Transesterification Using Ultrasonic Spray of Triolein Containing CaO Particles into Methanol Vapor in a 3-Phase Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Transesterification Reaction in the Three-Phase Reactor
2.3. FAME (Fatty Acid Methyl Ester) Analysis
3. Results and Discussion
3.1. Effect of the Flow Rate of Triolein on Methyl Ester
3.2. Effect of CaO Loading on Methyl Ester Yield
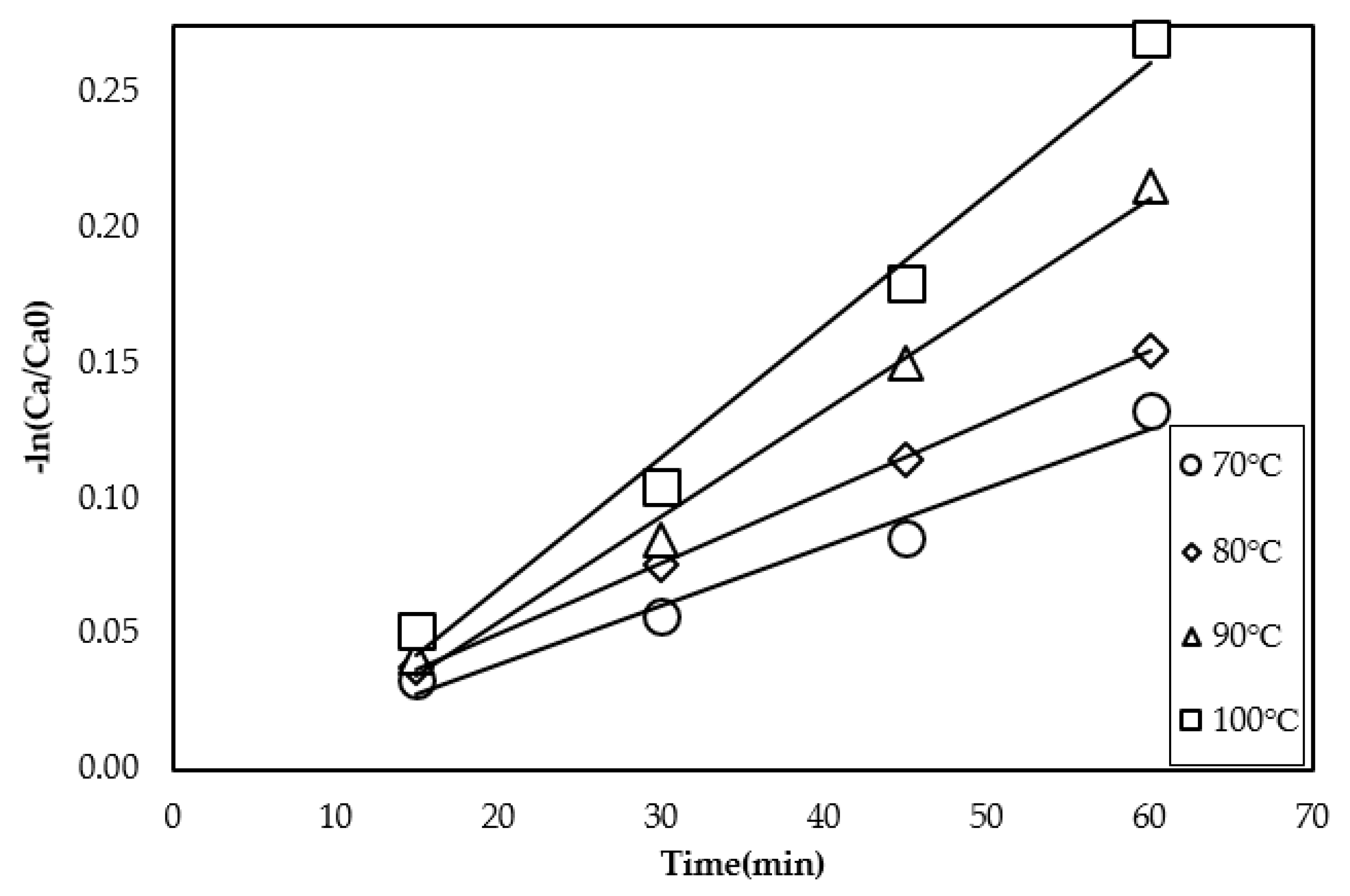
3.3. Effect of Temperature on the Methyl Ester Yield
3.4. Comparison between Three-Phase with Ultrasonic Spraying and a Conventional Reactor
3.5. Relationship between the Time-Totalized Surface Area of the Droplets and the Methyl Ester Yield
- Step 1: Methanol mass transfer happens from the vapor phase to the triolein liquid droplets;
- Step 2: The dissolved methanol diffuses and reaches the catalysts in droplets;
- Step 3: The methanol reacts with the triolein in the presence of the catalysts.
