Extraction of Chlorobenzenes and PCBs from Water by ZnO Nanoparticles
Abstract
:1. Introduction
2. Methods
2.1. Nanomaterials and Chemicals
2.2. Adsorption of Aromatic Organochlorines onto ZnO NPs
2.3. Adsorption of Aromatic Organochlorines onto Soil Particles
2.4. Working Solutions
2.5. Extraction of Aromatic Organochlorines
2.6. Analysis of Aromatic Organochlorines with GC/MS/MS
2.7. Molecular Models of Aromatic Organochlorines
2.8. Molecular Model of the Zinc Oxide Surface
2.9. Molecular Dynamics Methods
2.10. Free-Energy Calculations
3. Results and Discussion
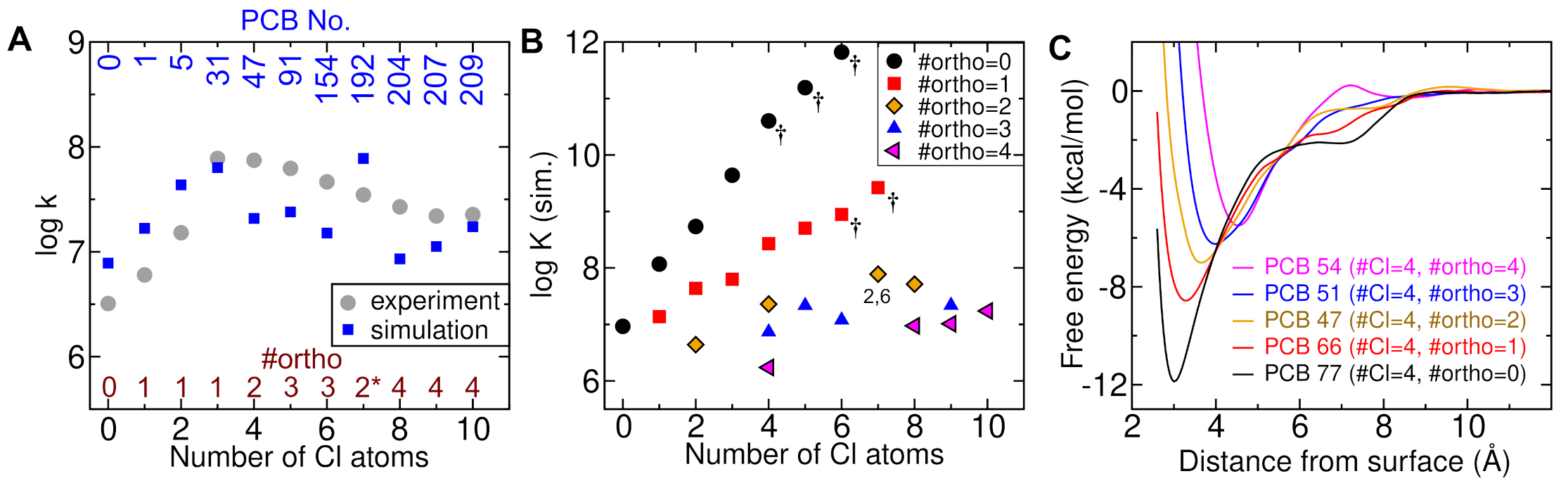
3.1. Effects of Chloro Substitutions
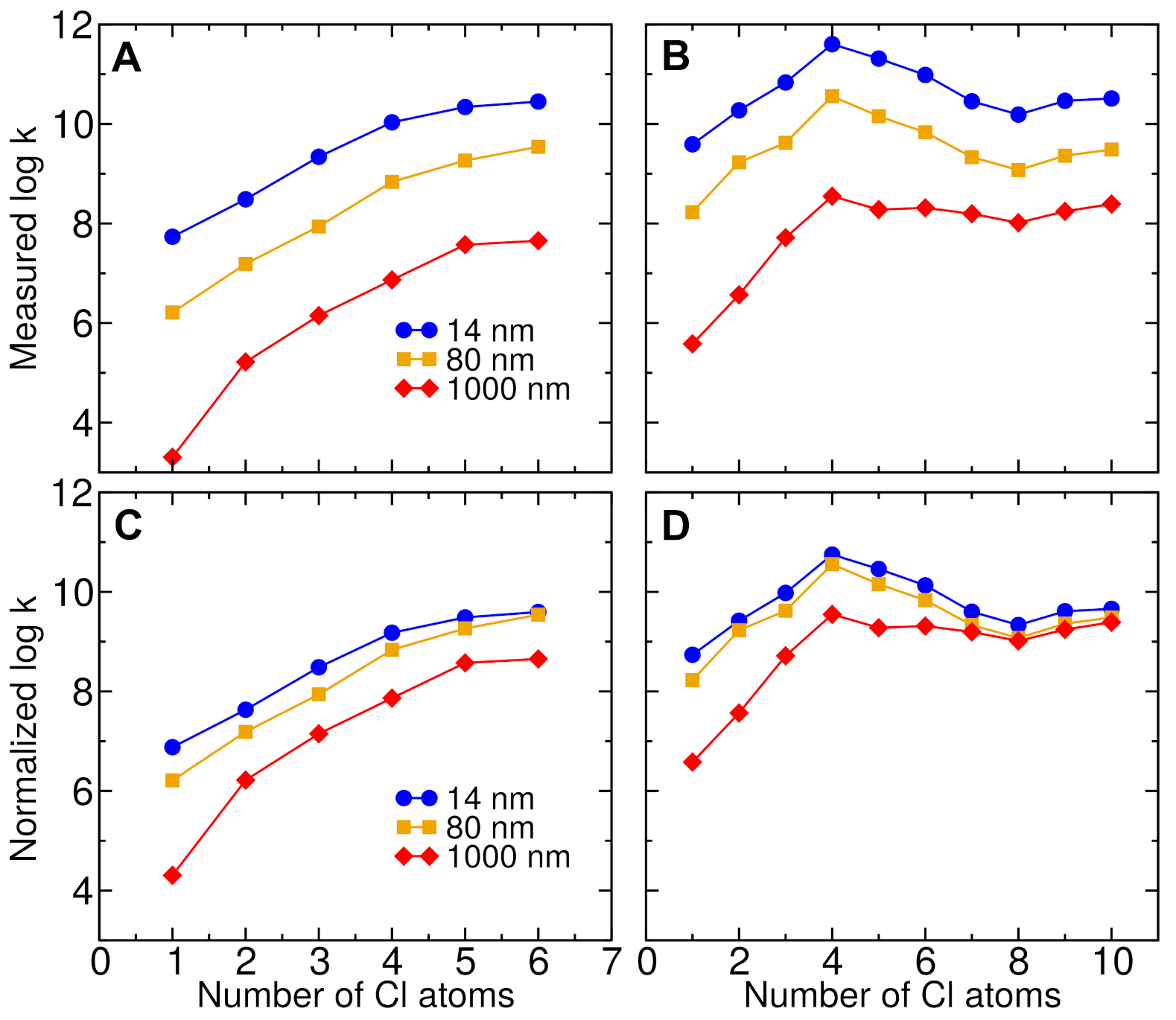
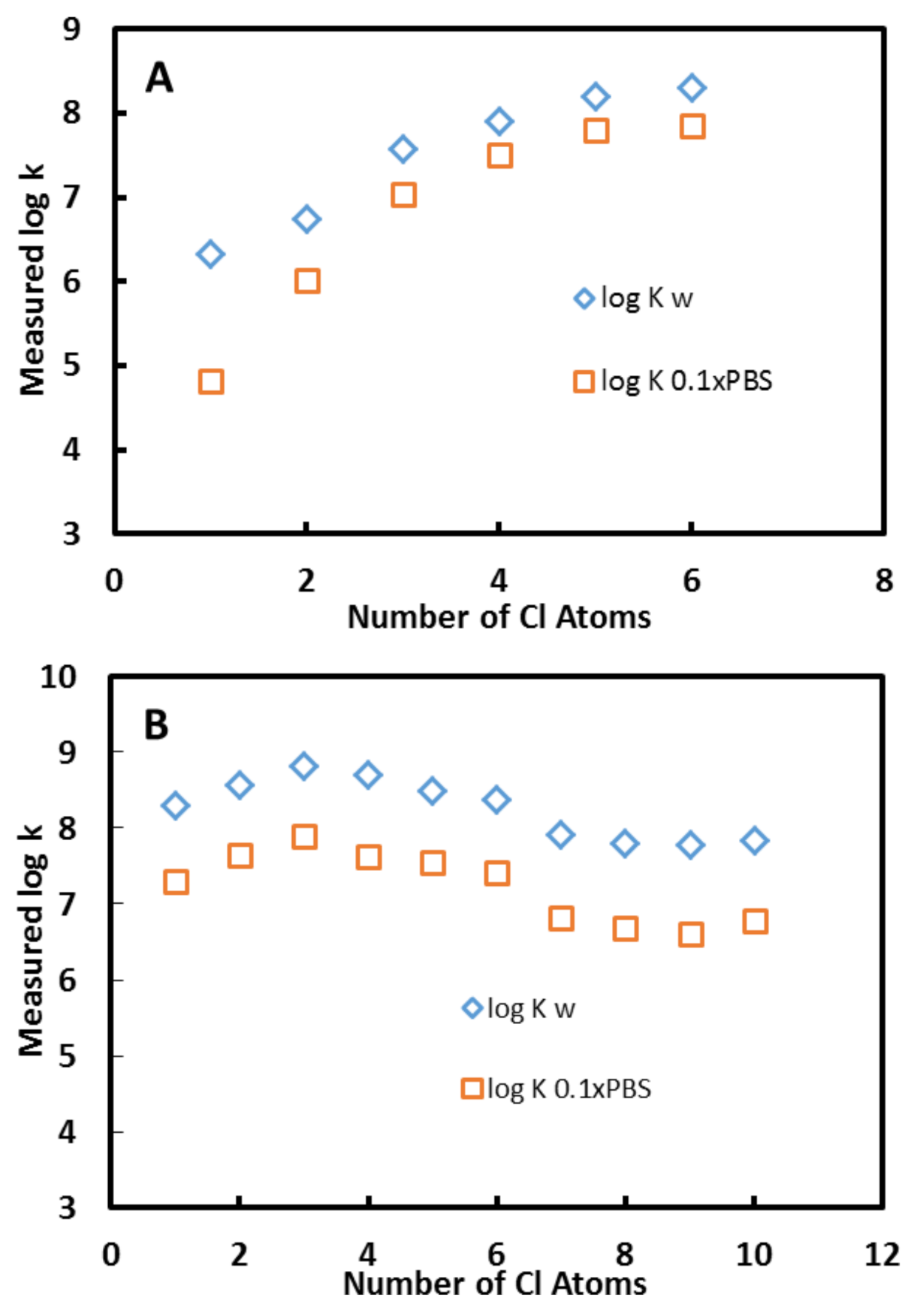
3.2. Effect of Chlorine Number
3.3. Effect of the Substitution Position in Chlorobenzenes
3.4. Effect of Substitution Position in PCBs
3.5. Substitution Position and PCB Conformation
3.6. Effect of Nanoparticle Size
3.7. Effect of Organochlorine Concentration
3.8. Effect of Nanoparticle Concentration
3.9. Effects of pH and Ionic Strength
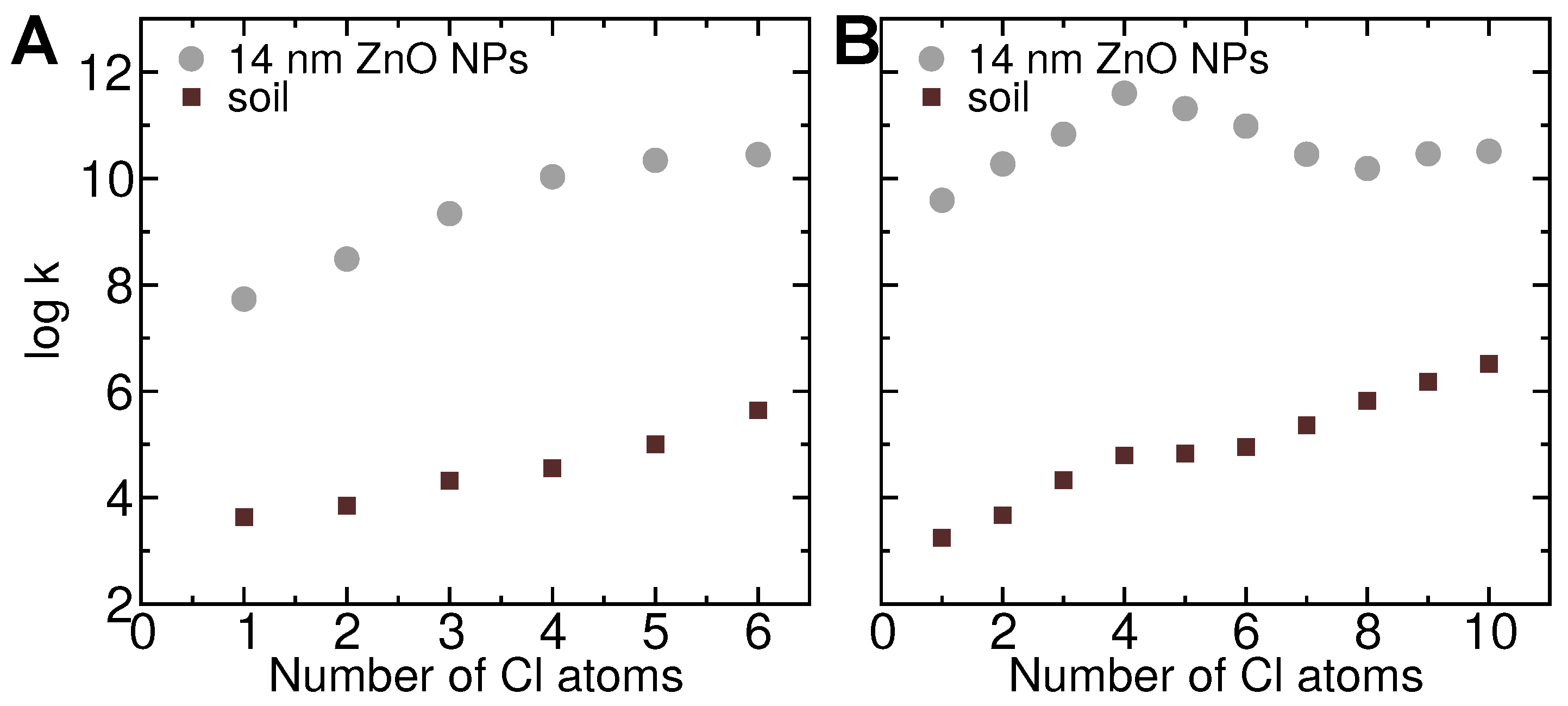
3.10. Partitioning between Soil and Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pirsaheb, M.; Limoee, M.; Namdari, F.; Khamutian, R. Organochlorine pesticides residue in breast milk: A systematic review. Med. J. Islam. Repub. Iran 2015, 29, 228. [Google Scholar] [PubMed]
- Saravi, S.S.S.; Dehpour, A.R. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: A review. Life Sci. 2016, 45, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.W.; Minegishi, T.; Cummiskey, C.C.; Fragala, M.A.; Hartman, R.; MacIntosh, D.L. PCB remediation in schools: A review. Environ. Sci. Pollut. Res. 2016, 23, 1986–1997. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Staimez, L.R. Association of persistent organic pollutants and non-persistent pesticides with diabetes and diabetes-related health outcomes in Asia: A systematic review. Environ. Int. 2015, 76, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Cha, E.S.; Ko, Y.H.; Wang, M.S.; Hong, J.H.; Lee, W.J. Exposure to Dichlorodiphenyltrichloroethane and the Risk of Breast Cancer: A Systematic Review and Meta-analysis. Osong Public Health Res. Perspect. 2014, 5, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Nadal, M.; Marquès, M.; Mari, M.; Domingo, J.L. Climate change and environmental concentrations of POPs: A review. Environ. Res. 2015, 143, 177–185. [Google Scholar] [CrossRef]
