Bioactive Compounds Produced by the Accompanying Microflora in Bulgarian Yoghurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yogurt Sample Collection
2.2. Bacterial Strains, Media and Cultivation Conditions
2.3. Total DNA Isolation from Yoghurt
2.4. Molecular Approaches in Strains’ Identification
2.4.1. Isolation of Chromosomal DNA, PCR Amplification of the 16S rRNA Gene and Sequencing
2.4.2. Randomly Amplified Polymorphic DNA–PCR (RAPD) Analysis
2.4.3. Multi-Locus Sequence Typing (MLST)
2.4.4. Pulse Field Gel Electrophoresis (PFGE)
2.5. Metagenome Library Construction, Sequencing and Bioinformatics Analysis
2.6. Data Availability
2.7. Liquid Chromatography and Mass Spectrometry (LC-MS) Analysis
3. Results and Discussion
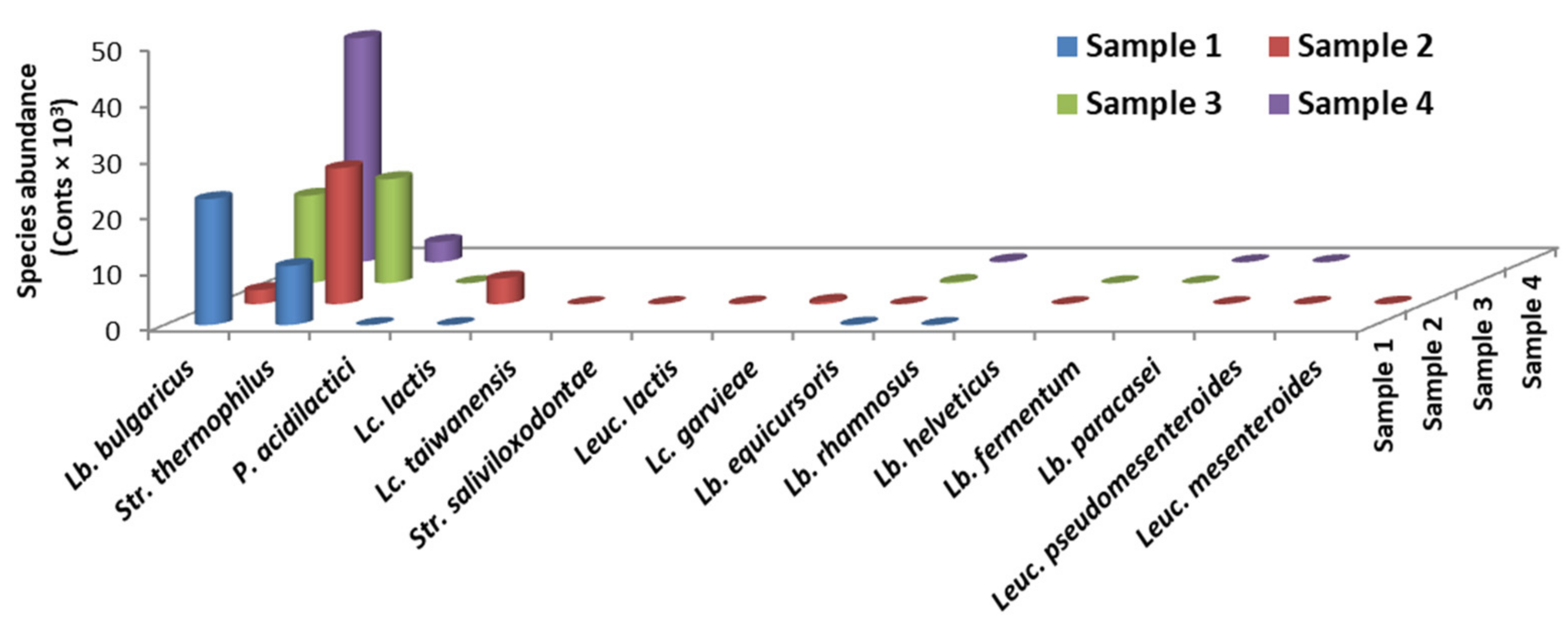
3.1. Metagenomes Analysis
3.2. LAB Strains Isolation and Identification
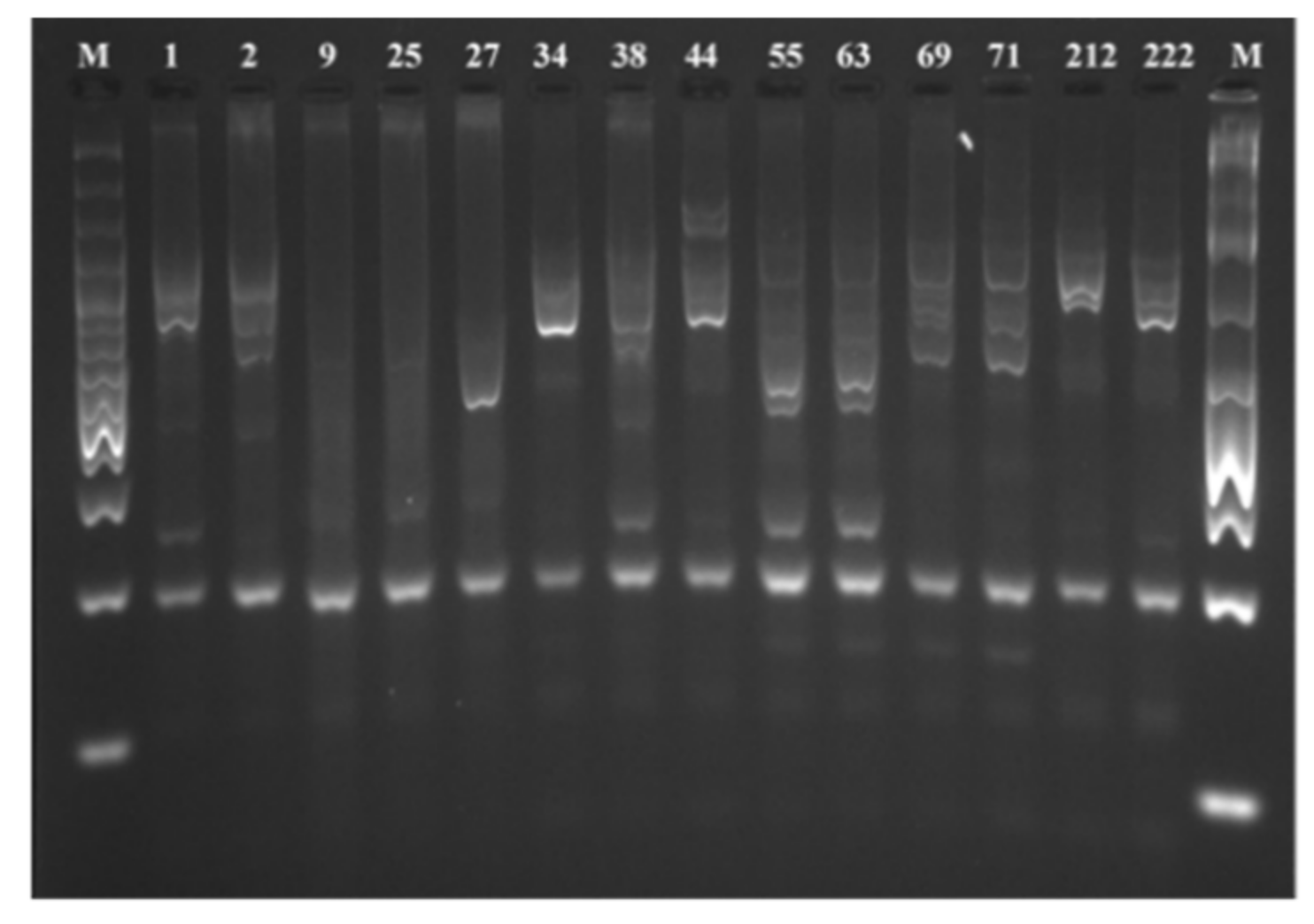
3.2.1. RAPD Analysis
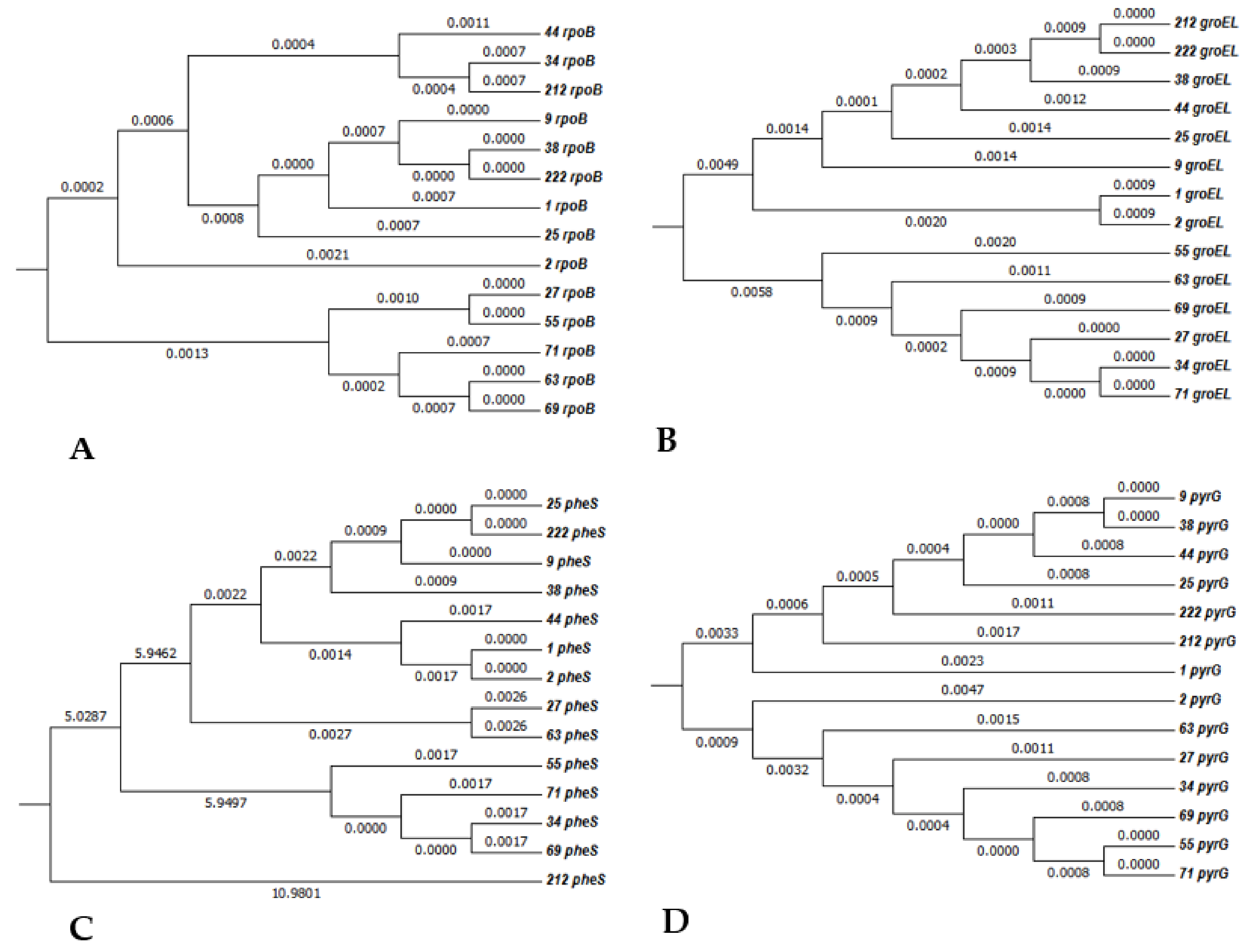
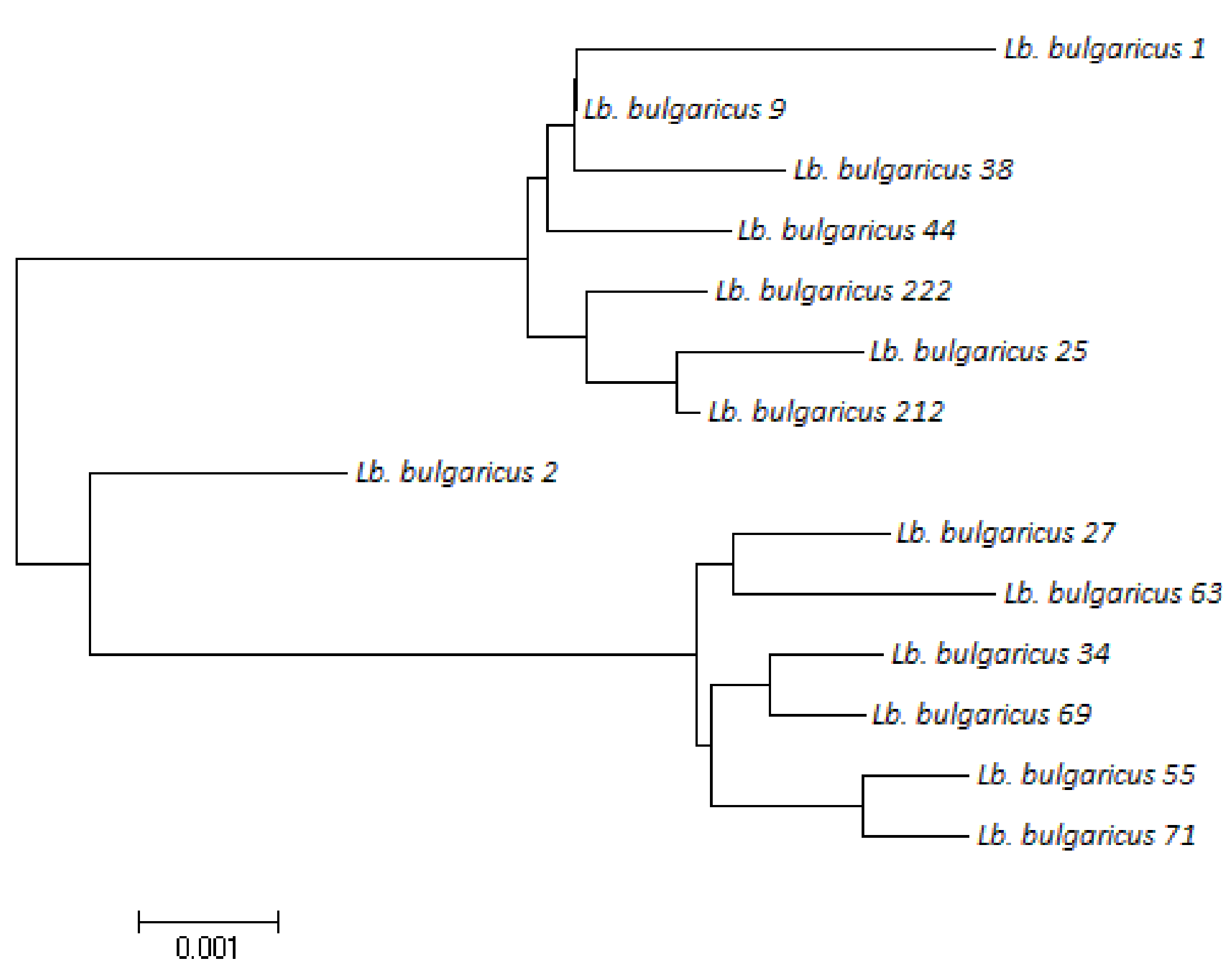
3.2.2. MLST Analysis
3.2.3. PFGE
3.3. Bioactive Metabolites Produced by the Accompanying Microflora of Bulgarian Yoghurts
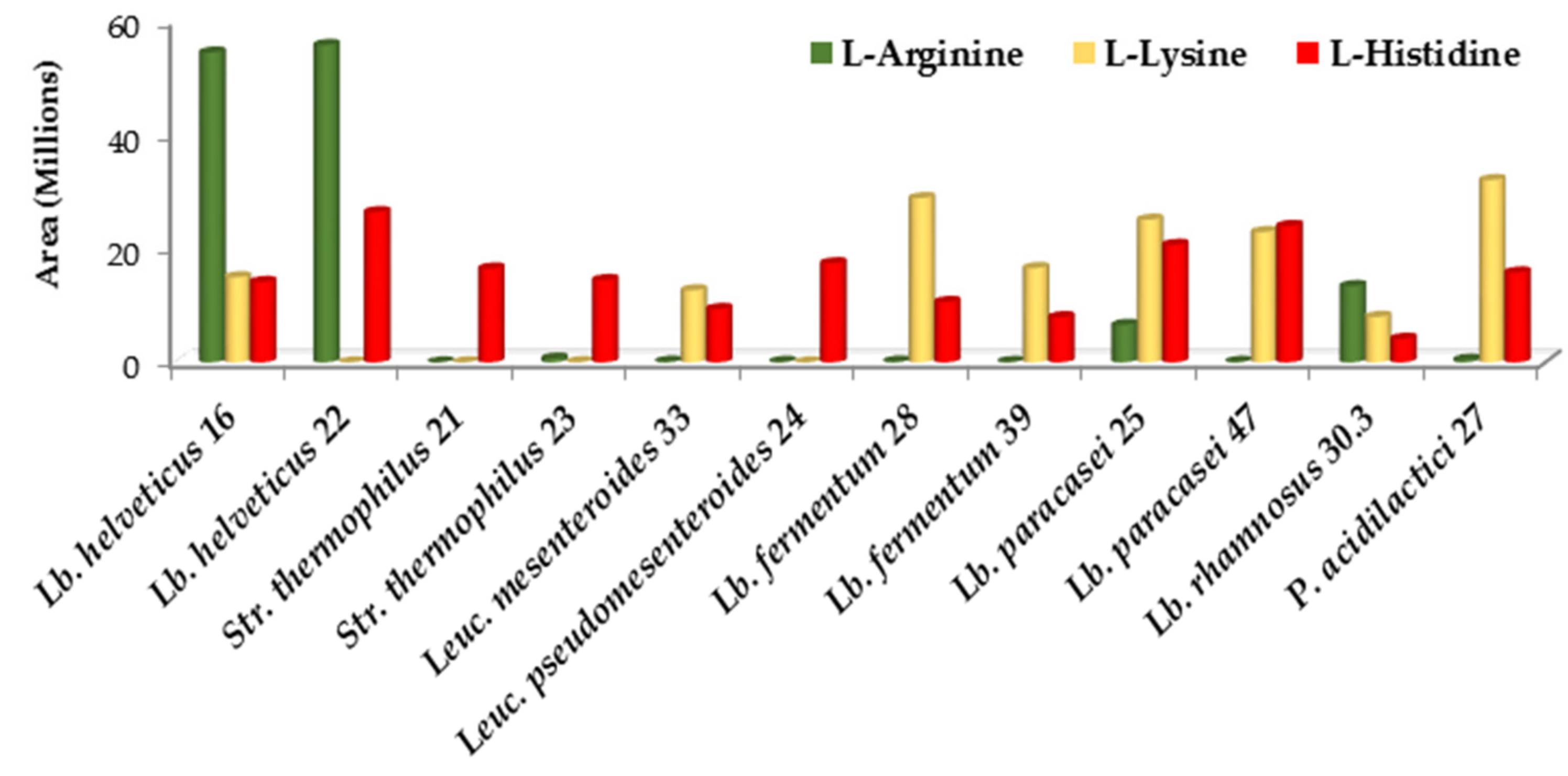
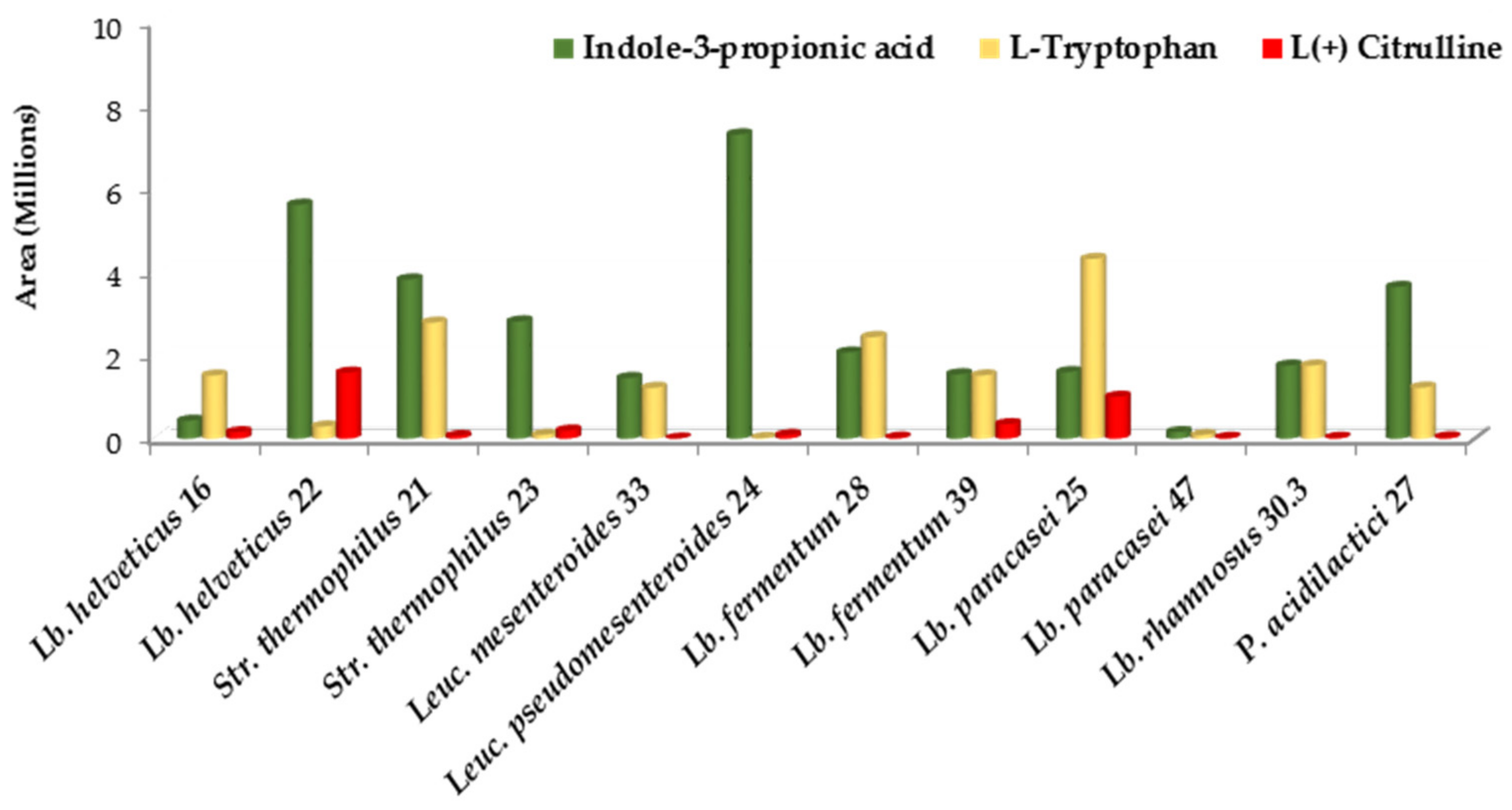
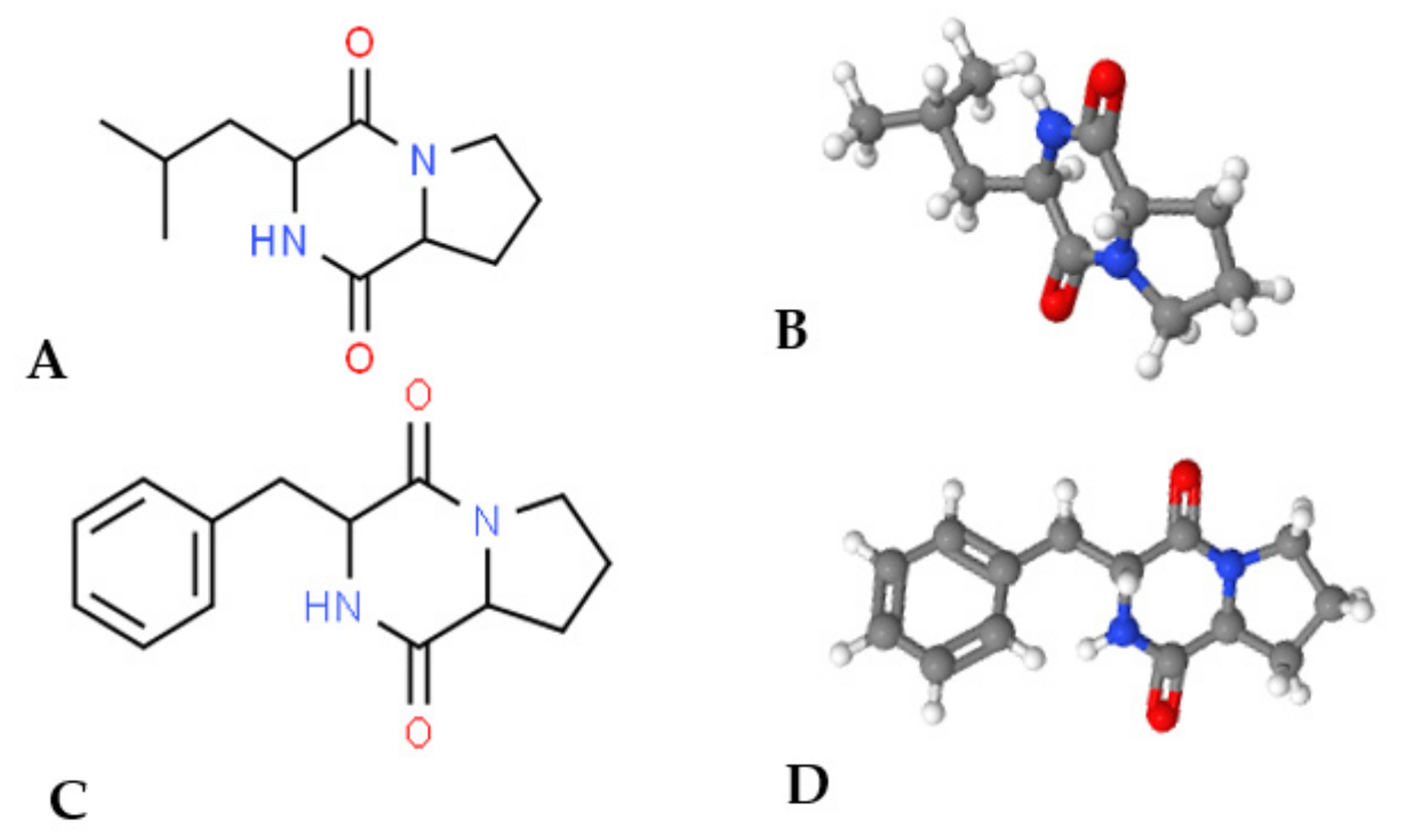
3.3.1. Amino Acids and Their Derivatives
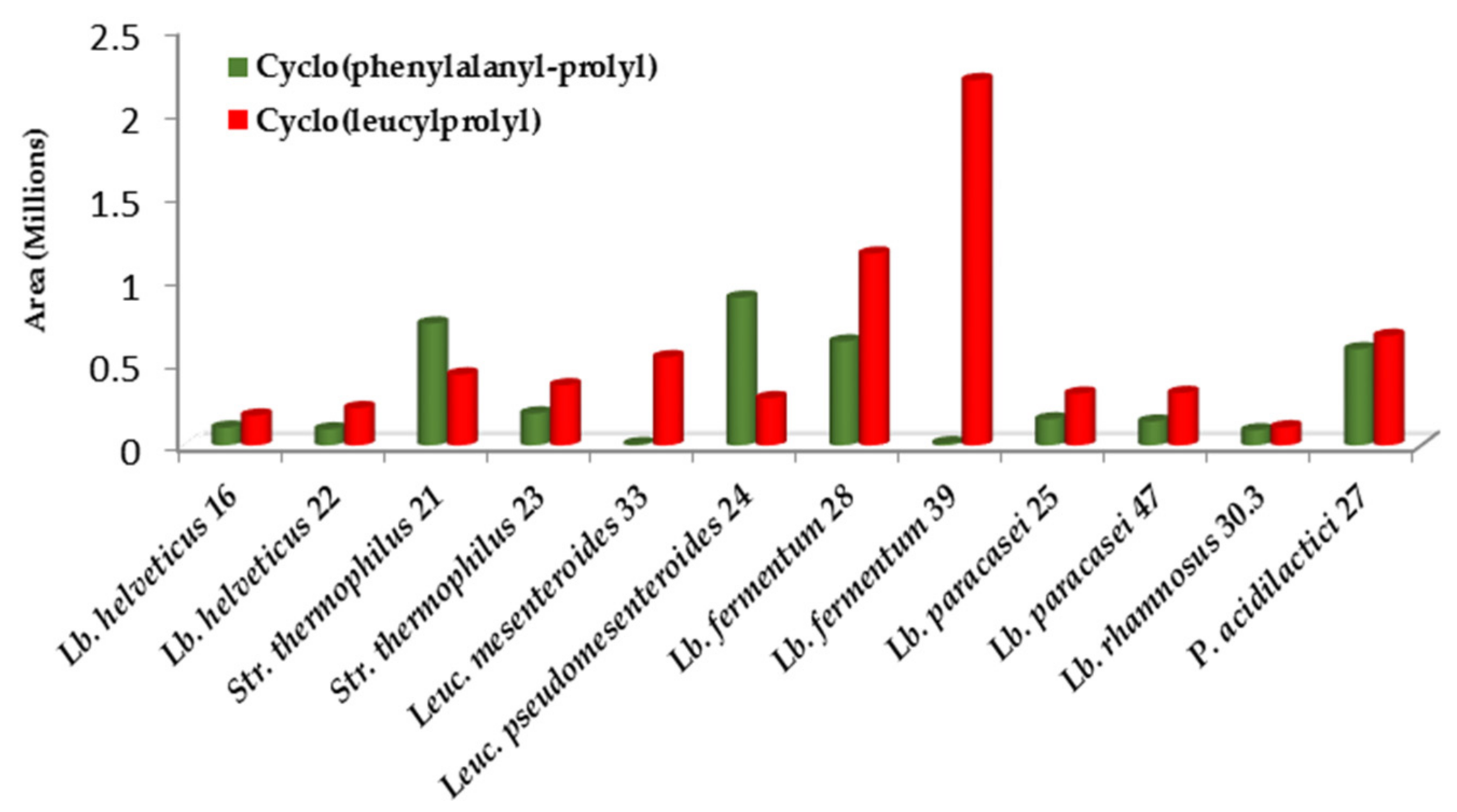
3.3.2. Antimicrobial Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernandez, M.A.; Panahi, S.; Daniel, N.; Tremblay, A.; Marette, A. Yogurt and cardiometabolic diseases: A critical review of potential mechanisms. Adv. Nutr. 2017, 8, 812–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthold-Pluta, A.M.; Pluta, A.S.; Garbowska, M.; Stasiak-Różańska, L. Exopolysaccharide-producing Lactic Acid Bacteria—Health-promoting properties and application in the dairy industry. Adv. Microbiol. 2019, 58, 191–204. [Google Scholar] [CrossRef] [Green Version]
- Popović, N.; Brdarić, E.; Đokić, J.; Dinić, M.; Veljović, K.; Golić, N.; Terzić-Vidojević, A. Yogurt produced by novel natural starter cultures improves gut epithelial barrier in vitro. Microorganisms 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Julio, P.D.; Carolina, G.L.; Luis, F.; Angel, G. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol. 2014, 20, 15632–15649. [Google Scholar]