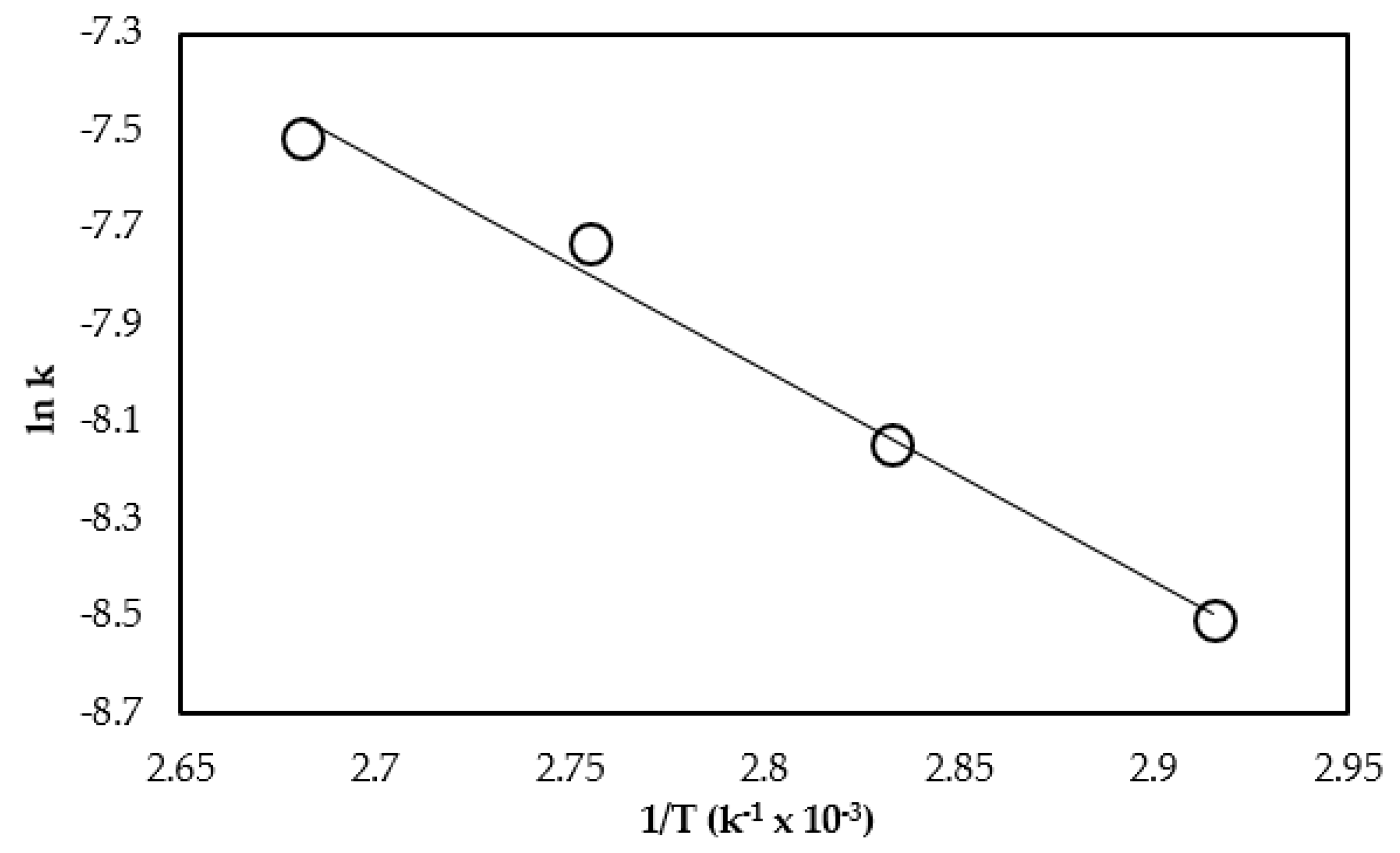
4. Determination of the Kinetic Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- LeTu, T.; Kenji, O.; Yasuhiro, S.; Norimichi, T.; Yasuaki, M.; Hiroshi, B. A two-step continuous ultrasound assisted production of biodiesel fuel from waste cooking oils: A practical andeconomical approach to produce high quality biodiesel fuel. Bioresour. Technol. 2010, 101, 5394–5401. [Google Scholar]
- Hattori, H. Solid base catalysts: Generation, characterization, and catalytic behavior of basic sites. J. Jpn. Pet. Inst. 2004, 47, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Kouzu, M.; Hidaka, J.; Komichi, Y. Calcium oxide functionalized with strontium as heterogeneous transesterification catalyst for biodiesel production. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.; Komichi, Y.; Nakano, H.; Yamamoto, M. A process to transesterify vegetable oil with methanol in the presence of quick lime bit functioning as solid base catalyst. Fuel 2009, 88, 1983–1990. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- Niu, S.L.; Huo, M.J.; Lu, C.M.; Liu, M.Q.; Li, H. An investigation on the catalytic capacity of dolomite in transesterification and the calculation of kinetic parameters. Bioresour. Technol. 2014, 158, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kouzu, M.; Hidaka, J. Transesterification of vegetable oil into biodiesel catalyzed CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, S. Biodiesel production through transesterification over natural calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Mun, Y.C.; Eng, S.C.; Cher, P.S. Biodiesel production catalysed by low-cost liquid enzyme Eversa® Transform 2.0: Effect of free fatty acid content on lipase methanol tolerance and kinetic model. Fuel 2021, 283, 119266. [Google Scholar]
- Stamenkovic, O.S.; Todorovic, Z.B.; Veljkovic, V.B. A kinetic study of sunflower oil methanolysis catalyzed by barium hydroxide. Chem. Eng. Technol. 2014, 37, 2143–2151. [Google Scholar] [CrossRef]
- Sarve, A.N.; Sonawane, S.S.; Varma, M.N. Ultrasound assisted biodiesel production from Sesame (Sesamum indicum L.) oil using barium hydroxide as a heterogeneous catalyst: Comparative assessment of prediction abilities between response surface methodology (RSM) and artificial neural network (ANN). Ultrason. Sonochemistry 2015, 26, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, B.; Cao, Y.; Li, J.; Liu, M.; Yan, F. Biodiesel production using cation exchange resin as heterogeneous catalyst. Bioresour. Technol. 2010, 101, 1518–1521. [Google Scholar]