- Mostafalou, S. Persistent Organic Pollutants and Concern Over the Link with Insulin Resistance Related Metabolic Diseases. Rev. Environ. Contam. Toxicol. 2016, 238, 69–89. [Google Scholar]
- Beyer, A.; Biziuk, M. Environmental fate and global distribution of polychlorinated biphenyls. Rev. Environ. Contam. Toxicol. 2009, 201, 137–158. [Google Scholar]
- Katagi, T. Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin, Germany, 2010; pp. 1–132. [Google Scholar]
- Tsuji, M.; Kawamoto, T.; Koriyama, C.; Yamamoto, M.; Tsuchiya, T.; Matsumura, F. Association of PCBs and allergies in children. Pestic. Biochem. Physiol. 2015, 120, 21–26. [Google Scholar] [CrossRef]
- Zani, C.; Toninelli, G.; Filisetti, B.; Donato, F. Polychlorinated biphenyls and cancer: An epidemiological assessment. J. Environ. Sci. Health Part C 2013, 31, 99–144. [Google Scholar] [CrossRef]
- Van den Berg, M.; Birnbaum, L.; Bosveld, A.; Brunström, B.; Cook, P.; Feeley, M.; Giesy, J.P.; Hanberg, A.; Hasegawa, R.; Kennedy, S.W.; et al. Toxic Equivalency Factors (TEFs) for PCBs, PCDDs, PCDFs for Humans and Wildlife. Environ. Health Perspect. 1998, 106, 775. [Google Scholar] [CrossRef]
- Silkworth, J.B.; Grabstein, E.M. Polychlorinated Biphenyl Immunotoxicity: Dependence on Isomer Planarity and the Ah Gene Complex. Toxicol. Appl. Pharmacol. 1982, 65, 109–115. [Google Scholar] [CrossRef]
- Zhang, W.; Sargis, R.M.; Volden, P.A.; Carmean, C.M.; Sun, X.J.; Brady, M.J. PCB 126 and Other Dioxin-like PCBs Specifically Suppress Hepatic PEPCK Expression via the Aryl Hydrocarbon Receptor. PLoS ONE 2012, 7, e37103. [Google Scholar] [CrossRef] [Green Version]
- Fischer, L.J.; Seegal, R.F.; Ganey, P.E.; Pessah, I.N.; Kodavanti, P.R.S. Symposium Overview: Toxicity of Non-coplanar PCBs. Toxicol. Sci. 1998, 41, 49–61. [Google Scholar]
