Morphology and Structure Controls of Single-Atom Fe–N–C Catalysts Synthesized Using FePc Powders as the Precursor
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
- (a)
- An electrodeposition method was used to deposit Cu foils on the surface of a steel sheet. Then, the Cu foils were peeled off from the sheet and put into a tube furnace.
- (b)
- The monolayer graphene was synthesized on the surface of the Cu foil (noted as G/Cu) through a chemical vapor deposition (CVD) process in a mixture atmosphere of CH4 (12 sccm) and H2 (48 sccm) gases at 1000 °C [30].
- (c)
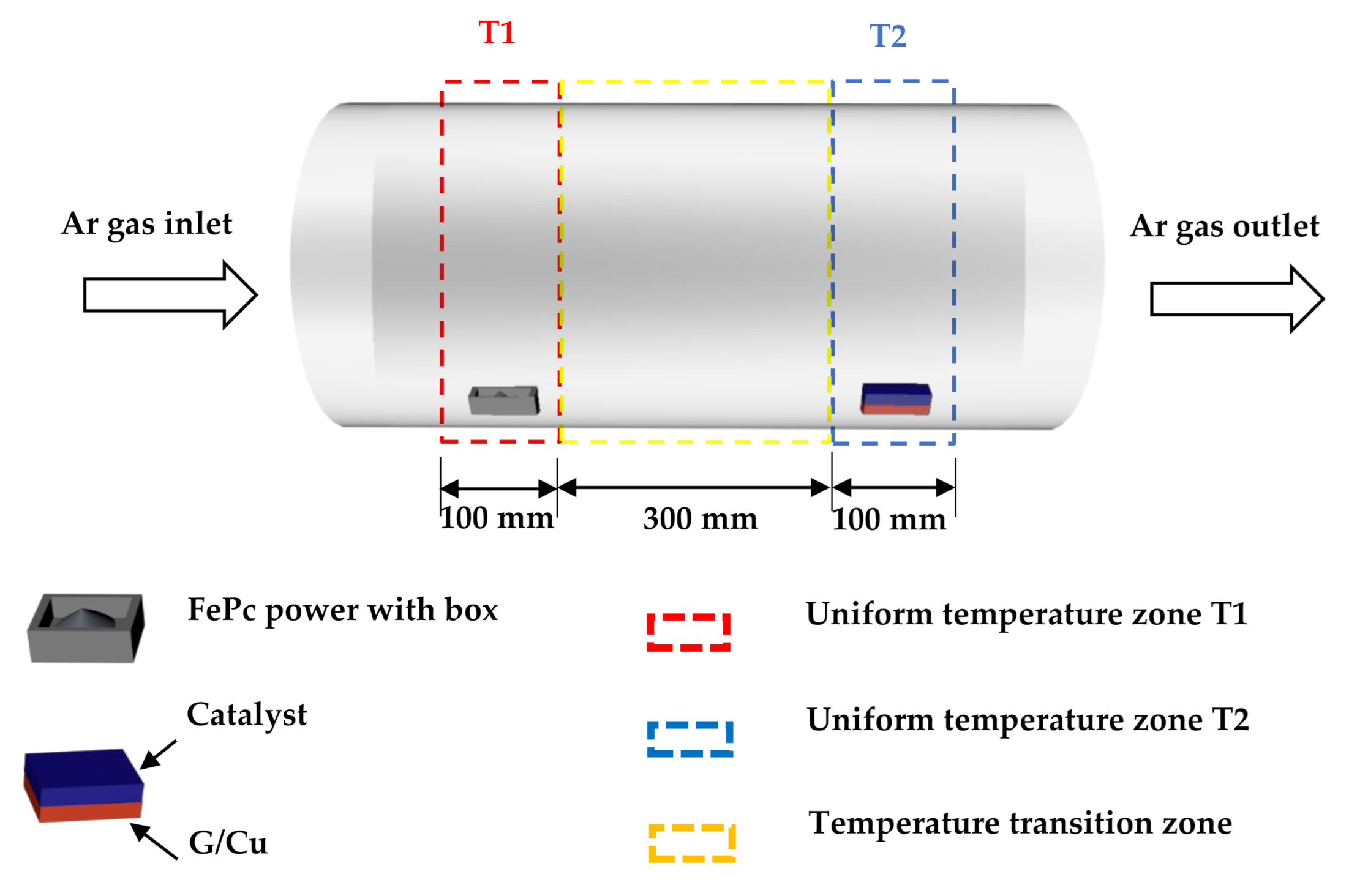
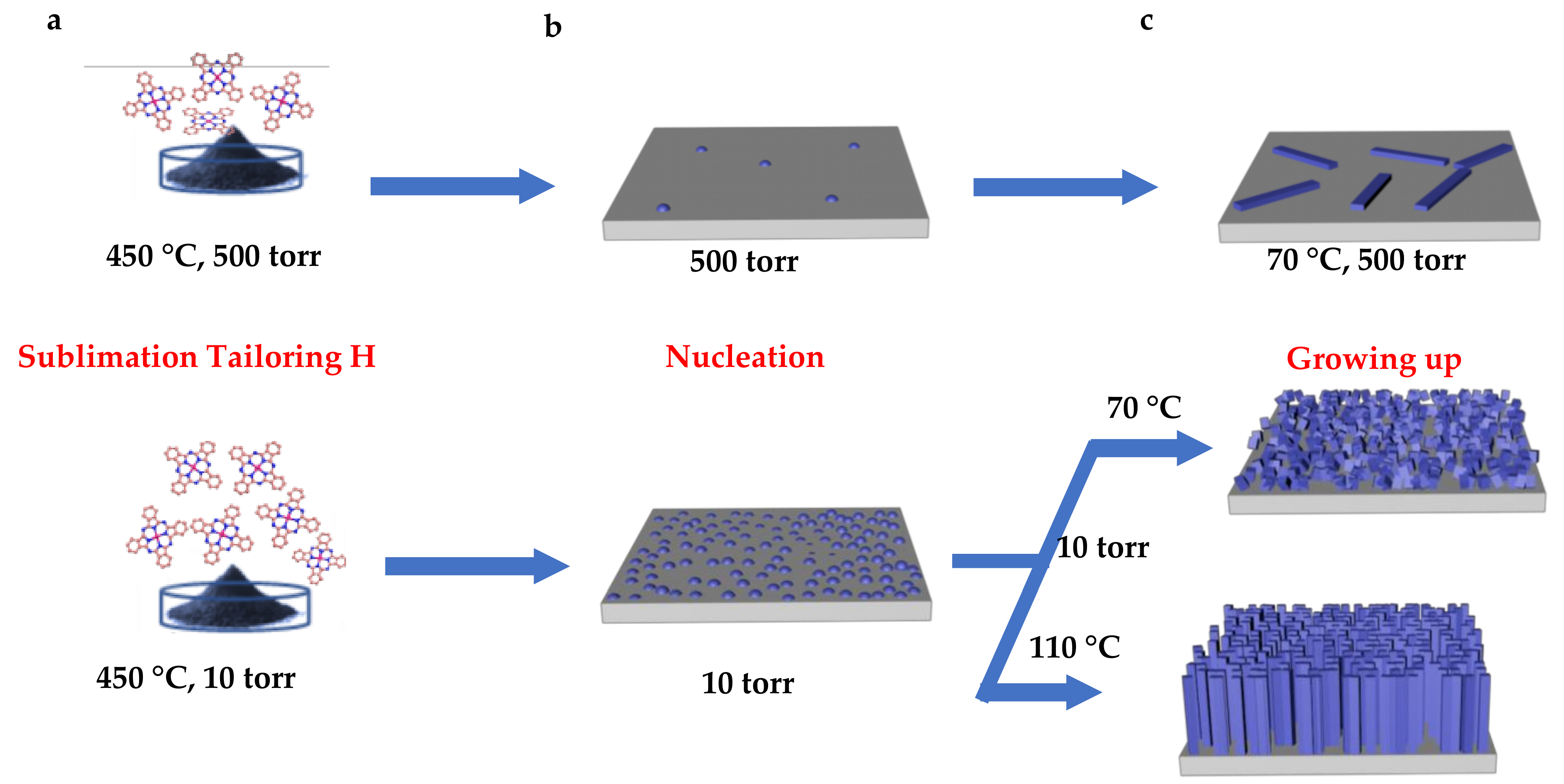
- FePc powders (the precursor) and G/Cu film were separately placed into another tube furnace with a distance of 300 mm (Figure 1). The synthetic strategy of Fe–N–C single-atom catalysts is described as follows: (i) FePc powder was put into the T1 (450 °C) temperature zone, G/Cu film was put in the T2 temperature zone. To investigate the effect of the temperature and pressure, we controlled the temperature T2 to be 110 °C and 70 °C, respectively. (ii) The pressure in the T2 temperature zones was 10 torr and 500 torr, respectively. Then, keeping the temperatures for 30 min with argon gas flowing through the tube furnace in the direction from FePc powders to G/Cu film, we synthesized the catalysts on the surface of the monolayer graphene and noted as Fe–N–C/G/Cu.
- (d)
- The copper layers in synthesized catalysts Fe–N–C/G/Cu were removed by floating the films on the surface of 0.1 M FeCl3 solution for more than 12 h. Then, the reserved film (noted as Fe–N–C) was washed with deionized water 3 times and dried at 60 °C.
