Novel Methods Using an Arthrobacter sp. to Create Anaerobic Conditions for Biobutanol Production from Sweet Sorghum Juice by Clostridium beijerinckii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Butanol Production Medium
2.3. Microorganisms and Inoculum Preparation
2.4. Experimental Procedures
2.4.1. Use of SSJ Medium for Butanol Production
2.4.2. Use of SDTN to Create Anaerobic Conditions for Butanol Production
2.4.3. Use of Arthrobacter sp. BCC 72131 to Create Anaerobic Conditions for Butanol Production
2.5. Analytical Methods
3. Results and Discussion
3.1. Butanol Production from SSJ Medium
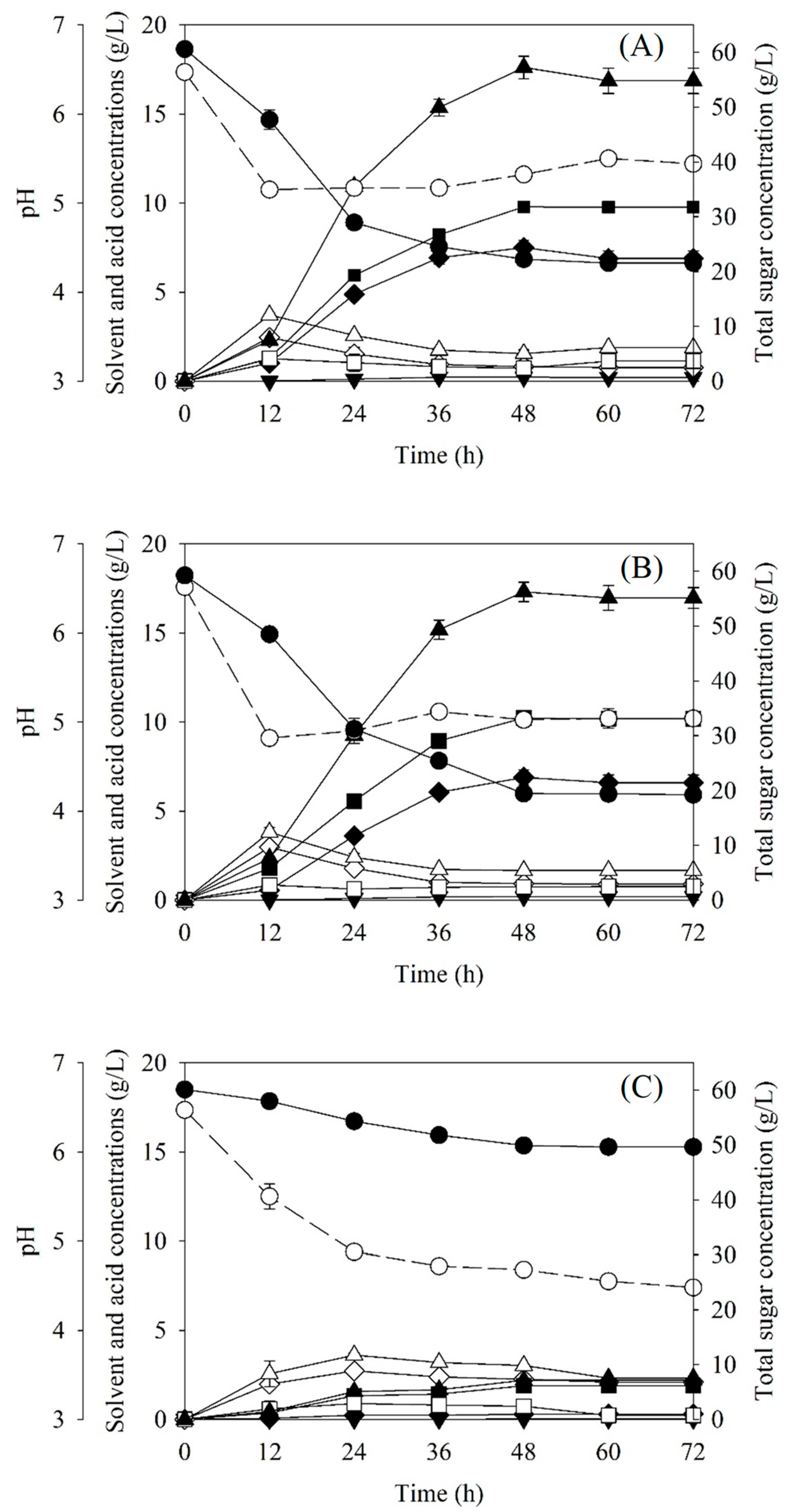
3.2. Batch Butanol Production from SSJ Medium by C. beijerinckii TISTR 1461 under Anaerobic Conditions Created Using SDTN
3.3. Batch Butanol Production from SSJ Medium by C. beijerinckii TISTR 1461 under Anaerobic Conditions Created Using Arthrobacter sp. BCC 72131
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Luo, Z.; Cui, Y.; Xu, Y.; Lin, L.; Zhao, M.; Guo, Y.; Pang, Z. Dual function of ammonium acetate in acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. Bioresour. Technol. 2018, 267, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Cheng, C. Butanol production by Clostridium. In Advances in Bioenergy, 1st ed.; Elsevier Inc.: Oxford, UK, 2019; Volume 4, pp. 35–77. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.; Xu, M.; Tang, I.C.; Zhao, J.; Bai, F.; Yang, S.T. Butanol production in acetone-butanol-ethanol fermentation with in situ product recovery by adsorption. Bioresour. Technol. 2016, 219, 158–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shorgani, N.K.N.; Ali, E.; Kalil, M.S.; Yusoff, W.M.W. Bioconversion of butyric acid to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) in a limited nutrient medium. Bioenergy Res. 2012, 5, 287–293. [Google Scholar] [CrossRef]
- Ponthein, W.; Cheirsilp, B. Development of Acetone Butanol Ethanol (ABE) Production from palm pressed fiber by mixed culture of Clostridium sp. and Bacillus sp. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 9, pp. 459–467. [Google Scholar] [CrossRef] [Green Version]