- Gafni, J.; Wong, P.W.; Pessah, I.N. Non-coplanar 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) Amplifies Ionotropic Glutamate Receptor Signaling in Embryonic Cerebellar Granule Neurons by a Mechanism Involving Ryanodine Receptors. Toxicol. Sci. 2004, 77, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Elnar, A.A.; Desor, F.; Marin, F.; Soulimani, R.; Nemos, C. Lactational Exposure to Low Levels of the Six Indicator Non-dioxin-like Polychlorinated Biphenyls Induces DNA Damage and Repression of Neuronal Activity, in Juvenile Male Mice. Toxicology 2015, 328, 57–65. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- El Bakouri, H.; Usero, J.; Morillo, J.; Rojas, R.; Ouassini, A. Drin pesticides removal from aqueous solutions using acid-treated date stones. Bioresour. Technol. 2009, 100, 2676–2684. [Google Scholar] [CrossRef]
- Perrard, A.; Descorme, C. Static and dynamic adsorption studies of PolyChloroBiphenyls (PCBs) over activated carbons. Chemosphere 2016, 145, 528–534. [Google Scholar] [CrossRef]
- Chys, M.; Depuydt, V.; Boeckaert, C.; Hulle, S.W.V. Treatment of rainwater runoff in recovery and recycling companies: Lab and pilot-scale testing. J. Environ. Sci. Health Part A 2013, 48, 446–452. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef] [Green Version]
- Mohmood, I.; Lopes, C.B.; Lopes, I.; Ahmad, I.; Duarte, A.C.; Pereira, E. Nanoscale materials and their use in water contaminants removal-a review. Environ. Sci. Pollut. Res. 2013, 20, 1239–1260. [Google Scholar] [CrossRef]
- Taka, A.L.; Pillay, K.; Mbianda, X.Y. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: A review. Carbohydr. Polym. 2017, 159, 94–107. [Google Scholar] [CrossRef]
- Bootharaju, M.S.; Pradeep, T. Understanding the Degradation Pathway of the Pesticide, Chlorpyrifos by Noble Metal Nanoparticles. Langmuir 2012, 28, 2671–2679. [Google Scholar] [CrossRef]
- Vasimalai, N.; Abraham, J.S. Biopolymer capped silver nanoparticles as fluorophore for ultrasensitive and selective determination of malathion. Talanta 2013, 115, 24–31. [Google Scholar] [CrossRef]