2.2. Electrochemical Measurements
2.3. Structural Characterization
3. Results
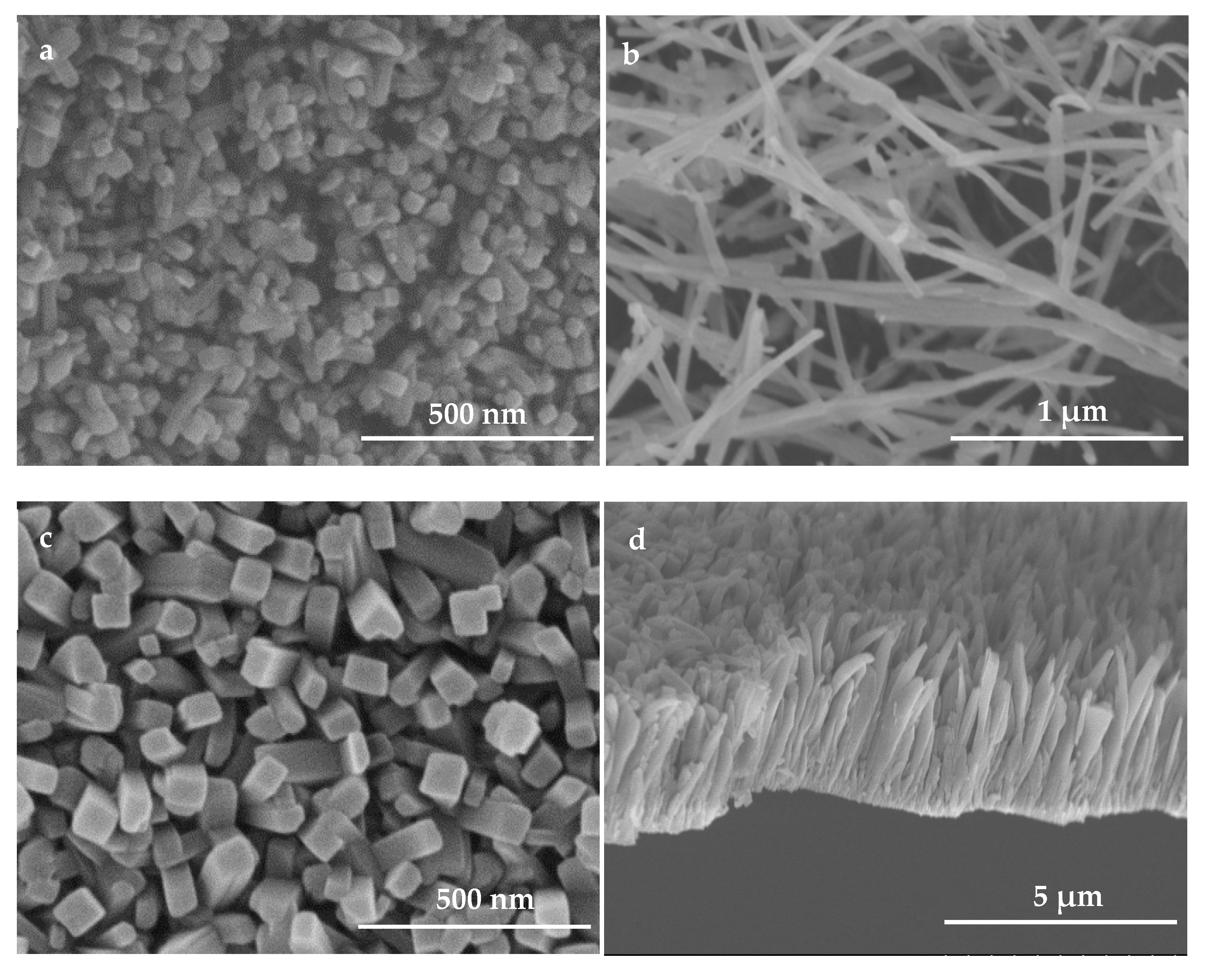
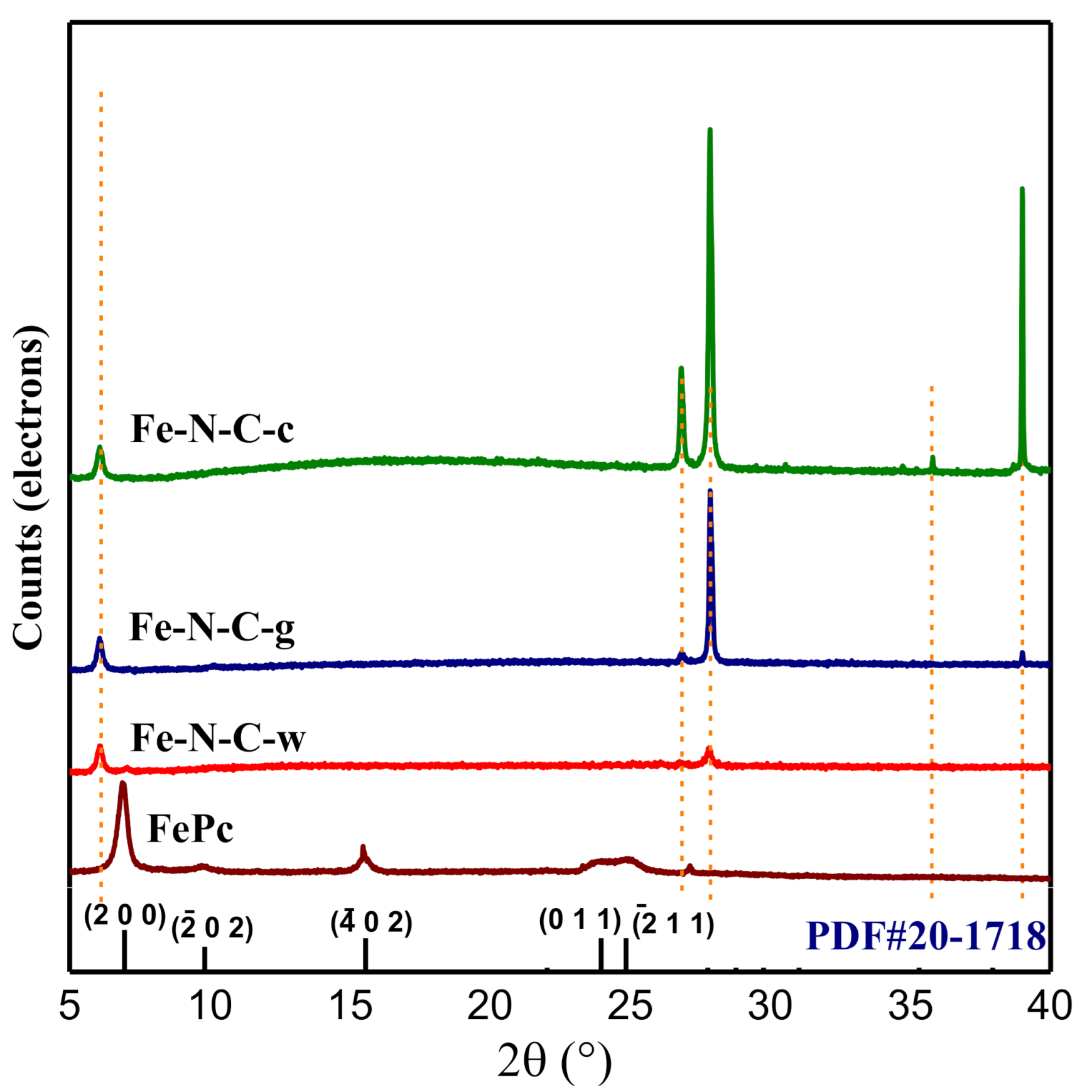
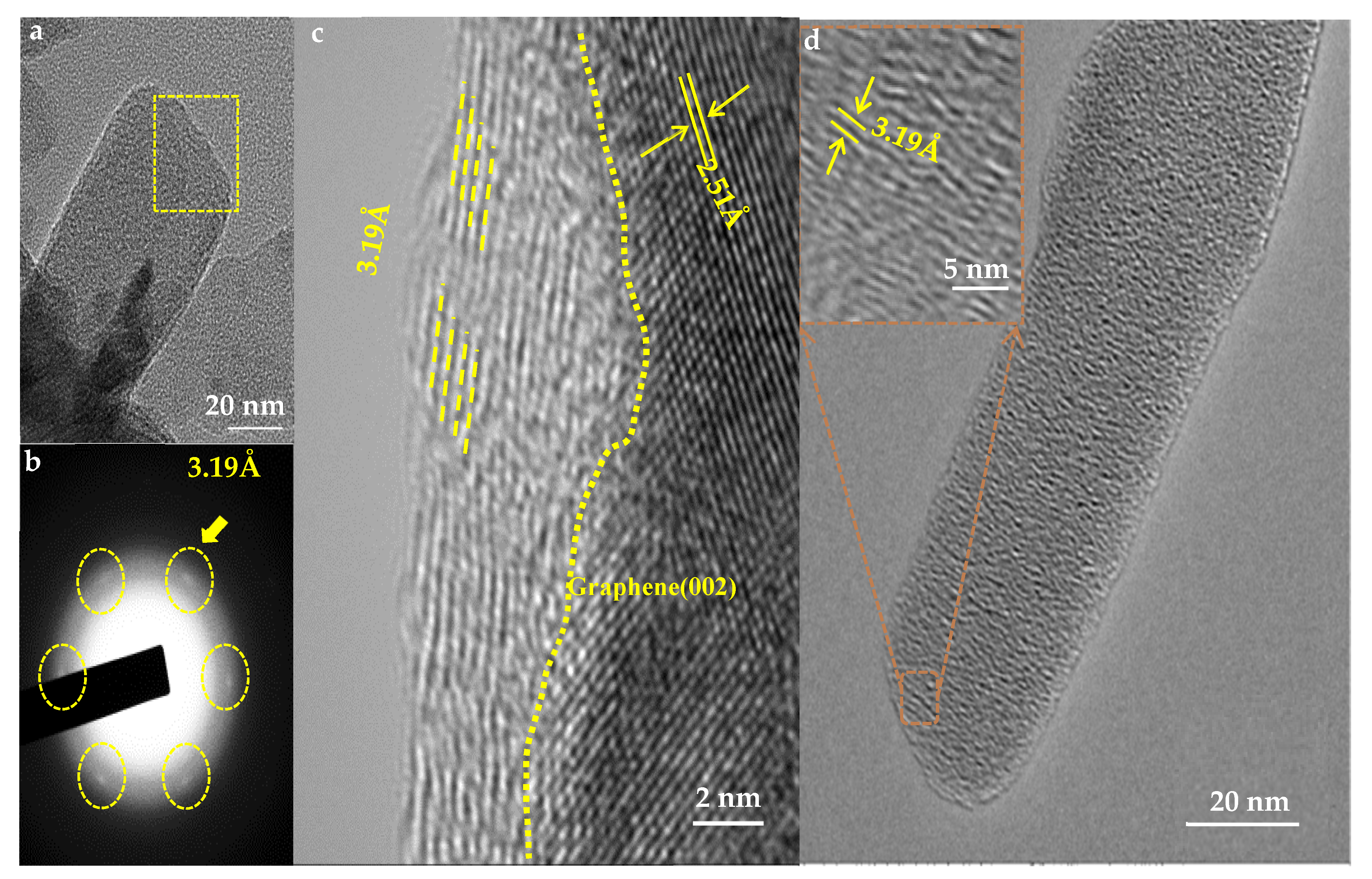
3.1. Analyses on the Morphology and Structure
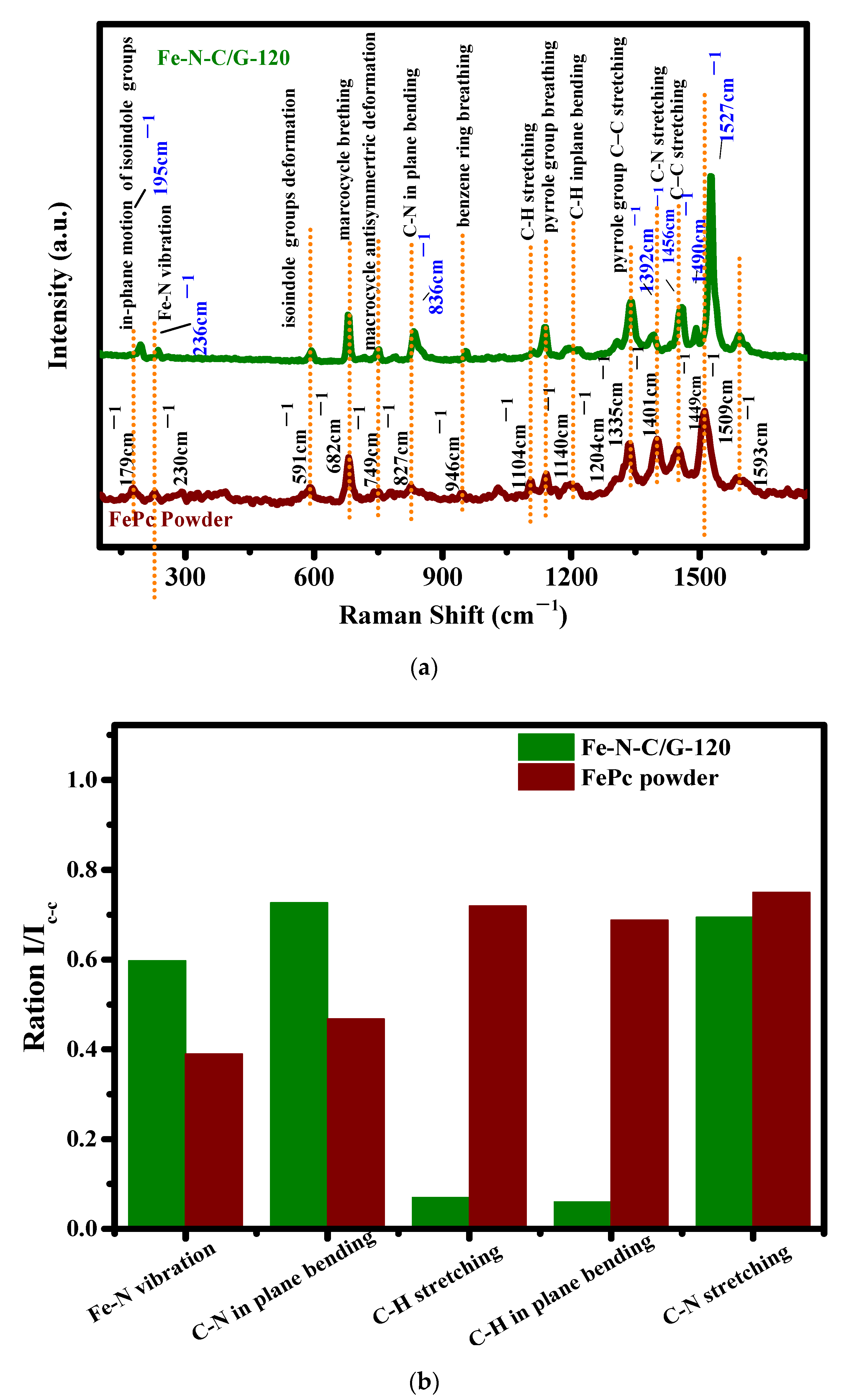
3.2. Raman and XPS Analyses
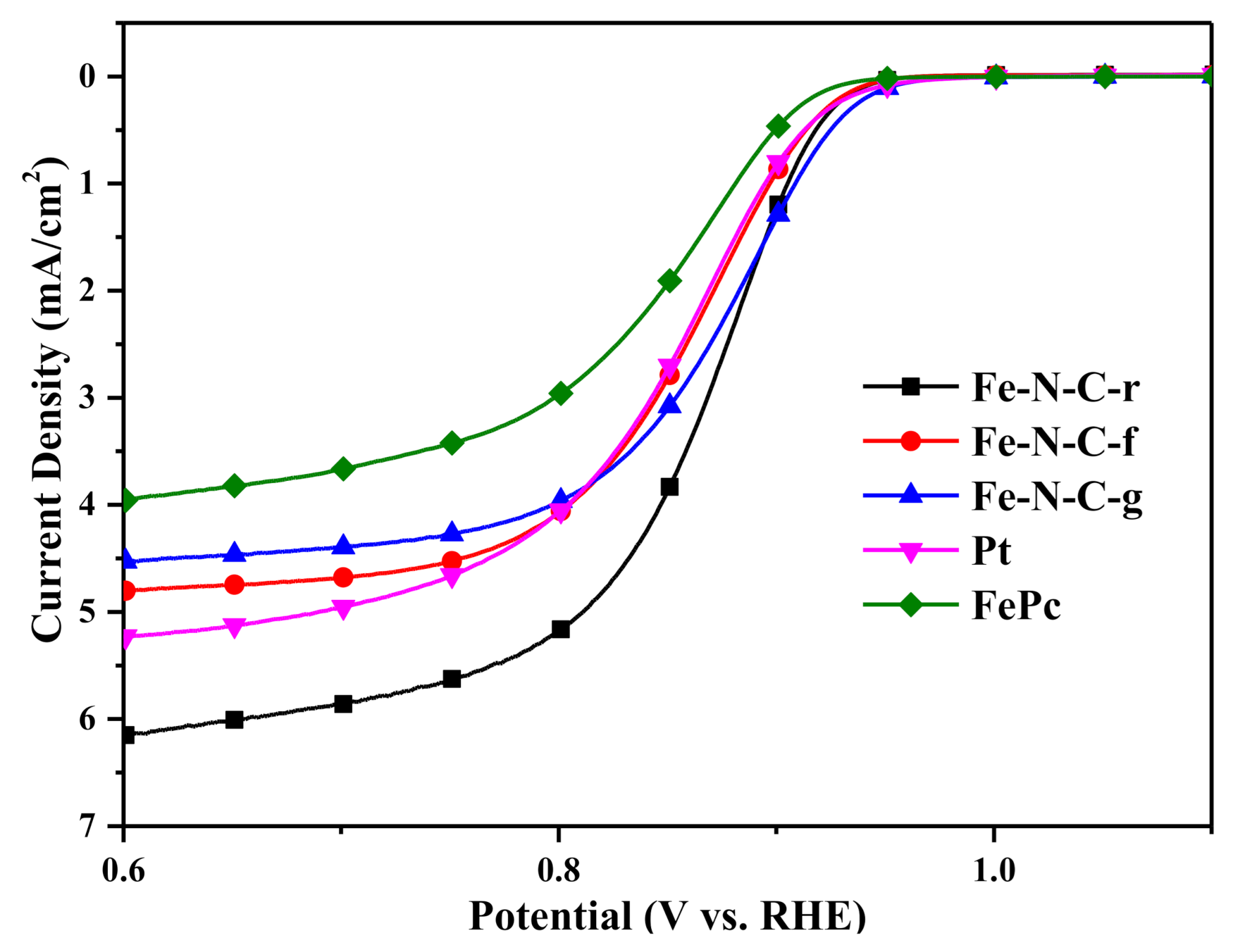
3.3. Analyses on the Performance
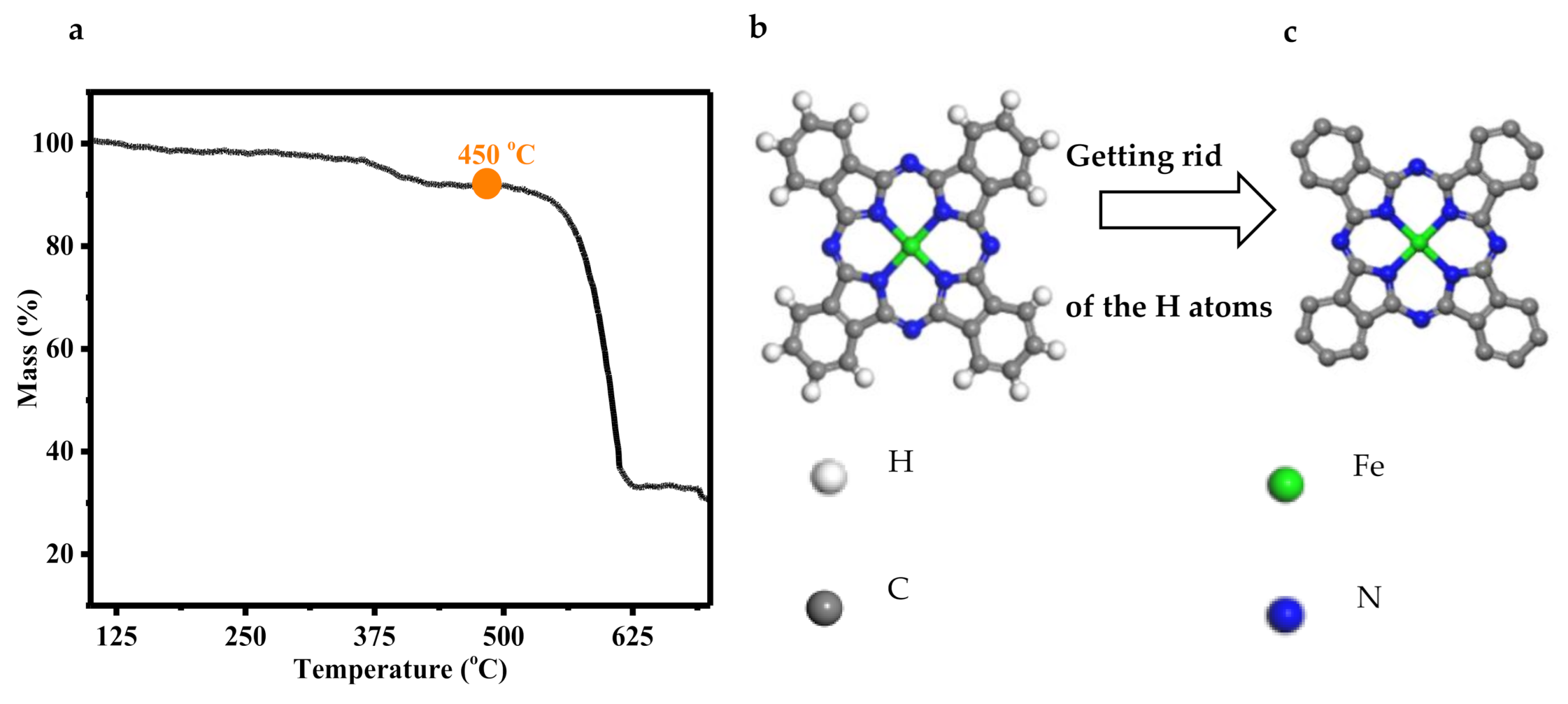
3.4. Thermogravimetric Analyses
3.5. Proposed Forming Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Future of Energy Supply: Challenges and Opportunities. Angew. Chem. Int. Ed. Engl. 2007, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Joya, K.S.; Joya, Y.F.; Ocakoglu, K.; van de Krol, R. Water-Splitting Catalysis and Solar Fuel Devices: Artificial Leaves on the Move. Angew. Chem. Int. Ed. Engl. 2013, 52, 10426–10437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Guo, C.; Zheng, Y.; Qiao, S.Z. Surface and Interface Engineering of Noble-Metal-Free Electrocatalysts for Efficient Energy Conversion Processes. Acc. Chem. Res. 2017, 50, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Chen, C.; You, W.; Zhang, J.; Liu, J.; Che, R. Yolk–Shell Fe/Fe4N@Pd/C Magnetic Nanocomposite as an Efficient Recyclable ORR Electrocatalyst and SERS Substrate. Small 2019, 15, e1805032. [Google Scholar] [CrossRef]
- Guo, S.; Li, D.; Zhu, H.; Zhang, S.; Markovic, N.M.; Stamenkovic, V.R.; Sun, S. FePt and CoPt Nanowires as Efficient Catalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. Engl. 2013, 52, 3465–3468. [Google Scholar] [CrossRef]
