A Review on the Role of Amorphous Aluminum Compounds in Catalysis: Avenues of Investigation and Potential Application in Petrochemistry and Oil Refining
Abstract
1. Introduction
2. Some Aspects of the Synthesis of Amorphous Aluminum Compounds: Unique Properties and Problems of Study
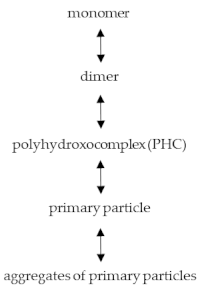
 where the minimum sizes reach 1.2–1.6 nm.
where the minimum sizes reach 1.2–1.6 nm.2.1. Mechanochemical Activation (MCA), Thermochemical Activation (TCA), and Thermal Centrifugal Activation (CTA)
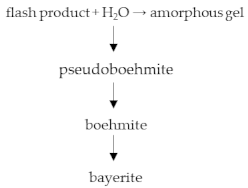
2.2. Precipitation Method
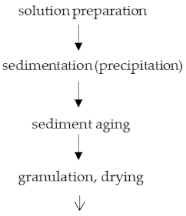
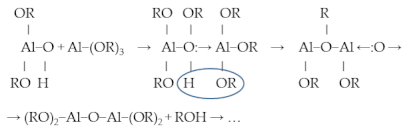
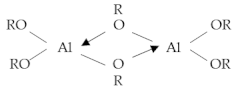
2.3. Sol-Gel Process (Alcoholate Technology)


3. The Effect of Amorphous Aluminum Compounds on Catalysts in Different Processes: The Introduction of Hydrothermal Treatment of Catalyst Precursors
4. The Role of Amorphous Aluminum Compounds in Petrochemistry
- Ambiguous terminology.
- 2.
- Ignoring the formation of amorphous aluminum compounds.
- 3.
- Crystallization of amorphous aluminum compounds.
- 4.
- Potential application amorphous aluminum compounds in oil refining
5. Concluding Remarks
- The role of amorphous aluminum compounds in the field of petrochemistry is currently ambiguous as their positive and negative sides are described. A negative effect is the impeded filtration during the synthesis of the alumina catalyst precursor, the increased coke formation at the active sites of the amorphous alumina as a consequence of the catalytic reaction. A positive effect is possible by introducing hydrothermal treatment of the amorphous aluminum compounds in the catalyst synthesis phase. The hydrothermal treatment promotes the forced crystallization of the amorphous compounds into aluminum hydroxides with the desired properties.
- However, the role of amorphous aluminum compounds in oil refining is also controversial due to the lack of knowledge on the subject. At the same time, the possibilities of using amorphous aluminum compounds represent a great potential due to the low cost of the initial reagents. The Bronsted acid sites on the surface of the aluminum compounds can act as proton donors in the aquathermolysis reaction. The aggregate state and structure of the gel enables the use of amorphous aluminum compounds as water-soluble catalysts for oil refining. Thus, amorphous aluminum compounds can play the role of a catalyst in oil refining under aquathermolysis conditions, which opens up new opportunities in the field of their potential applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Liu, Y.; Chen, D. Mechanisms of hydrolysis-oligomerization of aluminum alkoxide Al(OC3H7)3. J. Phys. Chem. A 2011, 115, 4719–4728. [Google Scholar] [CrossRef] [PubMed]
- Cardona, L.; Medina, O.E.; Santiago, C.; Lopera, S.H.; Cort, F.B.; Franco, C.A. Nanoparticle and Oil Recovery Assisted with PdO and NiO-Functionalized. Processes 2021, 9, 1009. [Google Scholar] [CrossRef]
- Lichtenberger, R.; Schubert, U. Chemical modification of aluminium alkoxides for sol-gel processing. J. Mater. Chem. 2010, 20, 9287–9296. [Google Scholar] [CrossRef]