- Manimegalai, G.; Shanthakumar, S.; Sharma, C. Silver Nanoparticles: Synthesis and Application in Mineralization of Pesticides Using Membrane Support. Int. Nano Lett. 2014, 4, 105. [Google Scholar] [CrossRef] [Green Version]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of Crystal Structure of Nanosized ZrO2 on Photocatalytic Degradation of Methyl Orange. Nanoscale Res. 2015, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Barakat, N.A.M.; Nassar, M.M.; Farrag, T.E.; Mahmoud, M.S. Effective Photodegradation of Methomyl Pesticide in Concentrated Solutions by Novel Enhancement of the Photocatalytic Activity of TiO2 Using CdSO4 Nanoparticles. Environ. Sci. Pollut. Res. 2013, 21, 1425–1435. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Litton, C.W.; Cantwell, G.; Harsch, W.C. Valence-band ordering in ZnO. Phys. Rev. B 1999, 60, 2340–2344. [Google Scholar] [CrossRef] [Green Version]
- Janotti, A.; de Walle, C.G.V. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.T.; Porter, P.E.; Schmedding, D.W. Partition Equilibriums of Nonionic Organic Compounds between Soil Organic Matter and Water. Environ. Sci. Technol. 1983, 17, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G.; van Noort, P.C.; Govers, H.A. Desorption Kinetics of Chlorobenzenes, Polycyclic Aromatic Hydrocarbons, and Polychlorinated Biphenyls: Sediment Extraction with Tenax® and Effects of Contact Time and Solute Hydrophobicity. Environ. Toxicol. Chem. 1997, 16, 1351–1357. [Google Scholar] [CrossRef]
- Uzgiris, E.E.; Edelstein, W.A.; Philipp, H.R.; Iben, I.T. Complex Thermal Desorption of PCBs from Soil. Chemosphere 1995, 30, 377–387. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Goss, K.U.; Ball, W.P. Polyparameter Linear Free Energy Relationships for Estimating the Equilibrium Partition of Organic Compounds between Water and the Natural Organic Matter in Soils and Sediments. Environ. Sci. Technol. 2005, 39, 913–924. [Google Scholar] [CrossRef]
- Xia, X.R.; Monteiro-Riviere, N.A.; Riviere, J.E. An Index for Characterization of Nanomaterials in Biological Systems. Nature Nanotech. 2010, 5, 671–675. [Google Scholar] [CrossRef]
- Xia, X.R.; Monteiro-Riviere, N.A.; Mathur, S.; Song, X.; Xiao, L.; Oldenberg, S.J.; Fadeel, B.; Riviere, J.E. Mapping the Surface Adsorption Forces of Nanomaterials in Biological Systems. ACS Nano 2011, 5, 9074–9081. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Zhang, Y.; Darabi Sahneh, F.; Scoglio, C.M.; Wohlleben, W.; Haase, A.; Monteiro-Riviere, N.A.; Riviere, J.E. Nanoparticle Surface Characterization and Clustering through Concentration-Dependent Surface Adsorption Modeling. ACS Nano 2014, 8, 9446–9456. [Google Scholar] [CrossRef]