- Mousavi, S.N.; Somayeh, S.; Asbaghi, O. Effect of daily probiotic yogurt consumption on inflammation: A systematic review and meta-analysis of randomized Controlled Clinical trials. Obes. Med. 2020, 18, 100221. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Metagenomics insights into food fermentations. Microb. Biotechnol. 2016, 10, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zheng, H.; Yu, Y.; Ding, G.; Gu, W.; Chen, S.; Yu, Z.; Ren, S.; Oda, M.; Konno, T.; et al. Complete sequencing and pan-genomic analysis of Lactobacillus delbrueckii subsp. bulgaricus reveal its genetic basis for industrial yogurt production. PLoS ONE 2011, 6, e15964. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Siezen, R.J.; Nauta, A. In Silico prediction of horizontal gene transfer events in Lactobacillus bulgaricus and Streptococcus thermophilus reveals protocooperation in yogurt manufacturing. Appl. Environ. Microbiol. 2009, 75, 4120–4129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beshkova, D.; Simova, E.; Frengova, G.; Simov, Z. Production of flavour compounds by yogurt starter cultures. J. Ind. Microbiol. Biotechnol. 1998, 20, 180–186. [Google Scholar] [CrossRef]
- Velikova, P.; Petrov, K.; Lozanov, V.; Tsvetanova, F.; Stoyanov, A.; Wu, Z.; Liu, Z.; Petrova, P. Microbial diversity and health-promoting properties of the traditional Bulgarian yogurt. Biotechnol. Biotechnol. Equip. 2018, 32, 1205–1217. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Ikeda, K.; Sakuma, K.; Kawai, S.; Sawaki, K.; Asahara, T.; Takahashi, T.; Tsuji, H.; Nomoto, K.; Nagpal, R.; et al. Association between yogurt consumption and intestinal microbiota in healthy young adults differs by host gender. Front. Microbiol. 2017, 8, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyanova, L.; Stephanova-Kondratenko, M.; Mitov, I. Anti-Helicobacter pylori activity of Lactobacillus delbrueckii subsp. bulgaricus strains: Preliminary report. Lett. Appl. Microbiol. 2009, 48, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Gergova, G.; Markovska, R.; Yordanov, D.; Mitov, I. Bacteriocin-like inhibitory activities of seven Lactobacillus delbrueckii subsp. bulgaricus strains against antibiotic susceptible and resistant Helicobacter pylori strains. Lett. Appl. Microbiol. 