Hemicellulosic Bioethanol Production from Fast-Growing Paulownia Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
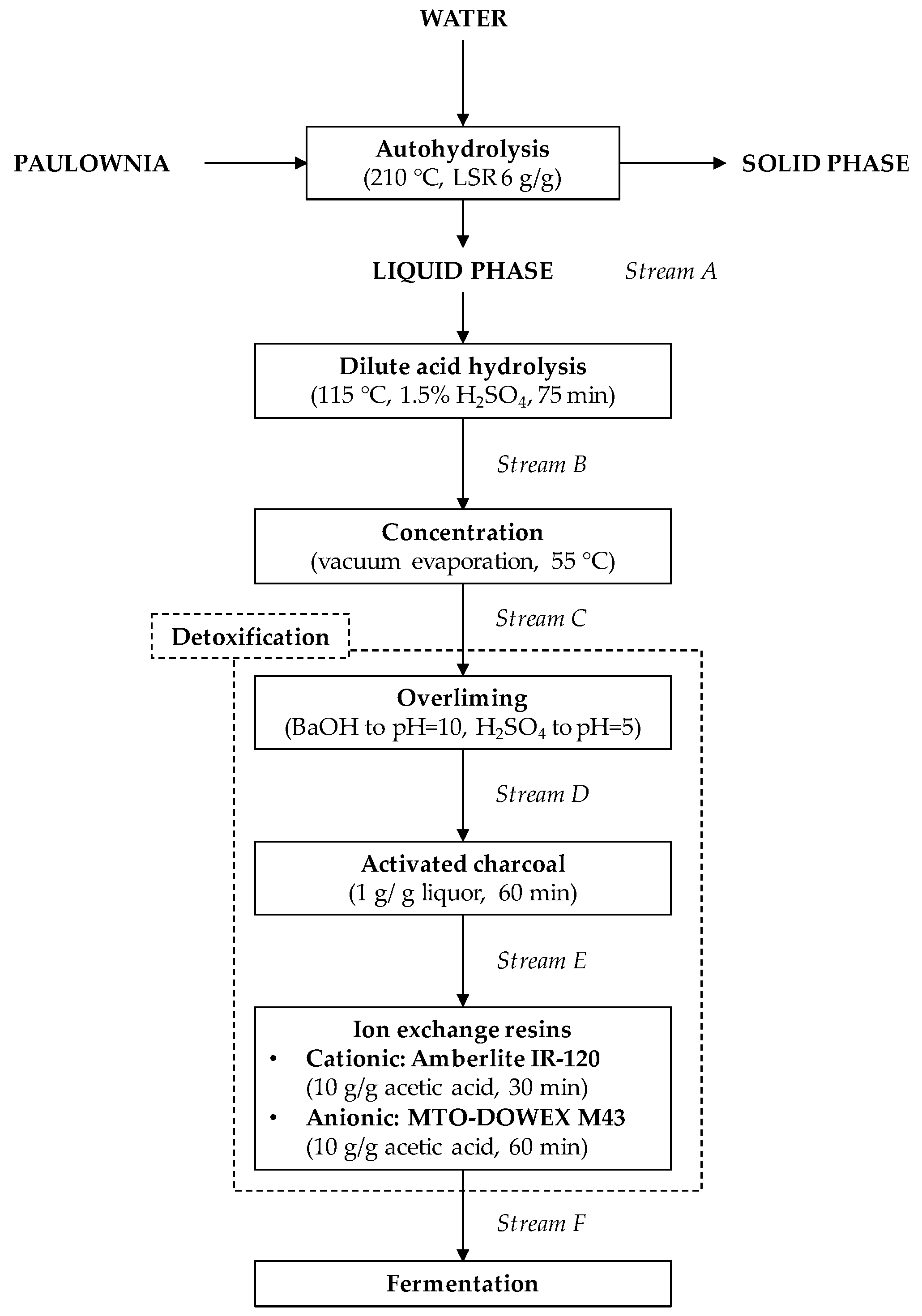
2.2. Preparation of Hemicellulosic Hydrolysate: Autohydrolysis and Dilute Acid Treatments
2.3. Concentration of Hemicellulosic Hydrolysate
2.4. Detoxification of Hemicellulosic Hydrolysate: Overliming, Activated Charcoal, and Ion Exchange
2.5. Yeast Species and Preparation of the Inoculum
2.6. Hemicellulosic Hydrolysate Fermentation
2.7. Analytical Methods and Composition of the Raw Material
2.8. Analytical Methods and Composition of the Raw Material
3. Results and Discussion
3.1. Autohydrolysis Treatment of Paulownia
3.2. Evaluation of Dilute Acid Hydrolysis Conditions on the Hemicellulosic Hydrolysate
3.3. Concentration and Detoxification of Hemicellulosic Hydrolysate
3.4. Hydrolysates Fermentation for Ethanol Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hinman, N.D.; Wright, J.D.; Hoagland, W.; Wyman, C.E. Xylose fermentation: An economic analysis. Appl. Biochem. Biotechnol. 1989, 20, 391–401. [Google Scholar] [CrossRef]
- Cuevas, M.; Saleh, M.; García-Martín, J.F.; Sánchez, S. Acid and enzymatic fractionation of olive stones for ethanol production using Pachysolen tannophilus. Processes 2020, 8, 195. [Google Scholar] [CrossRef] [Green Version]
- Susmozas, A.; Martín-Sampedro, R.; Ibarra, D.; Eugenio, M.E.; Iglesias, R.; Manzanares, P.; Moreno, A.D. Process strategies for the transition of 1G to advanced bioethanol production. Processes 2020, 8, 1310. [Google Scholar] [CrossRef]
- Moysés, D.N.; Reis, V.C.B.; de Almeida, J.R.M.; de Moraes, L.M.P.; Torres, F.A.G. Xylose fermentation by Saccharomyces cerevisiae: Challenges and prospects. Int. J. Mol. Sci. 2016, 17, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Río, P.G.; Gullón, P.; Rebelo, F.R.; Romaní, A.; Garrote, G.; Gullón, B. A whole-slurry fermentation approach to high-solid loading for bioethanol production from corn stover. Agronomy 2020, 10, 1790. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, J.T.; Romaní, A.; Costa, C.E.; Sá-Correia, I.; Domingues, L. Molecular and physiological basis of Saccharomyces cerevisiae tolerance to adverse lignocellulose-based process conditions. Appl. Microbiol. Biotechnol. 2019, 103, 159–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.J.; Deschatelets, L.; Nishikawa, N.K. Comparative fermentability of enzymatic and acid hydrolysates of steam-pretreated aspenwood hemicellulose by Pichia stipitis CBS 5776. Appl. Microbiol. Biotechnol. 1989, 31, 592–596. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; Alonso, J.L.; Parajó, J.C. Bioethanol production from hydrothermally pretreated Eucalyptus globulus wood. Bioresour. Technol. 2010, 101, 8706–8712. [Google Scholar] [CrossRef]