- Endo, S.; Goss, K.U. Applications of Polyparameter Linear Free Energy Relationships in Environmental Chemistry. Environ. Sci. Technol. 2014, 48, 12477–12491. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Monteiro-Riviere, N.A.; Riviere, J.E. Quantification of Nanoparticle Pesticide Adsorption: Computational Approaches Based on Experimental Data. Nanotoxicology 2016, 10, 1118–1128. [Google Scholar] [CrossRef]
- Abraham, M.H. Scales of Solute Hydrogen-bonding: Their Construction and Application to Physicochemical and Biochemical Processes. Chem. Soc. Rev. 1993, 22, 73–83. [Google Scholar] [CrossRef]
- Famini, G.R.; Wilson, L.Y. Using Theoretical Descriptors in Linear Free Energy Relationships: Characterizing Several Polarity, Acid and Basicity Scales. J. Phys. Org. Chem. 1999, 12, 645–653. [Google Scholar] [CrossRef]
- Comer, J.; Chen, R.; Poblete, H.; Vergara-Jaque, A.; Riviere, J.E. Predicting Adsorption Affinities of Small Molecules on Carbon Nanotubes Using Molecular Dynamics Simulation. ACS Nano 2015, 9, 11761–11774. [Google Scholar] [CrossRef] [Green Version]
- Poblete, H.; Miranda-Carvajal, I.; Comer, J. Determinants of Alanine Dipeptide Conformational Equilibria on Graphene and Hydroxylated Derivatives. J. Phys. Chem. B 2017, 121, 3895–3907. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Comer, J.; Chen, Z.; Chen, J.; Gumbart, J.C. Exploring Adsorption of Neutral Aromatic Pollutants onto Graphene Nanomaterials via Molecular Dynamics Simulations and Theoretical Linear Solvation Energy Relationships. Environ. Sci. Nano 2018, 5, 2117–2128. [Google Scholar] [CrossRef]
- Azhagiya Singam, E.R.; Zhang, Y.; Magnin, G.; Miranda-Carvajal, I.; Coates, L.; Thakkar, R.; Poblete, H.; Comer, J. Thermodynamics of Adsorption to Graphenic Surfaces from Aqueous Solution. J. Chem. Theory Comput. 2019, 15, 1302–1316. [Google Scholar] [CrossRef] [Green Version]
- Banks, M.; Schwab, A.; Henderson, C. Leaching and Reduction of Chromium in Soil as Affected by Soil Organic Content and Plants. Chemosphere 2006, 62, 255–264. [Google Scholar] [CrossRef]
