Dulieu, C.; Bazile, D. Influence of lipid nanocapsules composition on their aptness to freeze-drying. Pharm. Res. 2005, 22, 285–292. [Google Scholar] [CrossRef]
- Qi, M.; Gu, Y.; Sakata, N.; Kim, D.; Shirouzu, Y.; Yamamoto, C.; Hiura, A.; Sumi, S.; Inoue, K. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials 2004, 25, 5885–5892. [Google Scholar] [CrossRef] [Green Version]
- Burczak, K.; Gamian, E.; Kochman, A. Long-term in vivo performance and biocompatibility of poly(vinyl alcohol) hydrogel macrocapsules for hybrid-type artificial pancreas. Biomaterials 1996, 17, 2351–2356. [Google Scholar] [CrossRef]
- Oda, H.; Konno, T.; Ishihara, K. Efficient differentiation of stem cells encapsulated in a cytocompatible phospholipid polymer hydrogel with tunable physical properties. Biomaterials 2015, 56, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, S.; Rao, W.; Snyder, J.; Choi, J.K.; Wang, J.; Khan, I.A.; Saleh, N.B.; Mohler, P.J.; Yu, J.; et al. A Novel Core-Shell Microcapsule for Encapsulation and 3D Culture of Embryonic Stem Cells. J. Mater. Chem. B 2013, 2013, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Ozkale, B.; Shah, N.J.; Vining, K.H.; Descombes, T.; Zhang, L.; Tringides, C.M.; Wong, S.W.; Shin, J.W.; Scadden, D.T.; et al. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc. Natl. Acad. Sci. USA 2019, 116, 15392–15397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, A.E.; Lohan, P.; O’Flynn, L.; Treacy, O.; Chen, X.; Coleman, C.; Shaw, G.; Murphy, M.; Barry, F.; Griffin, M.D.; et al. Chondrogenic differentiation increases antidonor immune response to allogeneic mesenchymal stem cell transplantation. Mol. Ther. 2014, 22, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldana, L.; Bensiamar, F.; Valles, G.; Mancebo, F.J.; Garcia-Rey, E.; Vilaboa, N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell. Res. Ther. 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Swartzlander, M.D.; Blakney, A.K.; Amer, L.D.; Hankenson, K.D.; Kyriakides, T.R.; Bryant, S.J. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials 2015, 41, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leijs, M.J.; Villafuertes, E.; Haeck, J.C.; Koevoet, W.J.; Fernandez-Gutierrez, B.; Hoogduijn, M.J.; Verhaar, J.A.; Bernsen, M.R.; van Buul, G.M.; van Osch, G.J. Encapsulation of allogeneic mesenchymal stem cells in alginate extends local presence and therapeutic function. Eur. Cell Mater. 2017, 33, 43–58. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Khatab, S.; Leijs, M.J.; van Buul, G.; Haeck, J.; Kops, N.; Nieboer, M.; Bos, P.K.; Verhaar, J.A.N.; Bernsen, M.; van Osch, G. MSC encapsulation in alginate microcapsules prolongs survival after intra-articular injection, a longitudinal in vivo cell and bead integrity tracking study. Cell. Biol. Toxicol. 2020. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Li, C.; Chen, J.; Li, Y.; Xie, H.; Lin, C.; Fan, M.; Guo, Y.; Gao, E.; et al. Tailorable Hydrogel Improves Retention and Cardioprotection of Intramyocardial Transplanted Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. J. Am. Heart Assoc. 2020, 9, e013784. [Google Scholar] [CrossRef]