- Missoun, F.; Pérez de los Ríos, A.; Ortiz-Martínez, V.; Salar-García, M.J.; Hernández-Fernández, J.; Hernández-Fernández, F.J. Discovering low toxicity ionic liquids for Saccharomyces cerevisiae by using the agar well diffusion test. Processes 2020, 8, 1163. [Google Scholar] [CrossRef]
- Veras, H.C.T.; Parachin, N.S.; Almeida, J.R.M. Comparative assessment of fermentative capacity of different xylose-consuming yeasts. Microb. Cell Fact. 2017, 16, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.; He, M.; You, H.; Pan, L.; Hu, G.; Cui, Y.; Maeda, T. Enhanced fuel ethanol production from rice straw hydrolysate by an inhibitor-tolerant mutant strain of: Scheffersomyces stipitis. RSC Adv. 2017, 7, 31180–31188. [Google Scholar] [CrossRef] [Green Version]
- Cavka, A.; Jönsson, L.J. Detoxification of lignocellulosic hydrolysates using sodium borohydride. Bioresour. Technol. 2013, 136, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guigou, M.D.; Cebreiros, F.; Cabrera, M.N.; Ferrari, M.D.; Lareo, C. Bioethanol production from Eucalyptus grandis hemicellulose recovered before kraft pulping using an integrated biorefinery concept. Biomass Convers. Biorefin. 2017, 7, 191–197. [Google Scholar] [CrossRef]
- Pereira, F.B.; Romaní, A.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Industrial robust yeast isolates with great potential for fermentation of lignocellulosic biomass. Bioresour. Technol. 2014, 161, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Koppram, R.; Albers, E.; Olsson, L. Evolutionary engineering strategies to enhance tolerance of xylose utilizing recombinant yeast to inhibitors derived from spruce biomass. Biotechnol. Biofuels 2012, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cunha, J.T.; Aguiar, T.Q.; Romaní, A.; Oliveira, C.; Domingues, L. Contribution of PRS3, RPB4 and ZWF1 to the resistance of industrial Saccharomyces cerevisiae CCUG53310 and PE-2 strains to lignocellulosic hydrolysate-derived inhibitors. Bioresour. Technol. 2015, 191, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Cunha, J.T.; Costa, C.E.; Ferraz, L.; Romaní, A.; Johansson, B.; Sá-Correia, I.; Domingues, L. HAA1 and PRS3 overexpression boosts yeast tolerance towards acetic acid improving xylose or glucose consumption: Unravelling the underlying mechanisms. Appl. Microbiol. Biotechnol. 2018, 102, 4589–4600. [Google Scholar] [CrossRef]