- Yang, Z.K.; Lin, L.; Xu, A.W. 2D Nanoporous Fe−N/C Nanosheets as Highly Effificient Non-Platinum Electrocatalysts for Oxygen Reduction Reaction in Zn-Air Battery. Small 2016, 12, 5710–5719. [Google Scholar] [CrossRef]
- Becknell, N.; Son, Y.; Kim, D.; Li, D.; Yu, Y.; Niu, Z.; Lei, T.; Sneed, B.T.; More, K.L.; Markovic, N.M.; et al. Control of Architecture in Rhombic Dodecahedral Pt-Ni Nanoframe Electrocatalysts. J. Am. Chem. Soc. 2017, 139, 11678–11681. [Google Scholar] [CrossRef]
- Li, S.; Tian, Z.Q.; Liu, Y.; Jang, Z.; Hasan, S.W.; Chen, X.; Tsiakaras, P.; Shen, P.K. Hierarchically skeletal multi-layered Pt-Ni nanocrystals for highly efficient oxygen reduction and methanol oxidation reactions. Chin. J. Catal. 2021, 42, 648–657. [Google Scholar] [CrossRef]
- Liu, J.; Yin, J.; Feng, B.; Xu, T.; Wang, F. Enhanced Electrocatalytic Activity and Stability toward the Oxygen Reduction Reaction with Unprotected Pt Nanoclusters. Nanomaterials 2018, 8, 955. [Google Scholar] [CrossRef] [PubMed]
- Beermann, V.; Gocyla, M.; Kuhl, S.; Padgett, E.; Schmies, H.; Goerlin, M.; Erini, N.; Shviro, M.; Heggen, M.; Dunin-Borkowski, R.E.; et al. Tuning the Electrocatalytic Oxygen Reduction Reaction Activity and Stability of Shape-Controlled Pt−Ni Nanoparticles by Thermal Annealing-Elucidating the Surface Atomic Structural and Compositional Changes. J. Am. Chem. Soc. 2017, 139, 16536–16547. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.; Lee, J.; Han, K.; Kim, H. Performance and stability of Pt-based ternary alloy catalysts for PEMFC. Electrochim. Acta 2006, 52, 1603–1611. [Google Scholar] [CrossRef]