- Sarve, A.N.; Mahesh, N.V.; Shriram, S.S. Ultrasound assisted two-stage biodiesel synthesis from non-edible Schleichera triguga oil using heterogeneous catalyst: Kinetics and thermodynamic analysis. Ultrason. Sonochemistry 2016, 29, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Noureddini, H.; Zhu, D. Kinetics of transesterification of soybean oil. J. Am. Oil Chem. Soc. 1997, 74, 1457–1463. [Google Scholar] [CrossRef]
- Hingu, S.M.; Gogate, P.R.; Rathod, V.K. Synthesis of biodiesel from waste cooking oil using sonochemical reactors. Ultrason. Sonochemistry 2010, 17, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, V.B.; Stamenkovic, O.S.; Todorovic, Z.B.; Lazic, M.L.; Skela, D.U. Kinetics of sunflower oil methanolysis catalyzed by calcium oxide. Fuel 2009, 88, 1554–1562. [Google Scholar] [CrossRef]
- Vujicic, D.j.; Comic, D.; Zarubica, A.; Micic, R.; Boskovic, G. Kinetics of biodiesel synthesis from sunflower oil over CaO heterogeneous catalyst. Fuel 2010, 89, 2054–2061. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Waste cooking oil and waste chicken eggshells derived solid base catalyst for the biodiesel production: Optimization and kinetics. Waste Manag. 2018, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Parkar, P.A.; Choudhary, H.A.; Moholkar, V.S. Mechanistic and kinetic investigations in ultrasound assisted acid catalyzed biodiesel synthesis. Chem. Eng. J. 2012, 187, 248–260. [Google Scholar] [CrossRef]



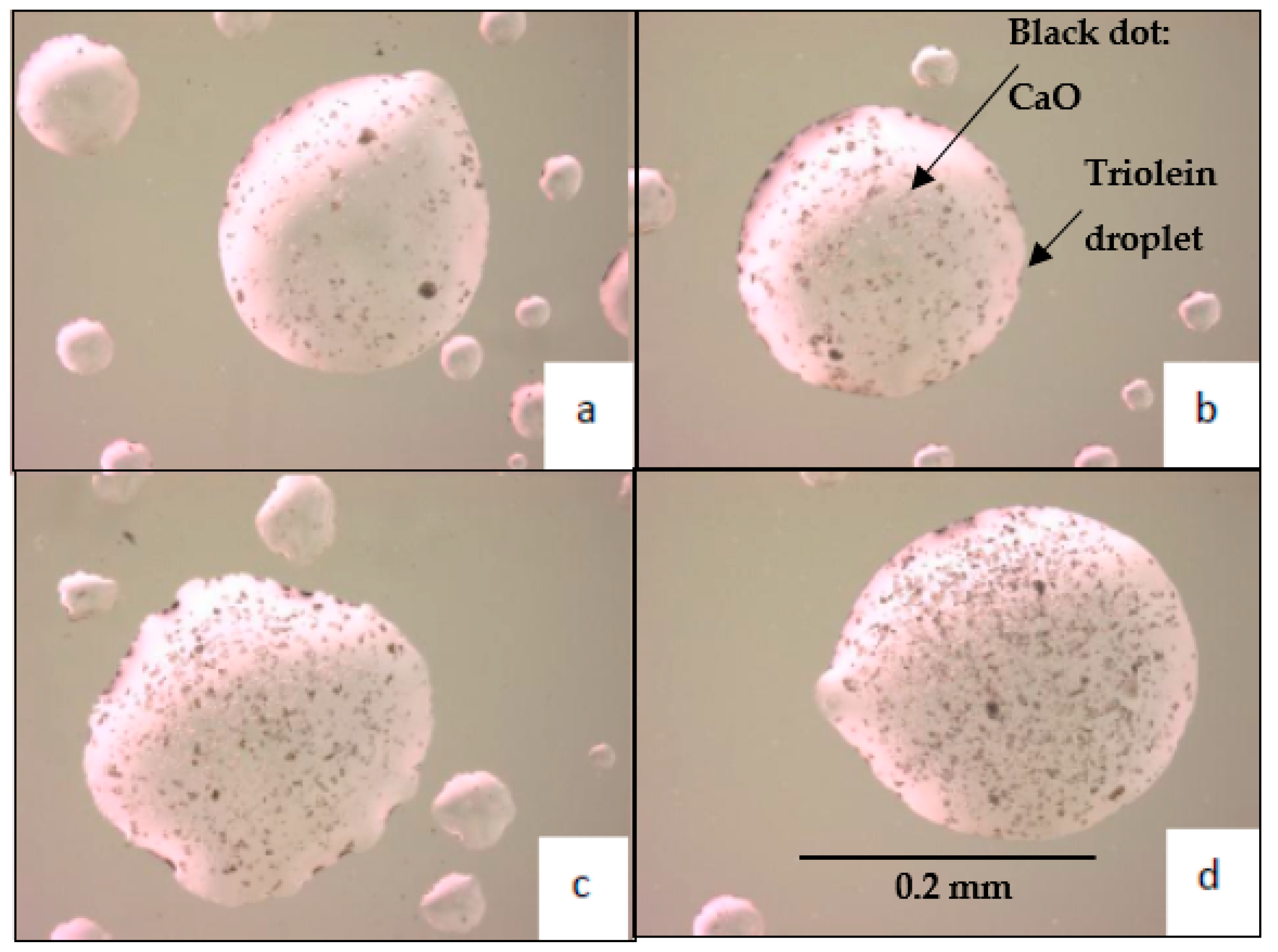
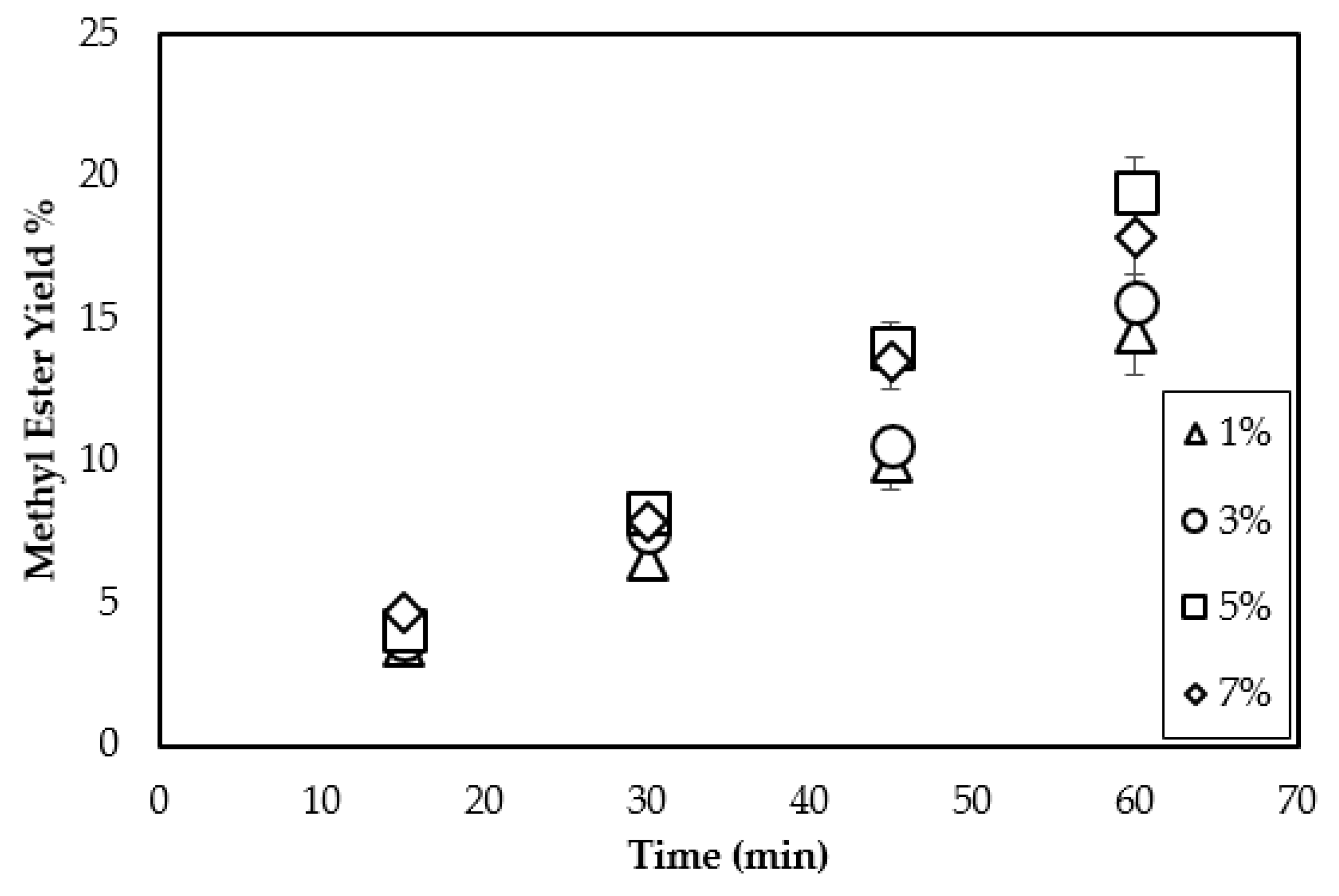
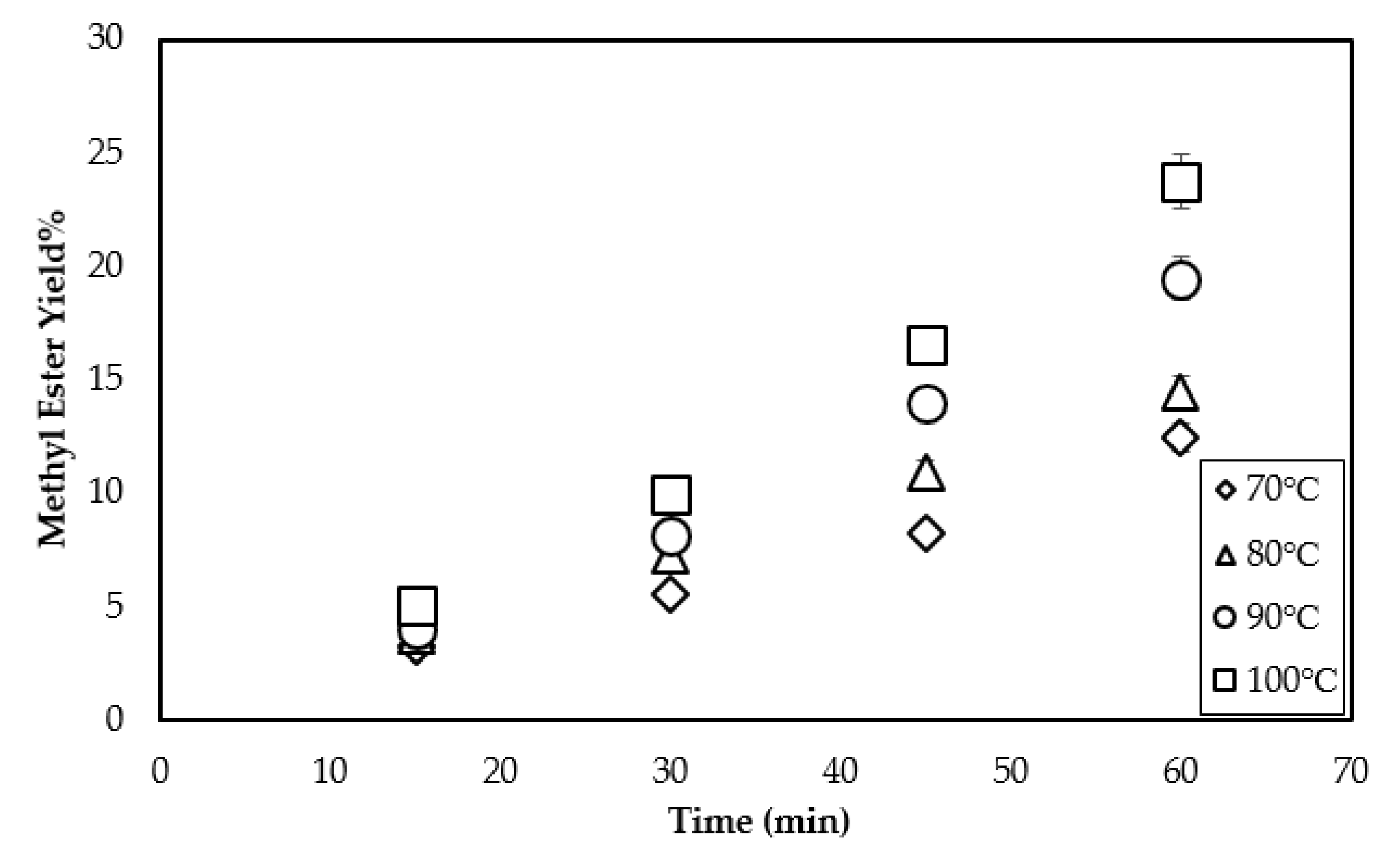

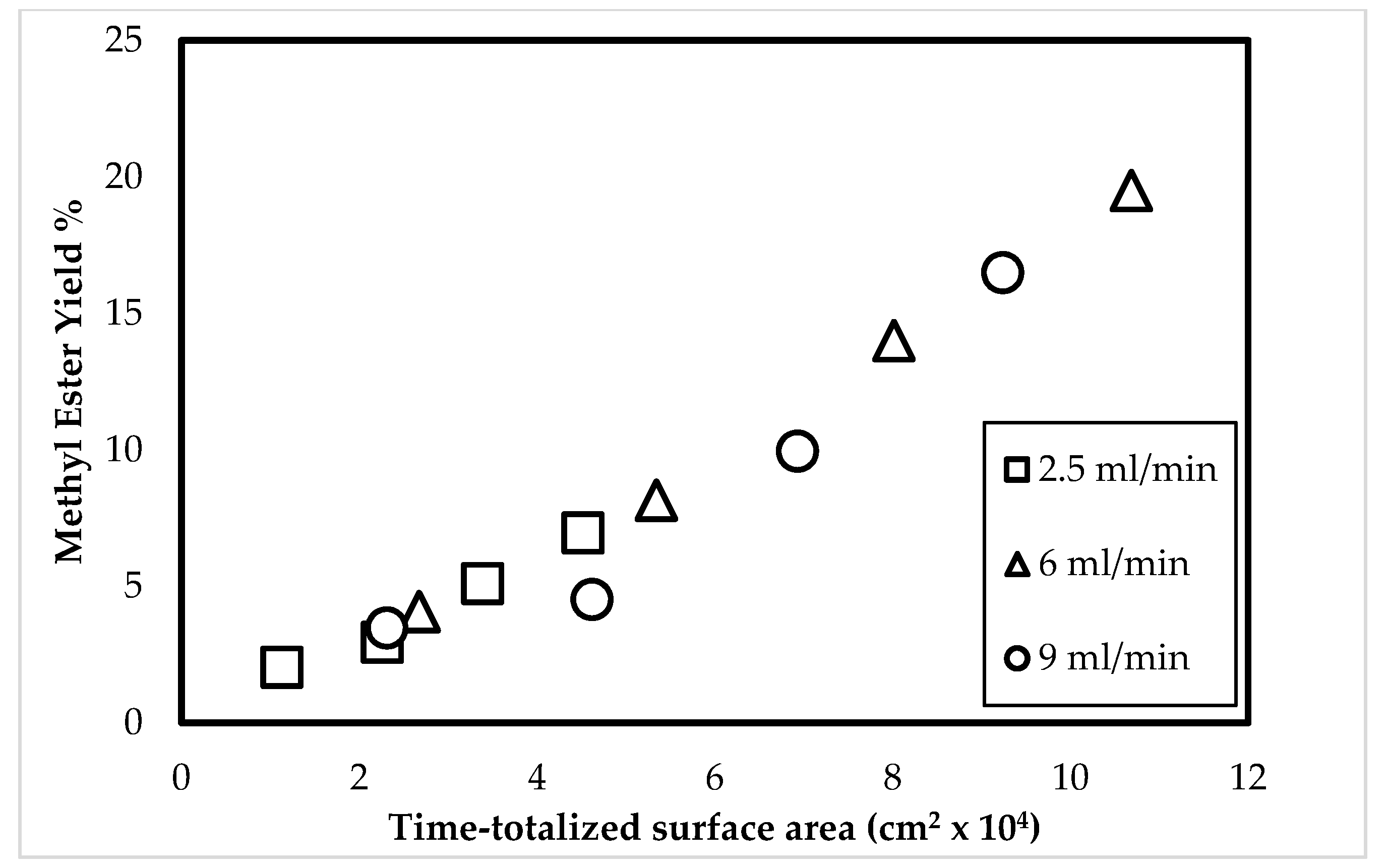
| Triolein Flow Rate (mL/min) | Average Diameter of Droplets (mm) | Volume of One Droplet (mL) | Number of Droplets Sprayed (1/min) | Surface Area of One Droplet (cm2) | Total Surface Area per min (cm2/min) |
|---|---|---|---|---|---|