- Pereira, F.B.; Teixeira, M.C.; Mira, N.P.; Sá-Correia, I.; Domingues, L. Genome-wide screening of Saccharomyces cerevisiae genes required to foster tolerance towards industrial wheat straw hydrolysates. J. Ind. Microbiol. Biotechnol. 2014, 41, 1753–1761. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.B.; Guimarães, P.M.R.; Gomes, D.G.; Mira, N.P.; Teixeira, M.C.; Sá-Correia, I.; Domingues, L. Identification of candidate genes for yeast engineering to improve bioethanol production in very high gravity and lignocellulosic biomass industrial fermentations. Biotechnol. Biofuels 2011, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.E.; Romaní, A.; Cunha, J.T.; Johansson, B.; Domingues, L. Integrated approach for selecting efficient Saccharomyces cerevisiae for industrial lignocellulosic fermentations: Importance of yeast chassis linked to process conditions. Bioresour. Technol. 2017, 227, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Generation of xylose solutions from Eucalyptus globulus wood by autohydrolysis-posthydrolysis processes: Posthydrolysis kinetics. Bioresour. Technol. 2001, 79, 155–164. [Google Scholar] [CrossRef]
- Rivas, B.; Domínguez, J.M.; Domínguez, H.; Parajó, J.C. Bioconversion of posthydrolysed autohydrolysis liquors: An alternative for xylitol production from corn cobs. Enzym. Microb. Technol. 2002, 31, 431–438. [Google Scholar] [CrossRef]
- Moldes, A.B.; Bustos, G.; Torrado, A.; Domínguez, J.M. Comparison between different hydrolysis processes of vine-trimming waste to obtain hemicellulosic sugars for further lactic acid conversion. Appl. Biochem. Biotechnol. 2007, 143, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, J.; Romaní, A.; González-Muñoz, M.J.; Garrote, G.; Parajó, J.C. Extracting value-added products before pulping: Hemicellulosic ethanol from Eucalyptus globulus wood. Holzforschung 2012, 66, 591–599. [Google Scholar] [CrossRef]
- Rivas, S.; Vila, C.; Santos, V.; Parajó, J.C. Furfural production from birch hemicelluloses by two-step processing: A potential technology for biorefineries. Holzforschung 2016, 70, 901–910. [Google Scholar] [CrossRef]
- Rivas, S.; González-Muñoz, M.J.; Vila, C.; Santos, V.; Parajó, J.C. Manufacture of levulinic acid from pine wood hemicelluloses: A kinetic assessment. Ind. Eng. Chem. Res. 2013, 52, 3951–3957. [Google Scholar] [CrossRef]
- Marsal, F.; Thevathasan, N.V.; Guillot, S.; Mann, J.; Gordon, A.M.; Thimmanagari, M.; Deen, W.; Silim, S.; Soolanayakanahally, R.; Sidders, D. Biomass yield assessment of five potential energy crops grown in southern Ontario, Canada. Agrofor. Syst. 2016, 90, 773–783. [Google Scholar] [CrossRef]
- López, F.; Pérez, A.; Zamudio, M.A.M.; De Alva, H.E.; García, J.C. Paulownia as raw material for solid biofuel and cellulose pulp. Biomass Bioenergy 2012, 45, 77–86. [Google Scholar] [CrossRef]
- Domínguez, E.; Nóvoa, T.; del Río, P.G.; Garrote, G.; Romaní, A. Sequential two-stage autohydrolysis biorefinery for the production of bioethanol from fast-growing Paulownia biomass. Energy Convers. Manag. 2020, 226. [Google Scholar] [CrossRef]