2017, 65, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, T.; Urshev, Z.; Dimitrov, Z.; Fatchikova, N.; Minkova, S. Species affiliation of dairy lactobacilli with angiotensin converting enzyme inhibitory activity. Biotechnol. Biotechnol. Equip. 2009, 23, 1250–1254. [Google Scholar] [CrossRef] [Green Version]
- Cocolin, L.; Ercolini, D. Zooming into food-associated microbial consortia: A “cultural” evolution. Curr. Opin. Food Sci. 2015, 2, 43–50. [Google Scholar] [CrossRef]
- Lee, A.; Lee, Y.J.; Yoo, H.J.; Kim, M.; Chang, Y.; Lee, D.S.; Lee, J.H. Consumption of dairy yogurt containing Lactobacillus paracasei ssp. paracasei, Bifidobacterium animalis ssp. lactis and heat-treated Lactobacillus plantarum improves immune function including natural killer cell activity. Nutrients 2017, 9, 558. [Google Scholar] [CrossRef]
- Sánchez, E.; Nieto, J.; Vidal, S.; Santiago, A.; Martinez, X.; Sancho, F.J.; Sancho-Bru, P.; Mirelis, B.; Corominola, H.; Juárez, C.; et al. Fermented milk containing Lactobacillus paracasei subsp. paracasei CNCM I-1518 reduces bacterial translocation in rats treated with carbon tetrachloride. Sci. Rep. 2017, 7, 45712. [Google Scholar]
- Wang, J.; Li, C.; Xue, J.; Yang, J.; Zhang, Q.; Zhang, H.; Chen, Y. Fermentation characteristics and angiotensin I-converting enzyme-inhibitory activity of Lactobacillus helveticus isolate H9 in cow milk, soy milk, and mare milk. J. Dairy Sci. 2015, 98, 3655–3664. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Huo, R.; Kwok, L.Y.; Li, C.; Ma, Y.; Mi, Z.; Chen, Y. Effects of applying Lactobacillus helveticus H9 as adjunct starter culture in yogurt fermentation and storage. J. Dairy Sci. 2019, 102, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Cavalheiro, G.F.; Baptista, D.P.; Galli, B.D.; Negrão, F.; Eberlin, M.N.; Gigante, M.L. High protein yogurt with addition of Lactobacillus helveticus: Peptide profile and angiotensin-converting enzyme ACE-inhibitory activity. Food Chem. 2020, 333, 127482. [Google Scholar] [CrossRef]