- Oliva-Rodríguez, A.G.; Quintero, J.; Medina-Morales, M.A.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, M.; Aroca, G.; Rios González, L.J. Clostridium strain selection for co-culture with Bacillus subtilis for butanol production from agave hydrolysates. Bioresour. Technol. 2019, 275, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Tri, C.L.; Kamei, I. Butanol production from cellulosic material by anaerobic co-culture of white-rot fungus Phlebia and bacterium Clostridium in consolidated bioprocessing. Bioresour. Technol. 2020, 305, 123065. [Google Scholar] [CrossRef] [PubMed]
- Kilroy, W.P. Anaerobic decomposition of sodium dithionite in alkaline solution. J. Inorg. Nucl. Chem. 1980, 42, 1071–1073. [Google Scholar] [CrossRef]
- Camacho, F.; Páez, M.P.; Jiménez, M.C.; Fernández, M. Application of the sodium dithionite oxidation to measure oxygen transfer parameters. Chem. Eng. Sci. 1997, 52, 1387–1391. [Google Scholar] [CrossRef]
- Holman, D.A.; Bennett, D.W. A multicomponent kinetics study of the anaerobic decomposition of aqueous sodium dithionite. J. Phys. Chem. 1994, 98, 13300–13307. [Google Scholar] [CrossRef]
- Luscombe, B.M.; Gray, T.R.G. Characteristics of Arthrobacter grown in continuous culture. J. Gen. Microbiol. 1974, 82, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Eschbach, M.; Möbitz, H.; Rompf, A.; Jahn, D. Members of the genus Arthrobacter grow anaerobically using nitrate ammonification and fermentative processes: Anaerobic adaptation of aerobic bacteria abundant in soil. FEMS Microbiol. Lett. 2003, 223, 227–230. [Google Scholar] [CrossRef]
- Silva, C.R.; Souza, J.C.; Araújo, L.S.; Kagohara, E.; Garcia, T.P.; Pelizzari, V.H.; Andrade, L.H. Exploiting the enzymatic machinery of Arthrobacter atrocyaneus for oxidative kinetic resolution of secondary alcohols. J. Mol. Catal. B Enzym. 2012, 83, 23–28. [Google Scholar] [CrossRef]
- Cascon, H.R.; Choudhari, S.K.; Nisola, G.M.; Vivas, E.L.; Lee, D.J.; Chung, W.J. Partitioning of butanol and other fermentation broth components in phosphonium and ammonium-based ionic liquids and their toxicity to solventogenic Clostridia. Sep. Purif. Technol. 2011, 78, 164–174. [Google Scholar] [CrossRef]
- Mathur, S.; Umakanth, A.V.; Tonapi, V.A.; Sharma, R.; Sharma, M.K. Sweet sorghum as biofuel feedstock: Recent advances and available resources. Biotechnol. Biofuels 2017, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sirisantimethakom, L.; Thanapornsin, T.; Laopaiboon, L.; Laopaiboon, P. Enhancement of butanol production efficiency from sweet sorghum stem juice by Clostridium beijerinckii using statistical experimental