- Ho, S.S.; Vollmer, N.L.; Refaat, M.I.; Jeon, O.; Alsberg, E.; Lee, M.A.; Leach, J.K. Bone Morphogenetic Protein-2 Promotes Human Mesenchymal Stem Cell Survival and Resultant Bone Formation When Entrapped in Photocrosslinked Alginate Hydrogels. Adv. Healthc. Mater. 2016, 5, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Hyzy, S.L.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. BMP2 induces osteoblast apoptosis in a maturation state and noggin-dependent manner. J. Cell. Biochem. 2012, 113, 3236–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vries-van Melle, M.L.; Tihaya, M.S.; Kops, N.; Koevoet, W.J.; Murphy, J.M.; Verhaar, J.A.; Alini, M.; Eglin, D.; van Osch, G.J. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in a simulated osteochondral environment is hydrogel dependent. Eur. Cell Mater. 2014, 27, 112–123; discussion 123. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zheng, J.; Wang, H.; Becker, M.L.; Leipzig, N.D. Neural stem cell encapsulation and differentiation in strain promoted crosslinked polyethylene glycol-based hydrogels. J. Biomater. Appl. 2018, 32, 1222–1230. [Google Scholar] [CrossRef]
- Hung, B.P.; Harvestine, J.N.; Saiz, A.M.; Gonzalez-Fernandez, T.; Sahar, D.E.; Weiss, M.L.; Leach, J.K. Defining hydrogel properties to instruct lineage- and cell-specific mesenchymal differentiation. Biomaterials 2019, 189, 1–10. [Google Scholar] [CrossRef]
- Prestwich, G.D. Engineering a clinically-useful matrix for cell therapy. Organogenesis 2008, 4, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef] [Green Version]
- Foster, A.A.; Dewi, R.E.; Cai, L.; Hou, L.; Strassberg, Z.; Alcazar, C.A.; Heilshorn, S.C.; Huang, N.F. Protein-engineered hydrogels enhance the survival of induced pluripotent stem cell-derived endothelial cells for treatment of peripheral arterial disease. Biomater. Sci. 2018, 6, 614–622. [Google Scholar] [CrossRef]
- Khetan, S.; Corey, O. Maintenance of stem cell viability and differentiation potential following cryopreservation within 3-dimensional hyaluronic acid hydrogels. Cryobiology 2019, 90, 83–88. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, X.; Zhu, K.; He, X. Hydrogel Encapsulation Facilitates Rapid-Cooling Cryopreservation of Stem Cell-Laden Core-Shell Microcapsules as Cell-Biomaterial Constructs. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Choi, J.K.; Rao, W.; Zhao, S.; Agarwal, P.; Zhao, G.; He, X. Alginate Hydrogel Microencapsulation Inhibits Devitrification and Enables Large-Volume Low-CPA Cell Vitrification. Adv. Funct. Mater. 2015, 25, 6839–6850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambu, S.; Xu, X.; Schiffer, H.A.; Cui, Z.F.; Ye, H. RGDS-fuctionalized alginates improve the survival rate of encapsulated embryonic stem cells during cryopreservation. Cryo Lett. 2011, 32, 389–401. [Google Scholar]
- Pravdyuk, A.I.; Petrenko, Y.A.; Fuller, B.J.; Petrenko, A.Y. Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology 2013, 66, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell Encapsulation within Alginate Microcapsules: Immunological Challenges and Outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Mendez, T.B.; Santos-Vizcaino, E.; Pedraz, J.L.; Hernandez, R.M.; Orive, G. Cell microencapsulation technologies for sustained drug delivery: Clinical trials and companies. Drug Discov. Today 2020. [Google Scholar] [CrossRef]
- Heile, A.; Brinker, T. Clinical translation of stem cell therapy in traumatic brain injury: The potential of encapsulated mesenchymal cell biodelivery of glucagon-like peptide-1. Dialogues Clin. Neurosci. 2011, 13, 279–286. [Google Scholar] [CrossRef]
- CellMed, A. GLP-1 CellBeads® for the Treatment of Stroke Patients with Space-Occupying Intracerebral Hemorrhage. NCT01298830. Available online: http://www.clinicaltrials.gov (accessed on 13 May 2016).

