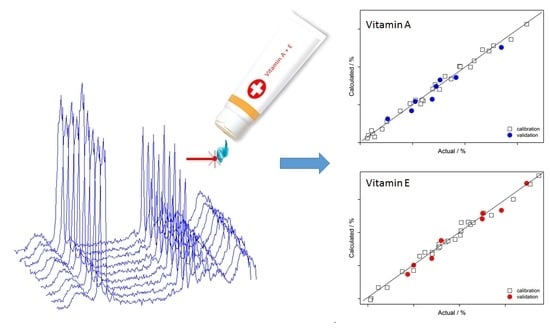
Quantitative Determination of Vitamins A and E in Ointments Using Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Chemicals
2.3. Sample Preparation
2.4. Modeling and Spectral Data Treatment
3. Results
3.1. Preparation of the Ointment Base
3.2. PLS Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, M.A. Retinoic acid–Independent actions of the naturally occuring retinoids: Retinol, the retroretinoids, and 4-oxoretinol. In Vitamin A: New Research; Loessing, I.T., Ed.; Nova Biomdical Books: New York, NY, USA, 2007; pp. 25–38. ISBN 9781600216688. [Google Scholar]
- Tomasetti, M.; Alleva, R.; Wang, X.; Neuzil, J. Vitamin E analogs and potentation of cancer therapy. In Vitamin E: New Research; Braunstein, M.H., Ed.; Nova Science Publishers: New York, NY, USA, 2006; pp. 21–38. ISBN 9781594549700. [Google Scholar]
- USP29-NF. Vitamin A Monograph, Vitamin E Monograph; US Pharmacopeial Convention: Rockville, MD, USA, 2006; pp. 2258,2260. ISBN 1889788392/9781889788395. [Google Scholar]
- Pelletier, M.J. Introduction to applied Raman spectroscopy. In Analytical Applications of Raman Spectroscopy; Pelletier, M.J., Ed.; Blackwell Science: Oxford, UK, 1999; pp. 1–52. ISBN 9780632053056. [Google Scholar]
- Paudel, A.; Raijada, D.; Rantanen, J. Raman spectroscopy in pharmaceutical product design. Adv. Drug Deliv. Rev. 2015, 89, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Esmonde-White, K.A.; Cuellar, M.; Uerpmann, C.; Lenain, B.; Lewis, I.R. Raman spectroscopy as a process analytical technology for pharmaceutical manufacturing and bioprocessing. Anal. Bioanal. Chem. 2017, 409, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Vankeirsbilck, T.; Vercauteren, A.; Baeyens, W.; Van der Weken, G.; Verpoort, F.; Vergote, G.; Remon, J.P. Applications of Raman spectroscopy in pharmaceutical analysis. TrAC Trends Anal. Chem. 2002, 21, 869–877. [Google Scholar] [CrossRef]
- Biancolillo, A.; Marini, F. Chemometric Methods for Spectroscopy-Based Pharmaceutical Analysis. Front. Chem. 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Szostak, R. Quantitative analysis of topical gels and ointments by FT-Raman spectroscopy. Vib. Spectrosc. 2016, 83, 1–7. [Google Scholar] [CrossRef]
- Mazurek, S.; Szostak, R. Quantification of active ingredients in pharmaceutical suspensions by FT Raman spectroscopy. Vib. Spectrosc. 2017, 93, 57–64. [Google Scholar] [CrossRef]
- Szostak, R.; Mazurek, S. Quantification of active ingredients in suppositories by FT-Raman spectroscopy. Drug Test. Anal. 2013, 5, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Hancewicz, T.M.; Petty, C. Quantitative analysis of vitamin A using Fourier transform Raman spectroscopy. Spectrochim. Acta A 1995, 51, 2193–2198. [Google Scholar] [CrossRef]