design. Chiang. Mai. J. Sci. 2018, 45, 1235–1246. [Google Scholar]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice using very high gravity technology: Effects of carbon and nitrogen supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Qureshi, N.; Blaschek, H.P. Butanol recovery from model solution/fermentation broth by pervaporation: Evaluation of membrane performance. Biomass Bioenergy 1999, 17, 175–184. [Google Scholar] [CrossRef]
- Sirisantimethakom, L.; Laopaiboon, L.; Sanchanda, P.; Chatleudmongkol, J.; Laopaiboon, P. Improvement of butanol production from sweet sorghum juice by Clostridium beijerinckii using an orthogonal array design. Ind. Crop. Prod. 2016, 79, 287–294. [Google Scholar] [CrossRef]
- Narueworanon, P.; Laopaiboon, L.; Phukoetphim, N.; Laopaiboon, P. Impacts of initial sugar, nitrogen and calcium carbonate on butanol fermentation from sugarcane molasses by Clostridium beijerinckii. Energies 2020, 13, 694. [Google Scholar] [CrossRef] [Green Version]
- Wechgama, K.; Laopaiboon, L.; Laopaiboon, P. Enhancement of batch butanol production from sugarcane molasses using nitrogen supplementation integrated with gas stripping for product recovery. Ind. Crop. Prod. 2017, 95, 216–226. [Google Scholar] [CrossRef]
- Camacho Rubio, F.; Paez Dueñas, M.P.; Blazquez Garcia, G.; Garrido Martin, J.M. Oxygen absorption in alkaline sodium dithionite solutions. Chem. Eng. Sci. 1992, 47, 4309–4314. [Google Scholar] [CrossRef]
- Qi, G.; Xiong, L.; Luo, M.; Huang, Q.; Huang, C.; Li, H.; Chen, X.; Chen, X. Solvents production from cassava by co-culture of Clostridium acetobutylicum and Saccharomyces cerevisiae. J. Environ. Chem. Eng. 2018, 6, 128–133. [Google Scholar] [CrossRef]
- Areesirisuk, A.; Laopaiboon, L.; Khongsay, N.; Laopaiboon, P. Improvement of gas chromatographic analysis for organic acids and solvents in acetone-butanolethanol fermentation from sweet sorghum juice. Afr. J. Biotechnol. 2010, 9, 6422–6429. [Google Scholar] [CrossRef]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol-sulphuric acid method assisted by multivariate calibration. Chemom. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Sriputorn, B.; Laopaiboon, P.; Phukoetphim, N.; Polsokchuak, N.; Butkun, K.; Laopaiboon, L. Enhancement of ethanol production efficiency in repeated-batch fermentation from sweet sorghum stem juice: Effect of initial sugar, nitrogen and aeration. Electron. J. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Yand, C.Y.; He, L. Neuroprotective effects of sinapine on PC12 cells apoptosis induced by sodium dithionite. Chin. J. Nat. Med. 2008, 6, 205–209. [Google Scholar] [CrossRef]