- Cameron, D.; Hock, Q.S.; Kadim, M.; Mohan, N.; Ryoo, E.; Sandhu, B.; Yamashiro, Y.; Jie, C.; Hoekstra, H.; Guarino, A. Probiotics for gastrointestinal disorders: Proposed recommendations for children of the Asia-Pacific region. World J. Gastroenterol. 2017, 23, 7952–7964. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Guarino, A.; Hojsak, I.; Indrio, F.; Kolacek, S.; Shamir, R.; Vandenplas, Y.; Weizman, Z. Use of probiotics for management of acute gastroenteritis: A position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J. Ped. Gastroenterol. Nutr. 2014, 58, 531–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szajewska, H.; Horvath, A. Lactobacillus rhamnosus GG in the primary prevention of eczema in children: A systematic review and meta-analysis. Nutrients 2018, 10, 1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigoroff, S. Étude sur une lait fermentée comestible. Le “Kissélo mléko” de Bulgarie. Rev. Méd. Suisse Romande 1905, 25, 714–720. [Google Scholar]
- Lick, S.; Keller, M.; Bockelmann, M.; Heller, K.J. Optimized DNA extraction method for starter cultures from yoghurt. Milchwissenschaft 1996, 51, 183–186. [Google Scholar]
- Cebeci, A.; Gurakan, C. Comparative typing of L. delbrueckii subsp. bulgaricus strains using multilocus sequence typing and RAPD–PCR. Eur. Food. Res. Technol. 2011, 233, 377–385. [Google Scholar] [CrossRef]
- Naser, S.M.; Thompson, F.L.; Hoste, B.; Gevers, D.; Dawyndt, P.; Vancanneyt, M.; Swings, J. Application of multilocus sequence analysis (MLSA) for rapid identification of Enterococcus species based on rpoA and pheS genes. Microbiology 2005, 151, 2141–2150. [Google Scholar] [CrossRef] [Green Version]
- Michaylova, M.; Minkova, S.; Kimura, K.; Sasaki, T.; Isawa, K. Isolation and characterization of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus from plants in Bulgaria. FEMS Microbiol. Lett. 2007, 269, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Undugoda, L.J.S.; Nilmini, A.H.L. Effect of lactic acid microbial ratio of yoghurt starter culture in yoghurt fermentation and reduction of post acidification. J. Food Ind. Microbiol. 2019, 5, 1. [Google Scholar]
- Surono, I.S.; Hosono, A. Fermented milks. Types and Standards of Identity. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: New York, NY, USA, 2011; pp. 470–476. [Google Scholar]