- Pablo, G.; Domínguez, V.D.; Domínguez, E.; Gullón, P.; Gullón, B.; Garrote, G.; Romaní, A. Comparative study of biorefinery processes for the valorization of fast-growing Paulownia wood. Bioresour. Technol. 2020, 314, 123722. [Google Scholar] [CrossRef]
- Srilekha Yadav, K.; Naseeruddin, S.; Sai Prashanthi, G.; Sateesh, L.; Venkateswar Rao, L. Bioethanol fermentation of concentrated rice straw hydrolysate using co-culture of Saccharomyces cerevisiae and Pichia stipitis. Bioresour. Technol. 2011, 102, 6473–6478. [Google Scholar] [CrossRef] [PubMed]
- Converti, A.; Perego, P.; Domínguez, J.M. Xylitol production from hardwood hemicellulose hydrolysates by Pachysolen tannophilus, Debaryomyces hansenii, and Candida guilliermondii. Appl. Biochem. Biotechnol.-Part A Enzym. Eng. Biotechnol. 1999, 82, 141–151. [Google Scholar] [CrossRef]
- Romaní, A.; Pereira, F.; Johansson, B.; Domingues, L. Metabolic engineering of Saccharomyces cerevisiae ethanol strains PE-2 and CAT-1 for efficient lignocellulosic fermentation. Bioresour. Technol. 2015, 179, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass. Laboratory Analytical Procedure (LAP); Technicl Report NREL/TP-510-42619; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. Laboratory Analytical Procedure (LAP); Technicl Report NREL/TP-510-42622; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples. Laboratory Analytical Procedure (LAP); Technicl Report NREL/TP-510-42621; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L. Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP); Technicl Report NREL/TP-510-42618; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uranic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Conde, E.; Castro López, M.D.; Moure, A.; Manuel López Vilariño, J.; Domínguez, H.; Abad López, M.J.; Gonzalez Rodriguez, V. An approach to assess the synergistic effect of natural antioxidants on the performance of the polypropylene stabilizing systems. J. Appl. Polym. Sci. 2012, 126, 1852–1858. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. The use of high-solids loadings in biomass pretreatment-a review. Biotechnol. Bioeng. 2012, 109, 1430–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Casey, E.; Sedlak, M.; Ho, N.W.Y.; Mosier, N.S. Effect of acetic acid and pH on the cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Romaní, A.; Domingues, L.; Garrote, G. Evaluation of strategies for second generation bioethanol production from fast growing biomass Paulownia within a biorefinery scheme. Appl. Energy 2017, 187, 777–789. [Google Scholar] [CrossRef] [Green Version]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Manufacture of xylose-based fermentation media from corncobs by posthydrolysis of autohydrolysis liquors. Appl. Biochem. Biotechnol.-Part A Enzym. Eng. Biotechnol. 2001, 95, 195–207. [Google Scholar] [CrossRef]