- Gutsche, C.; Moeller, C.J.; Knipper, M.; Borchert, H.; Parisi, J.; Plaggenborg, T. Synthesis, Structure, and Electrochemical Stability of Ir-Decorated RuO2 Nanoparticles and Pt Nanorods as Oxygen Catalysts. J. Phys. Chem. C 2016, 120, 1137–1146. [Google Scholar] [CrossRef]
- Muratsugu, S.; Miyamoto, S.; Sakamoto, K.; Ichihashi, K.; Kim, C.K.; Ishiguro, N.; Tada, M. Size Regulation and Stability Enhancement of Pt Nanoparticle Catalyst via Polypyrrole Functionalization of Carbon-Nanotube Supported Pt Tetranuclear Complex. Langmuir 2017, 33, 10271–10282. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Willinger, E.; Rudi, S.; Heggen, M.; Dunin-Borkowski, R.E.; Willinger, M.G.; Strasser, P. Rh-Doped Pt–Ni Octahedral Nanoparticles: Understanding the Correlation between Elemental Distribution, Oxygen Reduction Reaction, and Shape Stability. Nano Lett. 2016, 16, 1719–1725. [Google Scholar] [CrossRef]
- Silva, G.C.; Fernandes, M.R.; Ticianelli, E.A. Activity and Stability of Pt/IrO2 Bifunctional Materials as Catalysts for the Oxygen Evolution/Reduction Reactions. ACS Catal. 2018, 8, 2081–2092. [Google Scholar] [CrossRef]
- Firouzjaie, H.A.; Mustain, W.E. Catalytic Advantages, Challenges, and Priorities in Alkaline Membrane Fuel Cells. ACS Catal. 2019, 10, 225–234. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.-C.; Chen, C.; Yang, X.-D.; Zhou, Z.-Y.; Sun, S.-G. A mesoporous Fe/N/C ORR catalyst for polymer electrolyte membrane fuel cells. Chin. J. Catal. 2016, 37, 1103–1108. [Google Scholar] [CrossRef]