- Georgalaki, M.; Zoumpopoulou, G.; Mavrogonatou, E.; Van Driessche, G.; Alexandraki, V.; Anastasiou, R.; Papadelli, M.; Kazou, M.; Manolopoulou, E.; Kletsas, D.; et al. Evaluation of the antihypertensive angiotensin-converting enzyme inhibitory (ACE-I) activity and other probiotic properties of lactic acid bacteria isolated from traditional Greek dairy products. Int. Dairy J. 2017, 75, 10–21. [Google Scholar] [CrossRef]
- Urshev, Z.L.; Pashova-Baltova, K.N.; Dimitrov, Z.P. Tracing Streptococcus thermophilus strains in three-component yogurt starters. World J. Microbiol. Biotechnol. 2006, 22, 1223–1228. [Google Scholar] [CrossRef]
- Ma, C.; Chen, Z.; Gong, G.; Huang, L.; Li, S.; Ma, A. Starter culture design to overcome phage infection during yogurt fermentation. Food Sci. Biotechnol. 2015, 24, 521–527. [Google Scholar] [CrossRef]
- Sieuwerts, S.; De Bok, F.A.; Hugenholtz, J.; Van Hylckama Vlieg, J.E. Unraveling microbial interactions in food fermentations: From classical to genomics approaches. Appl. Environ. Microbiol. 2008, 74, 4997–5007. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, A.S.; Tap, J.; Chambaud, I.; Cools-Portier, S.; Quinquis, L.; Bourlioux, P.; Marteau, P.; Guillemard, E.; Schrezenmeir, J.; Derrien, M. Safety and functional enrichment of gut microbiome in healthy subjects consuming a multi-strain fermented milk product: A randomised controlled trial. Sci. Rep. 2020, 10, 15974. [Google Scholar] [CrossRef] [PubMed]
- Roder, T.; Wüthrich, D.; Bär, C.; Sattari, Z.; von Ah, U.; Ronchi, F.; Macpherson, A.J.; Ganal-Vonarburg, S.C.; Bruggmann, R.; Vergères, G. In Silico Comparison Shows that the Pan-Genome of a Dairy-Related Bacterial Culture Collection Covers Most Reactions Annotated to Human Microbiomes. Microorganisms 2020, 8, 966. [Google Scholar] [CrossRef]
- Miteva, V.I.; Abadjieva, A.N.; Stefanova, T.T. M13 DNA fingerprinting, a new tool for classification and identification of Lactobacillus spp. J. Appl. Bacteriol. 1992, 73, 349–354. [Google Scholar] [CrossRef]
- Dimitrov, Z.; Michaylova, M.; Mincova, S. Characterization of Lactobacillus helveticus strains isolated from Bulgarian yogurt, cheese, plants and human faecal samples by sodium dodecyl sulfate polyacrylamide gel electrophoresis of cell-wall proteins, ribotyping, and pulsed-field gel fingerprinting. Int. Dairy J. 2005, 15, 998–1005. [Google Scholar] [CrossRef]