- Carvalho, G.B.M.; Mussatto, S.I.; Cândido, E.J.; Almeida e Silva, J.B. Comparison of different procedures for the detoxification of eucalyptus hemicellulosic hydrolysate for use in fermentative processes. J. Chem. Technol. Biotechnol. 2006, 81, 152–157. [Google Scholar] [CrossRef]
- Zhao, J.; Xia, L. Ethanol production from corn stover hemicellulosic hydrolysate using immobilized recombinant yeast cells. Biochem. Eng. J. 2010, 49, 28–32. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- van Zyl, C.; Prior, B.A.; du Preez, J.C. Acetic acid inhibition of D-xylose fermentation by Pichia stipitis. Enzyme Microb. Technol. 1991, 13, 82–86. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, L.; Zhu, J.; Xu, Y.; Yu, S. Transcriptome and metabolome analysis of Pichia stipitis to three representative lignocellulosic inhibitors. Arch. Microbiol. 2019, 201, 581–589. [Google Scholar] [CrossRef]
- Brito, P.L.; de Azevedo Ferreira, C.M.; Silva, A.F.F.; de Araújo Pantoja, L.; Nelson, D.L.; dos Santos, A.S. Hydrolysis, detoxification and alcoholic fermentation of hemicellulose fraction from palm press fiber. Waste and Biomass Valorization 2018, 9, 957–968. [Google Scholar] [CrossRef]
- Nakasu, P.Y.S.; Ienczak, L.J.; Costa, A.C.; Rabelo, S.C. Acid post-hydrolysis of xylooligosaccharides from hydrothermal pretreatment for pentose ethanol production. Fuel 2016, 185, 73–84. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Dasu, V.V.; Goud, V.V.; Rao, P.S. Development of dilute sulfuric acid pretreatment method for the enhancement of xylose fermentability. Biocatal. Agric. Biotechnol. 2017, 11, 224–230. [Google Scholar] [CrossRef]
- Keshav, P.K.; Shaik, N.; Koti, S.; Linga, V.R. Bioconversion of alkali delignified cotton stalk using two-stage dilute acid hydrolysis and fermentation of detoxified hydrolysate into ethanol. Ind. Crops Prod. 2016, 91, 323–331. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Gómez-Cruz, I.; Ruiz, E.; Romero, I.; Castro, E. Production of ethanol from hemicellulosic sugars of exhausted olive pomace by Escherichia coli. Processes 2020, 8, 533. [Google Scholar] [CrossRef]
- Zhu, L.; Li, P.; Sun, T.; Kong, M.; Li, X.; Ali, S.; Liu, W.; Fan, S.; Qiao, J.; Li, S.; et al. Overexpression of SFA1 in engineered Saccharomyces cerevisiae to increase xylose utilization and ethanol production from different lignocellulose hydrolysates. Bioresour. Technol. 2020, 313, 123724. [Google Scholar] [CrossRef]