- He, F.; Chen, X.; Shen, Y.; Li, Y.; Liu, A.; Liu, S.; Mori, T.; Zhang, Y. Ionic liquid-derived Fe–N/C catalysts for highly efficient oxygen reduction reaction without any supports, templates, or multi-step pyrolysis. J. Mater. Chem. A 2016, 4, 6630–6638. [Google Scholar] [CrossRef]
- Serov, A.; Artyushkova, K.; Atanassov, P. Fe-N-C Oxygen Reduction Fuel Cell Catalyst Derived from Carbendazim: Synthesis, Structure, and Reactivity. Adv. Energy Mater. 2014, 4, 1301735. [Google Scholar] [CrossRef]
- Sgarbi, R.; Kumar, K.; Jaouen, F.; Zitolo, A.; Ticianelli, E.A.; Maillard, F. Oxygen reduction reaction mechanism and kinetics on M-NxCy and M@N-C active sites present in model M-N-C catalysts under alkaline and acidic conditions. J. Solid State Electrochem. 2019. [Google Scholar] [CrossRef]
- Li, J.; Ghoshal, S.; Liang, W.; Sougrati, M.; Jaouen, F.; Halevi, B.; McKinney, S.; McCool, G.; Ma, C.; Yuan, X.; et al. Structural and mechanistic basis for the high activity of Fe–N–C catalysts toward oxygen reduction. Energy Environ. Sci. 2016, 9, 2418–2432. [Google Scholar] [CrossRef]
- Kramm, U.I.; Herranz, J.; Larouche, N.; Arruda, T.M.; Lefevre, M.; Jaouen, F.; Bogdanoff, P.; Fiechter, S.; Abs-Wurmbach, I.; Mukerjee, S.; et al. Structure of the catalytic sites in Fe/N/C-catalysts for O2-reduction in PEM fuel cells. Phys. Chem. Chem. Phys. 2012, 14, 11673–11688. [Google Scholar] [CrossRef]