- Morita, H.; Shimazu, M.; Shiono, H.; Toh, H.; Nakajima, F.; Akita, H.; Takagi, M.; Takami, H.; Murakami, M.; Masaoka, T.; et al. Lactobacillus equicursoris sp. nov., isolated from the faeces of a thoroughbred racehorse. Int. J. Syst. Evol. Microbiol. 2010, 60, 109–112. [Google Scholar] [CrossRef]
- Beleza do Campo, K. Available online: https://www.belezadocampo.com.br (accessed on 10 December 2020).
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef] [Green Version]
- Verena, S.J.; Schmidt, V.K.; Kulozik, U.; Scherer, S.; Wenning, M. Microbial biodiversity, quality and shelf life of microfiltered and pasteurized extended shelf life (ESL) milk from Germany, Austria and Switzerland. Int. J. Food Microbiol. 2012, 154, 1–9. [Google Scholar]
- Kagkli, D.M.; Vancanneyt, M.; Hill, C.; Vandamme, P.; Cogan, T.M. Enterococcus and Lactobacillus contamination of raw milk in a farm dairy environment. Int. J. Food Microbiol. 2007, 114, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Agu, K.; Archibong, E.; Anekwe, D.; Ago, C.; Okafor, A.; Awah, N. Assessment of bacteria present in yoghurt sold on Awka Metropolis. Sch. J. Appl. Med. Sci. 2014, 2, 3071–3075. [Google Scholar]
- Pal, M.; Awel, H. Public health significance of Listeria monocytogenes in milk and milk products. J. Vet. Public Health 2014, 12, 1–5. [Google Scholar]
- Addis, M.F.; Tanca, A.; Uzzau, S.; Oikonomou, G.; Bicalho, R.C.; Moroni, P. The bovine milk microbiota: Insights and perspectives from -omics studies. Mol. Biosyst. 2016, 12, 2359–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, M.; Kaur, H.; Gupta, L.K.; Sood, N.K. Isolation of Yersinia enterocolitica from raw milk and pork in Ludhiana. Ind. J. Pathol. Microbiol. 2005, 48, 286–287. [Google Scholar]
- Lee, K.; Lee, J.; Kim, Y.H.; Moon, S.H.; Park, Y.H. Unique properties of four Lactobacilli in amino acid production and symbiotic mixed culture for lactic acid biosynthesis. Curr. Microbiol. 2001, 43, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Simova, E.; Simov, Z.; Beshkova, D.; Frengova, G.; Dimitrov, Z.; Spasov, Z. Amino acid profiles of lactic acid bacteria, isolated from kefir grains and kefir starter made from them. Int. J. Food Microbiol. 2006, 107, 112–123. [Google Scholar] [CrossRef]