- Yang, Y.; Hawthorne, S.B.; Miller, D.J.; Liu, Y.; Lee, M.L. Adsorption Versus Absorption of Polychlorinated Biphenyls onto Solid-phase Microextraction Coatings. Anal. Chem. 1998, 70, 1866–1869. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-like Molecules Compatible with the CHARMM All-atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [Green Version]
- Vanommeslaeghe, K.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) I: Bond Perception and Atom Typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) II: Assignment of Bonded Parameters and Partial Atomic Charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef] [Green Version]
- Ballschmiter, K.; Bacher, R.; Mennel, A.; Fischer, R.; Riehle, U.; Swerev, M. The Determination of Chlorinated Biphenyls, Chlorinated Dibenzodioxins, and Chlorinated Dibenzofurans by GC-MS. J. High Resolut. Chromatogr. 1992, 15, 260–270. [Google Scholar] [CrossRef]
- Raymand, D.; van Duin, A.C.; Baudin, M.; Hermansson, K. A Reactive Force Field (ReaxFF) for Zinc Oxide. Surf. Sci. 2008, 602, 1020–1031. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable Molecular Dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [Green Version]
- Feller, S.E.; Zhang, Y.H.; Pastor, R.W.; Brooks, B.R. Constant Pressure Molecular Dynamics Simulations—The Langevin Piston Method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Darden, T.A.; York, D.M.; Pedersen, L.G. Particle Mesh Ewald: An NlogN Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, C.W.; Le Grand, S.; Walker, R.C.; Roitberg, A.E. Long-time-step Molecular Dynamics through Hydrogen Mass Repartitioning. J. Chem. Theory Comput. 2015, 11, 1864–1874. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Darve, E.; Pohorille, A. Calculating Free Energies Using Average Force. J. Chem. Phys. 2001, 115, 9169–9183. [Google Scholar] [CrossRef] [Green Version]