| Application | Biomaterial |
|---|---|
| Angiogenesis | Gelan gum-HA [15], Alginate-Gelatin [16], PEG [17], HA-MAP [18] |
| Bone tissue engineering | Alginate [19], GelMA [20], Alginate-g-PNIPAAm [21], Collagen-Fibrin [22] |
| Breast cancer | Alginate [23] |
| Cartilage tissue engineering | CS-MA-PEGDA [24], CH-GP-HEC [25], PEG-PNIPAAm [26], Gelatin-HA [27] |
| Cartilage/bone tissue engineering | Poloxamer 407 [28], Gelatin-Methacrylamide [29] |
| Diabetes | PPS-b-PDMA-b-PNIPAAm [30] |
| Foreign body response | Alginate [31] |
| Ischemic stroke | CS-bFGF [32] |
| Ischemic tissue engineering | PNIPAAm-PHEMA-AOLA-PEGMA-PFO [33] |
| Muscle tissue engineering | Fibrin [34] |
| Myocardial infarction | PNIPAAm [35], PNIPAAm-PPAA-PHEMA-OTMC-PEGMA [36], PNIPAAm-PAA [37], PEG-fibrinogen [38], PNIPAAm-SWCNTs [39], HA-MAP [40] |
| Neural and visual repairment | HA and Methylecelluse [41] |
| Sensorineural hearing loss | Alginate (Ultra high viscous) [42] |
| Spine injury | Gelan gum [43], HA [44], PHEMA-APMA-PAMAM [45], L-PDMA-PNIPAAm [46], Alginate-Collagen [47], CS-MA [48] |
| Wound healing (skin) | PEGDA [49], Poloxamer 407-SAP [50] |
| Wound healing (diabetes) | Poloxamer 407 [51] |
| Wound healing (corneal) | Alginate [52] |
| Wound Healing (mucosal) | PEG-4MAL-IFN-γ [53] |
| Stem Cell Type | Biomaterial |
|---|---|
| Mesenchymal stem cells | Collagen-Fibrin [22], CH-GP-HEC [25], PHEMA-APMA-PAMAM [45], L-PDMA-PNIPAAm [46], PEG-PNIPAAm [26], Alginate [23], PEGDA [49], Fibrin [34], Gelatin-HA [27], PPS-b-PDMA-b-PNIPAAm [30], Alginate-Gelatin [16], PEG-4MAL-IFN-γ [53], HA-MAP [18,40] |
| Bone marrow mesenchymal stem cells | PNIPAAm-PHEMA-AOLA-PEGMA-PFO [33], CS-MA-PEGDA [24], Alginate-g-PNIPAAm [21] |
| Wharton’s jelly mesenchymal stem cells | Poloxamer 407-SAP [50] |
| BMP-2 transduced bone marrow mesenchymal stem cells | GelMA [20] |
| Brain-derived neurotrophic factor-producing mesenchymal stem cells | Alginate (ultrahigh viscous) [42] |
| Adipose-derived stem cells | Gelan gum-HA [15], Poloxamer 407 [51], Gelan gum [43], PNIPAAm-SWCNTs [39], Alginate [19], PEG [17] |
| Telomerase-immortalized human adipose-derived stem cells | Gelatin-Methacrylamide [29] |
| Cardiosphere-derived cells | PNIPAAm [35], PNIPAAm-PPAA-PHEMA-OTMC-PEGMA [36] |
| Human cardiac stem cells | PNIPAAm-PAA [37] |
| Neural stem cells-dental pulp stem cells | Alginate-Collagen [47], CS-MA [48] |
| Induced pluripotent stem cell-derived neural progenitor cells | CS-bFGF [32] |
| Human embryonic stem cell derived-neural stem cells | HA [44] |
| Retinal stem cell-derived rods and neural stem and progenitor cells | HA and methylecelluse [41] |
| Dental pulp stem cells | Poloxamer 407 [28] |
| Human placenta-derived mesenchymal stem cells | Alginate [31] |
| Multipotent adult progenitor cells | Alginate [52] |
| Mouse embryonic stem cells | PEG-fibrinogen [38] |
| Encapsulation Methods | Cross-Linking (Gelation) Methods | |
|---|---|---|
Lithography
| Chemical cross-linking
| Physical cross-linking
|
Extrusion
| ||
Microfluidics
| ||
Bioprinting
| ||
| Emulsification | ||
| Cryogelation | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandilogiannakis, L.; Filidou, E.; Kolios, G.; Paspaliaris, V. Ad-Dressing Stem Cells: Hydrogels for Encapsulation. Processes 2021, 9, 11. https://doi.org/10.3390/pr9010011
Kandilogiannakis L, Filidou E, Kolios G, Paspaliaris V. Ad-Dressing Stem Cells: Hydrogels for Encapsulation. Processes. 2021; 9(1):11. https://doi.org/10.3390/pr9010011
Chicago/Turabian StyleKandilogiannakis, Leonidas, Eirini Filidou, George Kolios, and Vasilis Paspaliaris. 2021. "Ad-Dressing Stem Cells: Hydrogels for Encapsulation" Processes 9, no. 1: 11. https://doi.org/10.3390/pr9010011