- Milessi, T.S.; Perez, C.L.; Zangirolami, T.C.; Corradini, F.A.; Sandri, J.P.; Foulquié-Moreno, M.R.; Giordano, R.C.; Thevelein, J.M.; Giordano, R.L. Repeated batches as a strategy for high 2G ethanol production from undetoxified hemicellulose hydrolysate using immobilized cells of recombinant Saccharomyces cerevisiae in a fixed-bed reactor. Biotechnol. Biofuels 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Álvarez, C.; Manzanares, P.; Moreno, A.D. Fermentation strategies for the efficient use of olive tree pruning biomass from a flexible biorefinery approach. Fuel 2020, 277. [Google Scholar] [CrossRef]


| Components | Stream A | Stream B | Stream C | Stream D | Stream E | Stream F |
|---|---|---|---|---|---|---|
| Glucooligosaccharides | 1.60 ± 0.04 | - | - | - | - | - |
| Xylooligosaccharides | 13.0 ± 0.75 | - | - | - | - | - |
| Acetyl groups | 2.87 ± 0.03 | - | - | - | - | - |
| Glucose | 1.09 ± 0.06 | 3.15 ± 0.16 | 9.86 ± 0.493 | 5.75 ± 0.29 | 5.11 ± 0.26 | 4.34 ± 0.22 |
| Xylose | 4.12 ± 0.12 | 20.14 ± 0.81 | 60.69 ± 2.43 | 61.98 ± 2.48 | 59.95 ± 2.40 | 52.19 ± 2.09 |
| Arabinose | 0.19 ± 0.01 | 0.59 ± 0.02 | 1.62 ± 0.049 | - | - | - |
| Formic Acid | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.71 ± 0.032 | 0.29 ± 0.01 | 0.18 ± 0.01 | 0.05 ± 0.00 |
| Acetic Acid | 4.48 ± 0.013 | 7.28 ± 0.18 | 5.67 ± 0.142 | 2.95 ± 0.07 | 2.48 ± 0.06 | 0.26 ± 0.07 |
| Levulinic Acid | 0.04 ± 0.00 | 0.12 ± 0.01 | 1.03 ± 0.038 | 0.87 ± 0.03 | 0.33 ± 0.01 | - |
| HMF | 0.28 ± 0.01 | 0.22 ± 0.01 | 0.69 ± 0.009 | 0.11 ± 0.03 | 0.05 ± 0.00 | - |
| Furfural | 1.14 ± 0.05 | 1.16 ± 0.06 | 0.65 ± 0.033 | 0.10 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.00 |
| Total phenolic compounds (expressed as gallic acid equivalents) | 3.28 ± 0.00 | 3.05 ± 0.01 | 8.25 ± 0.002 | 0.65 ± 0.00 | 0.32 ± 0.01 | 0.21 ± 0.02 |
| Hydrolysate Media Abbreviation | Yeast | [X]0 (g/L) | [X]f (g/L) | [Et]MAX (g/L) | EtYieldMAX (get/gX0-Xf) * | QP 24h (g/L·h) |
|---|---|---|---|---|---|---|
| a | S. stipitis CECT 1922 | 57.0 ± 0.10 | 46.5 ± 0.60 | 1.92 ± 0.14 a | 0.18 ± 0.01 a | 0.00 |
| b | 43.8 ± 0.05 | 11.7 ± 0.11 | 10.2 ± 0.45 b | 0.32 ± 0.01 b,c | 0.00 | |
| c | 39.9 ± 0.73 | 3.56 ± 0.12 | 9.16 ± 0.27 b | 0.25 ± 0.00 a,b | 0.00 | |
| d | 27.5 ± 0.18 | 3.83 ± 0.17 | 8.74 ± 0.14 b | 0.37 ± 0.03 c | 0.18 | |
| e | 46.8 ± 0.79 | 1.19 ± 0.02 | 14.2 ± 0.40 c | 0.31 ± 0.01 b,c | 0.53 | |
| a | S. cerevisiae MEC 1133 | 55.8 ± 0.53 | 12.3 ± 0.03 | 14.2 ± 0.31 c | 0.33 ± 0.02 a | 0.40 |
| b | 42.8 ± 1.67 | 9.35 ± 0.61 | 11.9 ± 0.21 b | 0.36 ± 0.03 a | 0.34 | |
| c | 37.1 ± 2.27 | 4.12 ± 0.61 | 10.9 ± 0.29 b | 0.33 ± 0.03 a | 0.37 | |
| d | 27.6 ± 0.97 | 2.51 ± 0.00 | 7.95 ± 0.05 a | 0.32 ± 0.04 a | 0.31 | |
| e | 47.6 ± 1.27 | 0.18 ± 0.05 | 12.5 ± 0.52 b,c | 0.26 ± 0.01 a | 0.51 |
| Raw Material | Pretreatment | Posthydrolysis and/or Detoxification | Strains | [X]0 (g/L) | [Et]MAX (g/L) | EtYieldMAX (get/gsugar) | Refs. |