- Yang, D.S.; Song, M.Y.; Singh, K.P.; Yu, J.S. The role of iron in the preparation and oxygen reduction reaction activity of nitrogen-doped carbon. Chem. Commun. 2015, 51, 2450–2453. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, S.; Qiu, Y. Effect of carbonization temperature on bimetallic FeCo-N/C nanofifiber electrocatalysts for oxygen reduction reaction in sulfuric acid solution. Int. J. Hydrog. Energy 2017, 42, 29274–29282. [Google Scholar] [CrossRef]
- Morozan, A.; Jegou, P.; Campidelli, S.; Palacin, S.; Jousselme, B. Relationship between polypyrrole morphology and electrochemical activity towards oxygen reduction reaction. Chem. Commun. 2012, 48, 4627–4629. [Google Scholar] [CrossRef]
- Kramm, U.I.; Lefevre, M.; Larouche, N.; Schmeisser, D.; Dodelet, J.P. Correlations between Mass Activity and Physicochemical Properties of Fe/N/C Catalysts for the ORR in PEM Fuel Cell via 57Fe Mossbauer Spectroscopy and Other Techniques. J. Am. Chem. Soc. 2014, 136, 978–985. [Google Scholar] [CrossRef]
- Li, X.S.; Cai, W.W.; An, J.H.; Kim, S.; Nah, J.; Yang, D.X.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Tatar, B.; Demiroğlu, D. Electrical properties of FePc organic semiconductor thin films obtained by CSP technique for photovoltaic applications. Mater. Sci. Semicond. Process. 2015, 31, 644–650. [Google Scholar] [CrossRef]
- Zhao, Q.; Hou, M.; Jiang, S.; Wang, S.; Ai, J.; Zheng, L.; Shao, Z. Investigation of a Fe–N–C catalyst for sulfur dioxide electrooxidation. RSC Adv. 2016, 6, 80024–80028. [Google Scholar] [CrossRef]
- Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. Chemistry of Multitudinous Active Sites for Oxygen Reduction Reaction in Transition Metal−Nitrogen−Carbon Electrocatalysts. J. Phys. Chem. C 2015, 119, 25917–25928. [Google Scholar] [CrossRef]