| 2.5 | 0.20 | 4.2 × 10−6 | 6.0 × 106 | 1.2 × 10−3 | 7.5 × 102 |
| 6.0 | 0.20 | 4.3 × 10−6 | 1.4 × 107 | 1.3 × 10−3 | 1.8 × 103 |
| 9.0 | 0.35 | 2.3 × 10−5 | 4.0 × 106 | 3.5 × 10−3 | 1.5 × 103 |
| Temperature | 70 °C | 80 °C | 90 °C | 100 °C |
|---|---|---|---|---|
| k, min−1 | 1.21 × 10−2 | 1.75 × 10−2 | 2.63 × 10−2 | 3.27 × 10−2 |
| r2 | 0.99 | 0.99 | 0.99 | 0.97 |
| Raw Material or Type of Transesterification | Catalyst | Rate Constant k (min−1) | Activation Energy Ea (kJmol−1) | Reference |
|---|---|---|---|---|
| Triolein | CaO | 1.21–3.70 × 10−2 | 36.1 | Present work |
| Sunflower | CaO | 0.27–5.92 × 10−2 | 101.0 | [18] |
| Waste cooking oil | CaO from eggshells | 0.92–2.25 × 10−2 | 54.1 | [19] |
| Soybean oil (ultrasound-assisted) | NaOH (homogenous) | 0.37–5.2 × 10−3 | 55.4 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitidsant, R.; Kodama, S.; Sekiguchi, H. Transesterification Using Ultrasonic Spray of Triolein Containing CaO Particles into Methanol Vapor in a 3-Phase Reactor. Processes 2021, 9, 181. https://doi.org/10.3390/pr9010181
Vitidsant R, Kodama S, Sekiguchi H. Transesterification Using Ultrasonic Spray of Triolein Containing CaO Particles into Methanol Vapor in a 3-Phase Reactor. Processes. 2021; 9(1):181. https://doi.org/10.3390/pr9010181
Chicago/Turabian StyleVitidsant, Ravisut, Satoshi Kodama, and Hidetoshi Sekiguchi. 2021. "Transesterification Using Ultrasonic Spray of Triolein Containing CaO Particles into Methanol Vapor in a 3-Phase Reactor" Processes 9, no. 1: 181. https://doi.org/10.3390/pr9010181
APA StyleVitidsant, R., Kodama, S., & Sekiguchi, H. (2021). Transesterification Using Ultrasonic Spray of Triolein Containing CaO Particles into Methanol Vapor in a 3-Phase Reactor. Processes, 9(1), 181. https://doi.org/10.3390/pr9010181