- Nascimento, M.M. Potential uses of arginine in dentistry. Adv. Dent. Res. 2018, 29, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- Ho, S.W.; El-Nezami, H.; Shah, N.P. Effects of supplementation of citrulline and Lactobacillus helveticus ASCC 511 on intestinal epithelial cell integrity. J. Funct. Foods 2020, 64, 103571. [Google Scholar] [CrossRef]
- Stanislavov, R.; Nikolova, V. Treatment of erectile dysfunction with pycnogenol and L-arginine. J. Sex Marital Ther. 2003, 29, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Cormio, L.; De Siati, M.; Lorusso, F.; Selvaggio, O.; Mirabella, L.; Sanguedolce, F.; Carrieri, G. Oral L-citrulline supplementation improves erection hardness in men with mild erectile dysfunction. Urology 2011, 77, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Cao, W.; Ying, H.; Chen, K.; Ouyang, P. Efficient production of enantiopure d-lysine from l-lysine by a two-enzyme cascade system. Catalysts 2016, 6, 168. [Google Scholar] [CrossRef]
- Lim, Y.H.; Foo, H.L.; Loh, T.C.; Mohamad, R.; Abdul-Rahim, R. Rapid evaluation and optimization of medium components governing tryptophan production by Pediococcus acidilactici tp-6 isolated from Malaysian food via statistical approaches. Molecules 2020, 25, 779. [Google Scholar] [CrossRef] [Green Version]
- Mimori, S.; Kawada, K.; Saito, R.; Takahashi, M.; Mizoi, K.; Okuma, Y.; Hosokawa, M.; Kanzaki, T. Indole-3-propionic acid has chemical chaperone activity and suppresses endoplasmic reticulum stress-induced neuronal cell death. Biochem. Biophys. Res. Commun. 2019, 517, 623–628. [Google Scholar] [CrossRef]
- Pappolla, M. Indole-3-propionic Acids, Salts and Esters Thereof Used as Medicaments. U.S. Patent WO1999042102A1, 26 August 1999. [Google Scholar]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- De Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Bergdahl, I.A. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish diabetes prevention study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Chemspider: Search and Share Chemistry, a Site of Royal Society of Chemistry. Available online: http://www.chemspider.com (accessed on 6 December 2020).