- Failloux, N.; Bonnet, I.; Baron, M.H.; Perrier, E. Quantitative analysis of vitamin A degradation by Raman spectroscopy. Appl. Spectrosc. 2003, 57, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Kochan, K.; Marzec, K.M.; Maslak, E.; Chlopicki, S.; Baranska, M. Raman spectroscopic studies of vitamin A content in the liver: A biomarker of healthy liver. Analyst 2015, 140, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Legesse, F.B.; Heuke, S.; Galler, K.; Hoffmann, P.; Schmitt, M.; Neugebauer, U.; Bauer, M.; Popp, J. Hepatic Vitamin A Content Investigation Using Coherent Anti-Stokes Raman Scattering Microscopy. Chem. Phys. Chem. 2016, 17, 4043–4051. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.J.; Li, J.J.; Jannasch, A.; Mutlu, A.S.; Wang, M.C.; Cheng, J.X. Fingerprint Stimulated Raman Scattering Imaging Reveals Retinoid Coupling Lipid Metabolism and Survival. Chem. Phys. Chem. 2018, 19, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Q.; Sun, D.W.; Wei, Q.Y.; Pu, H.B. Quantification and visualization of alpha-tocopherol in oil-in-water emulsion based delivery systems by Raman microspectroscopy. LWT Food Sci. Technol. 2018, 96, 66–74. [Google Scholar] [CrossRef]
- Feng, S.L.; Gao, F.; Chen, Z.W.; Grant, E.; Kitts, D.D.; Wang, S.; Lu, X.N. Determination of alpha-tocopherol in vegetable oils using a molecularly imprinted polymers-surface-enhanced Raman spectroscopic biosensor. J. Agric. Food. Chem. 2013, 61, 10467–10475. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.R.; Maguire, C.; Gilchrist, S.; Barrett, L.J.; Cross, C.E.; Possmayer, F.; Ennis, M.; Elborn, J.S.; Curry, W.J.; McGarvey, J.J.; et al. The use of Raman microscopy to determine and localize vitamin E in biological samples. FASEB J. 2007, 21, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Geladi, P.; Kowalski, B.R. Partial least-squares regression–A tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Li, B.; Calvet, A.; Casamayou-Boucau, Y.; Morris, C.; Ryder, A.G. Low-Content Quantification in Powders Using Raman Spectroscopy: A Facile Chemometric Approach to Sub 0.1% Limits of Detection. Anal. Chem. 2015, 87, 3419–3428. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, B.; Scrivens, G. Sample prepartion for solid oral dosage forms. In Sample Preparation of Pharmaceutical Dosage Forms; Nickerson, B., Ed.; Springer: New York, NY, USA, 2011; pp. 145–178. [Google Scholar] [CrossRef]





| Ointment Base System | Petrolatum | Eucerin | |
|---|---|---|---|
| API | Vitamin A | Vitamin A | Vitamin E |
| Concentration range (%) | 0.016–0.079 | 0.022–0.084 | 0.317–1.419 |
| R2 | 0.980 | 0.990 | 0.988 |
| RCV | 0.836 | 0.853 | 0.817 |
| RSEPcal (%) | 4.96 | 3.83 | 3.71 |
| RSEPval (%) | 5.91 | 5.69 | 4.38 |
| Number of LV | 4 | 4 | 5 |
| Determined content (%) | 0.046 ± 0.003 | 0.045 ± 0.002 | 0.78 ± 0.02 |
| Recovery (%) | 104.3 | 102.8 | 98.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, S.; Pichlak, K.; Szostak, R. Quantitative Determination of Vitamins A and E in Ointments Using Raman Spectroscopy. Processes 2021, 9, 8. https://doi.org/10.3390/pr9010008
Mazurek S, Pichlak K, Szostak R. Quantitative Determination of Vitamins A and E in Ointments Using Raman Spectroscopy. Processes. 2021; 9(1):8. https://doi.org/10.3390/pr9010008
Chicago/Turabian StyleMazurek, Sylwester, Kamil Pichlak, and Roman Szostak. 2021. "Quantitative Determination of Vitamins A and E in Ointments Using Raman Spectroscopy" Processes 9, no. 1: 8. https://doi.org/10.3390/pr9010008
APA StyleMazurek, S., Pichlak, K., & Szostak, R. (2021). Quantitative Determination of Vitamins A and E in Ointments Using Raman Spectroscopy. Processes, 9(1), 8. https://doi.org/10.3390/pr9010008