|---|---|---|---|---|---|---|---|
| Palm press fiber | Acid pret. SL * 30%, 5% H2SO4, 121 °C, 60 min |
| S. stipitis NRRLY 7124 | 18.6 | 6.13 | 0.33 | [51] |
| Eucalyptus grandis | 2% (v/v) green liquor (Na2S, NaOH, Na2CO3) pret. LSR * 3.5 (w/w), 155–160 °C for 150 min |
| S. stipitis NBRC 10063 | 19.1 | 5.00 | 0.21 ** | [14] |
| Sugarcane bagasse | Hydrothermal pret. SL * 9% (w/w), 190 °C, 10 min at 150 rpm |
| S. stipitis NRRLY 7124 | 33.5 | 10.6 | 0.32 | [52] |
| Sugarcane bagasse | Hydrothermal pret. SL * 9% (w/w), 190 °C, 10 min at 150 rpm |
| S. stipitis NRRLY 7124 | 35.1 | 10.6 | 0.30 | [52] |
| Sorghum stalks | Acid pret. SL 5% (w/v), 0.2 M H2SO4, 121 °C, 120 min |
| S. stipitis NCIM 3948 (CBS 6054) | 20.0 | 11.6 | 0.46 ** | [53] |
| Cotton stalks | Alkali pret. SL * 10% (w/v), 3% NaOH; room temperature, 24 h |
| S. stipitis NCIM 3948 (CBS 6054) | 29.4 | 10.1 | 0.45 ** | [54] |
| Exhausted olive pomace | Water extraction at 100 °C 30 min, and acid pret. 2% H2SO4 170 °C |
| E. coli SL100 | 23.6 | 13.6 | 0.47 ** | [55] |
| Exhausted olive pomace | Water extraction at 100 °C 30 min, and acid pret. 2% H2SO4 170 °C |
| E. coli SL100 | 23.3 | 14.5 | 0.46 ** | [55] |
| Sweet sorghum bagasse | Alkaline treatment and distillation | - | S. cerevisiae SFA1OE | - | 17.77 | 0.49 ** | [56] |
| Olive tree pruning | 1% Phosphoric-acid-catalyzed steam explosion (195 °C for 10 min) |
| S. cerevisiae F12 | 15.9 | 7.5 | 0.32 | [58] |
| Sugarcane bagasse | Supplied by University of São Paulo |
| Encapsulated GSE16-T18S.1 (T18) S. cerevisiae | - | - | 0.38 | [57] |
| Paulownia elongata x fortunei | Hydrothermal pret.: LSR * 6 g/g, 210 °C, 150 rpm |
| S. stipitis CECT 1922 | 46.8 | 14.2 | 0.31 | This work |
| Paulownia elongata x fortunei | Hydrothermal pret.: LSR * 6 g/g, 210 °C, 150 rpm |
| Recombinant S. cerevisiae MEC 1133 | 55.8 | 14.2 | 0.33 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, E.; Río, P.G.d.; Romaní, A.; Garrote, G.; Domingues, L. Hemicellulosic Bioethanol Production from Fast-Growing Paulownia Biomass. Processes 2021, 9, 173. https://doi.org/10.3390/pr9010173
Domínguez E, Río PGd, Romaní A, Garrote G, Domingues L. Hemicellulosic Bioethanol Production from Fast-Growing Paulownia Biomass. Processes. 2021; 9(1):173. https://doi.org/10.3390/pr9010173
Chicago/Turabian StyleDomínguez, Elena, Pablo G. del Río, Aloia Romaní, Gil Garrote, and Lucília Domingues. 2021. "Hemicellulosic Bioethanol Production from Fast-Growing Paulownia Biomass" Processes 9, no. 1: 173. https://doi.org/10.3390/pr9010173
APA StyleDomínguez, E., Río, P. G. d., Romaní, A., Garrote, G., & Domingues, L. (2021). Hemicellulosic Bioethanol Production from Fast-Growing Paulownia Biomass. Processes, 9(1), 173. https://doi.org/10.3390/pr9010173