| Constituents | Contents |
|---|---|
| Total soluble solids (ºBx) | 18 |
| Total protein a (g/100 mL) | 0.82 |
| Sulfur b (mg/L) | 2.41 |
| Phosphorus c (mg/L) | 380.40 |
| Potassium c (mg/L) | 4800.50 |
| Sodium c (mg/L) | 59.59 |
| Calcium c (mg/L) | 888.95 |
| Magnesium c (mg/L) | 290.50 |
| Iron c (mg/L) | 0.351 |
| Manganese c (mg/L) | 0.167 |
| Copper c (mg/L) | 0.025 |
| Zinc c (mg/L) | 0.078 |
| Molybdenum c (mg/L) | 0.055 |
| Nickel c (mg/L) | 0.007 |
| Boron c (mg/L) | 0.060 |
| Cobalt c (mg/L) | Not Detected |
| Medium | PB (g/L) | PABE (g/L) | SC (g/L) | SC (%) | t (h) | YB/S (g/g) | QB (g/L∙h) |
|---|---|---|---|---|---|---|---|
| SSJ + (NH4)2SO4 | 9.88 ± 0.38 a | 17.61 ± 0.63 a | 32.18 ± 0.01 b | 53.11 ± 0.12 b | 48 | 0.30 ± 0.01 a | 0.21 ± 0.01 a |
| P2 medium (Positive control) | 10.23 ± 0.31 a | 17.30 ± 0.55 a | 39.80 ± 1.50 a | 66.13 ± 0.59 a | 48 | 0.26 ± 0.00 b | 0.21 ± 0.01 a |
| SSJ (Negative control) | 1.90 ± 0.16 b | 2.20 ± 0.06 b | 10.22 ± 0.57 c | 28.25 ± 0.56 c | 36 | 0.19 ± 0.01 c | 0.05 ± 0.00 b |
| Medium | PB (g/L) | PABE (g/L) | Pacid (g/L) | SC (g/L) | SC (%) | t (h) | YB/S (g/g) | QB (g/L∙h) | YABE/S (g/g) |
|---|---|---|---|---|---|---|---|---|---|
| 0.125 mM SDTN | 2.30 ± 0.02 d | 3.24 ± 0.16 d | 4.19 ± 0.17 b | 12.20 ± 0.55 d | 19.69 ± 0.89 d | 48 | 0.18 ± 0.01 c | 0.05 ± 0.00 d | 0.25 ± 0.01 e |
| 0.25 mM SDTN | 8.51 ± 0.44 b | 14.07 ± 0.52 b | 1.25 ± 0.05 d | 33.89 ± 0.14 a | 55.99 ± 0.23 a | 84 | 0.26 ± 0.00 b | 0.10 ± 0.00 b | 0.41 ± 0.01 b |
| 0.50 mM SDTN | 4.26 ± 0.09 c | 5.80 ± 0.16 c | 5.00 ± 0.19 a | 17.10 ± 0.30 c | 27.52 ± 0.48 c | 72 | 0.25 ± 0.00 b | 0.06 ± 0.00 c | 0.34 ± 0.00 c |
| 0.75 mM SDTN | 0 ± 0.00 e | 0 ± 0.00 f | 0 ± 0.00 e | 0 ± 0.00 f | - | 84 | - | - | - |
| 1.00 mM SDTN | 0 ± 0.00 e | 0 ± 0.00 f | 0 ± 0.00 e | 0 ± 0.00 f | - | 84 | - | - | - |
| OFN gas flushing(Positive control) | 9.88 ± 0.38 a | 17.61 ± 0.63 a | 1.54 ± 0.21 c | 32.18 ± 0.01 b | 53.11 ± 0.12 b | 48 | 0.30 ± 0.01 a | 0.21 ± 0.01 a | 0.45 ± 0.00 a |
| No OFN gas flushing (Negative control) | 1.26 ± 0.35 d | 1.56 ± 0.12 e | 4.22 ± 0.02 b | 4.75 ± 0.35 e | 7.91 ± 0.58 e | 48 | 0.31 ± 0.01 a | 0.04 ± 0.00 e | 0.31 ± 0.00 d |