- Cai, W.; Zhou, J.; Li, G.; Zhang, K.; Liu, X.; Wang, C.; Zhou, H.; Zhu, Y.; Qian, Y. B, N-co-doped graphene supported sulfur for superior stable Li-S half cell and Ge-S full battery. ACS Appl. Mater. Interfaces 2016, 8, 27679–27687. [Google Scholar] [CrossRef]
- Jin, L.; Zhu, B.; Wang, X.; Zhang, L.; Song, D.; Guo, J.; Tao, H. Facile Synthesis of the Amorphous Carbon Coated Fe-N-C Nanocatalyst with Effiffifficient Activity for Oxygen Reduction Reaction in Acidic and Alkaline Media. Materials 2020, 13, 4551. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, N.; Liu, F.; Meng, X.; Qin, M.; Zhu, G.; Bu, L.; Liu, Z.; Wang, W. Morphology and Structure Controls of Single-Atom Fe–N–C Catalysts Synthesized Using FePc Powders as the Precursor. Processes 2021, 9, 109. https://doi.org/10.3390/pr9010109
Yan N, Liu F, Meng X, Qin M, Zhu G, Bu L, Liu Z, Wang W. Morphology and Structure Controls of Single-Atom Fe–N–C Catalysts Synthesized Using FePc Powders as the Precursor. Processes. 2021; 9(1):109. https://doi.org/10.3390/pr9010109
Chicago/Turabian StyleYan, Ning, Fan Liu, Xu Meng, Meng Qin, Guangqi Zhu, Luxia Bu, Zigeng Liu, and Wei Wang. 2021. "Morphology and Structure Controls of Single-Atom Fe–N–C Catalysts Synthesized Using FePc Powders as the Precursor" Processes 9, no. 1: 109. https://doi.org/10.3390/pr9010109
APA StyleYan, N., Liu, F., Meng, X., Qin, M., Zhu, G., Bu, L., Liu, Z., & Wang, W. (2021). Morphology and Structure Controls of Single-Atom Fe–N–C Catalysts Synthesized Using FePc Powders as the Precursor. Processes, 9(1), 109. https://doi.org/10.3390/pr9010109