- Comer, J.; Gumbart, J.C.; Hénin, J.; Lelièvre, T.; Pohorille, A.; Chipot, C. The Adaptive Biasing Force Method: Everything You Always Wanted to Know But Were Afraid to Ask. J. Phys. Chem. B 2015, 119, 1129–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Yalkowsky, S.H.; Valvani, C. Water Solubility and Octanol/water Partition Coefficients of Organics. Limitations of the Solubility-partition Coefficient Correlation. Environ. Sci. Technol. 1980, 14, 1227–1229. [Google Scholar] [CrossRef]
- Bahadur, N.P.; Shiu, W.Y.; Boocock, D.G.; Mackay, D. Temperature Dependence of Octanol-Water Partition Coefficient for Selected Chlorobenzenes. J. Chem. Eng. Data 1997, 42, 685–688. [Google Scholar] [CrossRef]
- Williams, J.W.; Krchma, I.J. The Dielectric Constants of Binary Mixtures. J. Am. Chem. Soc. 1926, 48, 1888–1896. [Google Scholar] [CrossRef]
- Dimitriu, M.; Ivan, L.M.; Dorohoi, D.O. Electro-optical Parameters of Some Chlorobenzene Derivatives Obtained from the Molecular Orbital Calculation. Rom. J. Phys 2008, 53, 79–84. [Google Scholar]
- Miller, M.M.; Ghodbane, S.; Wasik, S.P.; Tewari, Y.B.; Martire, D.E. Aqueous Solubilities, Octanol Water Partition Coefficients, and Entropies of Melting of Chlorinated Benzenes and Biphenyls. J. Chem. Eng. Data 1984, 29, 184–190. [Google Scholar] [CrossRef]
- Hawker, D.W.; Connell, D.W. Octanol-water Partition Coefficients of Polychlorinated Biphenyl Congeners. Environ. Sci. Technol. 1988, 22, 382–387. [Google Scholar] [CrossRef]
- Almenningen, A.; Bastiansen, O.; Fernholt, L.; Cyvin, B.N.; Cyvin, S.J.; Samdal, S. Structure and Barrier of Internal Rotation of Biphenyl Derivatives in the Gaseous State: Part 1. The Molecular Structure and Normal Coordinate Analysis of Normal Biphenyl and Pedeuterated Biphenyl. J. Mol. Struct. 1985, 128, 59–76. [Google Scholar] [CrossRef]
- Grein, F. Twist Angles and Rotational Energy Barriers of Biphenyl and Substituted Biphenyls. J. Phys. Chem. A 2002, 106, 3823–3827. [Google Scholar] [CrossRef]
- Romming, C.; Seip, H.; Oymo, I. Structure of Gaseous and Crystalline 2,2′–dichlorobiphenyl. Acta Chem. Scand. A 1974, 28, 507–514. [Google Scholar] [CrossRef] [Green Version]
- US Environmental Protection Agency (EPA). Recommended Toxicity Equivalence Factors (TEFs) for Human Health Risk Assessments of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Dioxin-like Compounds (EPA/600/R-10/005); Risk Assessment Forum: Washington, DC, USA, 2010; pp. 13–14.