| Condition | PB (g/L) | PABE (g/L) | Pacid (g/L) | SC (g/L) | SC (%) | t (h) | YB/S (g/g) | QB (g/L∙h) |
|---|---|---|---|---|---|---|---|---|
| Mixed Cultures of C. beijerinckii TISTR 1461 and Arthrobacter sp. BCC 72131 | ||||||||
| Simultaneous inoculation | 10.72 ± 0.31 a | 18.63 ± 0.22 a | 1.40 ± 0.18 a | 33.50 ± 0.38 a | 55.47 ± 0.42 a | 60 | 0.33 ± 0.01 a | 0.18 ± 0.01 b |
| Inoculation of Clostridium 4 h after Arthrobacter | 10.39 ± 0.25 a | 18.03 ± 0.23 b | 1.77 ± 0.24 a | 32.02 ± 0.47 b | 52.01 ± 0.82 b | 52 | 0.32 ± 0.01 a | 0.20 ± 0.00 a |
| Pure Culture of C. beijerinckii TISTR 1461 | ||||||||
| OFN flushing | 9.88 ± 0.38 b | 17.61 ± 0.63 b | 1.54 ± 0.21 a | 32.18 ± 0.01 b | 53.11 ± 0.12 c | 48 | 0.30 ± 0.01 b | 0.21 ± 0.01 a |
| Air flushing | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 b | 3.96 ± 0.14 d | 6.63 ± 0.05 e | 12 | - | - |
| Pure Culture of Arthrobacter sp. BCC 72131 | ||||||||
| No gas flushing | 0 ± 0.00 c | 0 ± 0.00 c | 0 ± 0.00 b | 5.77 ± 0.75 c | 9.60 ± 1.67 d | 48 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daengbussadee, C.; Laopaiboon, L.; Kaewmaneewat, A.; Sirisantimethakom, L.; Laopaiboon, P. Novel Methods Using an Arthrobacter sp. to Create Anaerobic Conditions for Biobutanol Production from Sweet Sorghum Juice by Clostridium beijerinckii. Processes 2021, 9, 178. https://doi.org/10.3390/pr9010178
Daengbussadee C, Laopaiboon L, Kaewmaneewat A, Sirisantimethakom L, Laopaiboon P. Novel Methods Using an Arthrobacter sp. to Create Anaerobic Conditions for Biobutanol Production from Sweet Sorghum Juice by Clostridium beijerinckii. Processes. 2021; 9(1):178. https://doi.org/10.3390/pr9010178
Chicago/Turabian StyleDaengbussadee, Chalida, Lakkana Laopaiboon, Anuphon Kaewmaneewat, Likit Sirisantimethakom, and Pattana Laopaiboon. 2021. "Novel Methods Using an Arthrobacter sp. to Create Anaerobic Conditions for Biobutanol Production from Sweet Sorghum Juice by Clostridium beijerinckii" Processes 9, no. 1: 178. https://doi.org/10.3390/pr9010178
APA StyleDaengbussadee, C., Laopaiboon, L., Kaewmaneewat, A., Sirisantimethakom, L., & Laopaiboon, P. (2021). Novel Methods Using an Arthrobacter sp. to Create Anaerobic Conditions for Biobutanol Production from Sweet Sorghum Juice by Clostridium beijerinckii. Processes, 9(1), 178. https://doi.org/10.3390/pr9010178