- Lee, K.H.; Rhee, K.H. Radioprotective effect of cyclo(l-phenylalanyl-l-prolyl) on irradiated rat lung. J. Microbiol. Biotechnol. 2008, 18, 369–376. [Google Scholar]
- Ravi, L.; Ragunathan, A.; Krishnan, K. Marine Streptomyces paradoxus VITALK03 derived gancidin W mediated cytotoxicity through Ras-Raf-MEK-ERK signalling pathway. Ind. J. Biotechnol. 2017, 16, 164–175. [Google Scholar]
- Nweze, J.A.; Mbaoji, F.N.; Huang, G.; Li, Y.; Yang, L.; Zhang, Y.; Huang, S.; Pan, L.; Yang, D. Antibiotics development and the potentials of marine-derived compounds to stem the tide of multidrug-resistant pathogenic bacteria, fungi, and protozoa. Mar. Drugs 2020, 18, 145. [Google Scholar] [CrossRef] [Green Version]


| Gene | Primer Name | Sequence (5′→3′) | Annealing T °C | Product Position in Gene | Position in Genome 1 | Template Size (bp) |
|---|---|---|---|---|---|---|
| rpoB | rpoB_F rpoB_R | GGCGGAAAGAGTTATCGT GATGTCGGCTGGAGTGAT | 58 | 399–1170 | 334519–338184 | 772 |
| groEL | groEL_F groEL_R | TCGGCAAGGACGGTGTT GTGGATTACGGCTACGC | 57 | 500–1134 | 1392354–1393967 | 635 |
| pheS | pheS_F pheS_R | CATCGGCATGAGCTACCA CCTCCTGACGGAATTGTTG | 55 | 369–1042 | 1283032–1284081 | 674 |
| pyrG | pyrG_F pyrG_R | AAGCCGACCCAGCAATC AGCCCAGACGCAAGGTG | 57 | 568–1309 | 301679–303298 | 742 |
| 1 | 1 | 2 | 9 | 25 | 27 | 34 | 38 | 44 | 55 | 63 | 69 | 71 | 212 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 0.004 | ||||||||||||
| 9 | 0.003 | 0.004 | |||||||||||
| 25 | 0.003 | 0.004 | 0.001 | ||||||||||
| 27 | 0.008 | 0.009 | 0.010 | 0.010 | |||||||||
| 34 | 0.007 | 0.008 | 0.009 | 0.009 | 0.002 | ||||||||
| 38 | 0.003 | 0.004 | 0.001 | 0.001 | 0.009 | 0.008 | |||||||
| 44 | 0.002 | 0.004 | 0.002 | 0.002 | 0.009 | 0.007 | 0.002 | ||||||
| 55 | 0.008 | 0.008 | 0.009 | 0.009 | 0.001 | 0.001 | 0.009 | 0.009 | |||||
| 63 | 0.008 | 0.009 | 0.010 | 0.010 | 0.002 | 0.001 | 0.009 | 0.008 | 0.001 | ||||
| 69 | 0.009 | 0.009 | 0.010 | 0.010 | 0.002 | 0.001 | 0.009 | 0.009 | 0.002 | 0.001 | |||
| 71 | 0.008 | 0.009 | 0.010 | 0.010 | 0.002 | 0.001 | 0.009 | 0.008 | 0.001 | 0.001 | 0.001 | ||
| 212 | 0.003 | 0.004 | 0.001 | 0.001 | 0.010 | 0.008 | 0.001 | 0.001 | 0.009 | 0.009 | 0.009 | 0.009 | |
| 222 | 0.003 | 0.004 | 0.001 | 0.001 | 0.009 | 0.009 | 0.001 | 0.001 | 0.009 | 0.009 | 0.010 | 0.009 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, I.; Petrov, K.; Lozanov, V.; Hristov, I.; Wu, Z.; Liu, Z.; Petrova, P. Bioactive Compounds Produced by the Accompanying Microflora in Bulgarian Yoghurt. Processes 2021, 9, 114. https://doi.org/10.3390/pr9010114
Ivanov I, Petrov K, Lozanov V, Hristov I, Wu Z, Liu Z, Petrova P. Bioactive Compounds Produced by the Accompanying Microflora in Bulgarian Yoghurt. Processes. 2021; 9(1):114. https://doi.org/10.3390/pr9010114
Chicago/Turabian StyleIvanov, Ivan, Kaloyan Petrov, Valentin Lozanov, Iassen Hristov, Zhengjun Wu, Zhenmin Liu, and Penka Petrova. 2021. "Bioactive Compounds Produced by the Accompanying Microflora in Bulgarian Yoghurt" Processes 9, no. 1: 114. https://doi.org/10.3390/pr9010114
APA StyleIvanov, I., Petrov, K., Lozanov, V., Hristov, I., Wu, Z., Liu, Z., & Petrova, P. (2021). Bioactive Compounds Produced by the Accompanying Microflora in Bulgarian Yoghurt. Processes, 9(1), 114. https://doi.org/10.3390/pr9010114