- Lai, L.; Irene, E. Area Evaluation of Microscopically Rough Surfaces. J. Vac. Sci. Technol. B 1999, 17, 33–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.S.P.; Westerhoff, K.; Hristovski, J.C.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.S.P.; Westerhoff, J.C.C. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res. 2009, 43, 4249–4257. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.M.; Grassian, V.H. Dissolution of ZnO nanoparticles at circumneutral pH: A study of size effects in the presence and absence of citric acid. Langmuir 2012, 28, 396–403. [Google Scholar] [CrossRef]
- Zhou, D.; Keller, A.A. Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res. 2010, 44, 2948–2956. [Google Scholar] [CrossRef]
- Cupi, D.; Hartmann, N.B.; Baun, A. Influence of pH and media composition on suspension stability of silver, zinc oxide, and titanium dioxide nanoparticles and immobilization of Daphnia magna under guideline testing conditions. Ecotoxicol. Environ. Saf. 2016, 127, 144–152. [Google Scholar] [CrossRef]
- Omar, F.M.; Aziz, H.A.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of Suwannee River humic acid. Sci. Total Environ. 2014, 468, 195–201. [Google Scholar] [CrossRef]
- Peng, Y.H.; Tso, C.P.; Tsai, Y.C.; Zhuang, C.M.; Shih, Y.H. The effect of electrolytes on the aggregation kinetics of three different ZnO nanoparticles in water. Sci. Total Environ. 2015, 530, 183–190. [Google Scholar] [CrossRef]
- DeLong, R.K.; Mitchell, J.A.; Morris, R.T.; Comer, J.; Hurst, M.N.; Ghosh, K.; Wanekaya, A.; Mudge, M.; Schaeffer, A.; Washington, L.L.; et al. Enzyme and Cancer Cell Selectivity of Nanoparticles: Inhibition of 3-D Metastatic Phenotype and Experimental Melanoma by Zinc Oxide. J. Biomed. Nanotechnol. 2017, 13, 221–231. [Google Scholar] [CrossRef]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef]
- Ahlborg, U.G.; Becking, G.; Birnbaum, L.; Brouwer, A.A.; Derks, H.; Feeley, M.; Golor, G.; Hanberg, A.; Larsen, J.; Liem, A.; et al. Toxic equivalency factors for dioxin-like PCBs: Report on WHO-ECEH and IPCS consultation, December 1993. Chemosphere 1994, 28, 1049–1067. [Google Scholar] [CrossRef]







| PCB | Compound Prefix | #Cl | #ortho |
|---|---|---|---|
| 0 | biphenyl * | 0 | 0 |
| 1 | 2-chloro * | 1 | 1 |
| 2 | 3-chloro | 1 | 0 |
| 4 | 2,2-dichloro | 1 | 2 |
| 5 | 2,3-dichloro * | 2 | 1 |
| 11 | 3,3-dichloro | 2 | 0 |
| 31 | 2,4,5-trichloro * | 3 | 1 |
| 35 | 3,3,4-trichloro | 3 | 0 |
| 47 | 2,2,4,4-tetrachloro * | 4 | 2 |
| 51 | 2,2,4,6-tetrachloro | 4 | 3 |
| 54 | 2,2,6,6-tetrachloro | 4 | 4 |
| 66 | 2,3,4,4-tetrachloro | 4 | 1 |
| 77 | 3,3,4,4-tetrachloro | 4 | 0 |
| 91 | 2,2,3,4,6-pentachloro * | 5 | 3 |
| 105 | 2,3,3,4,4-pentachloro | 5 | 1 |
| 126 | 3,3,4,4,5-pentachloro | 5 | 0 |
| 154 | 2,2,4,4,5,6-hexachloro * | 6 | 3 |
| 157 | 2,3,3,4,4,5-hexachloro | 6 | 1 |
| 169 | 3,3,4,4,5,5-hexachloro | 6 | 0 |
| 189 | 2,3,3,4,4,5,5-heptachloro | 7 | 1 |
| 192 | 2,3,3,4,5,5,6-heptachloro * | 7 | 2 |
| 194 | 2,2,3,3,4,4,5,5-octachloro | 8 | 2 |
| 204 | 2,2,3,4,4,5,6,6-octachloro * | 8 | 4 |
| 206 | 2,2,3,3,4,4,5,5,6-nonachloro | 9 | 3 |
| 207 | 2,2,3,3,4,4,5,6,6-nonachloro * | 9 | 4 |
| 209 | decachloro * | 10 | 4 |
| Atom | q (Elementary) | (kcal/mol) | (Å) |
|---|---|---|---|
| O | −0.15 | 0.28 | 3.0 |
| Zn | −0.15 | 0.08 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, R.; Riviere, J.E.; Comer, J. Extraction of Chlorobenzenes and PCBs from Water by ZnO Nanoparticles. Processes 2021, 9, 1764. https://doi.org/10.3390/pr9101764
Zhang Y, Chen R, Riviere JE, Comer J. Extraction of Chlorobenzenes and PCBs from Water by ZnO Nanoparticles. Processes. 2021; 9(10):1764. https://doi.org/10.3390/pr9101764
Chicago/Turabian StyleZhang, Yuntao, Ran Chen, Jim E. Riviere, and Jeffrey Comer. 2021. "Extraction of Chlorobenzenes and PCBs from Water by ZnO Nanoparticles" Processes 9, no. 10: 1764. https://doi.org/10.3390/pr9101764
APA StyleZhang, Y., Chen, R., Riviere, J. E., & Comer, J. (2021). Extraction of Chlorobenzenes and PCBs from Water by ZnO Nanoparticles. Processes, 9(10), 1764. https://doi.org/10.3390/pr9101764