- He, L.; Ren, Y.; Yue, B.; Tsang, S.C.E.; He, H. Tuning metal–support interactions on Ni/Al2O3 catalysts to improve catalytic activity and stability for dry reforming of methane. Processes 2021, 9, 706. [Google Scholar] [CrossRef]
- Porta-I-Batalla, M.; Xifré-Pérez, E.; Eckstein, C.; Ferré-Borrull, J.; Marsal, L.F. 3D nanoporous anodic alumina structures for sustained drug release. Nanomaterials 2017, 7, 227. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.V.H.; Perez, G.; Passos, F.B. Alumina supported bimetallic Pt-Fe catalysts applied to glycerol hydrogenolysis and aqueous phase reforming. Appl. Catal. B Environ. 2016, 185, 77–87. [Google Scholar] [CrossRef]
- Brieger, G.; Watson, S.W.; Barar, D.G.; Shene, A.L. Thermal Decomposition of Aluminum Alkoxides. J. Org. Chem. 1979, 44, 1340–1342. [Google Scholar] [CrossRef]
- Park, Y.K.; Tadd, E.H.; Zubris, M.; Tannenbaum, R. Size-controlled synthesis of alumina nanoparticles from aluminum alkoxides. Mater. Res. Bull. 2005, 45, 5667–5669. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Ishutenko, D.I.; Mozhaev, A.A.; Maslakov, K.I.; Pimerzin, A.A. Effects of composition and morphology of active phase of CoMo/Al2O3 catalysts prepared using Co2Mo 10-heteropolyacid and chelating agents on their catalytic properties in HDS and HYD reactions. J. Catal. 2014, 312, 152–169. [Google Scholar] [CrossRef]
- Huang, B.; Bartholomew, C.H.; Woodfield, B.F. Facile synthesis of mesoporous γ-alumina with tunable pore size: The effects of water to aluminum molar ratio in hydrolysis of aluminum alkoxides. Microporous Mesoporous Mater. 2014, 183, 37–47. [Google Scholar] [CrossRef]
- Lamberov, A.A.; Levin, O.V.; Egorova, S.R.; Evstyagin, D.A.; Aptikasheva, A.G. Effect of deposition and stabilization conditions on the texture properties of aluminum hydroxides. Russ. J. Appl. Chem. 2003, 76, 50–56. [Google Scholar]
- Pacewska, B.; Keshr, M. Thermal transformations of aluminium nitrate hydrate. Thermochem. Acta 2002, 385, 73–80. [Google Scholar] [CrossRef]
- Kennedy, G.C. Phase relations in the system of Al2O3-H2O at high temperatures and pressures. Am. J. Sci. 1959, 257, 563–573. [Google Scholar] [CrossRef]
- Kryukova, G.N.; Klenov, D.O.; Ivanova, A.S.; Tsybulya, S.V. Vacancy ordering in the structure of γ-Al2O3, J. Eur. Ceram. Soc. 2000, 20, 1187–1189. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Litvak, G.S.; Kryukova, G.N.; Tsybulya, S.V.; Paukshtis, E.A. Real structure of metastable forms of aluminum oxide. Kinet. Catal. 2000, 41, 122–126. [Google Scholar] [CrossRef]
- Novak, C.; Pokol, G.; Izvekov, V.; Gal, T. Studies on the reactions of aluminium oxides and hydroxides. J. Therm. Anal. 1990, 36, 1895–1909. [Google Scholar] [CrossRef]
- Lima, R.W.S.; Hewer, T.L.R.; Alves, R.M.B.; Schmal, M. Surface Analyses of adsorbed and deposited species on the Ni-Mo catalysts surfaces after Guaiacol HDO. Influence of the alumina and SBA-15 supports. Mol. Catal. 2021, 511, 111724. [Google Scholar] [CrossRef]
- Ullmann, K.; Ádám, P.; Sinkó, K. Chemical tailoring of porous aluminum oxide xerogels. J. Non-Cryst. Solids 2018, 499, 394–400. [Google Scholar] [CrossRef]
- Paglia, G. Determination of the Structure of γ-Alumina using Empirical and First Principes Calculations combined with Supporting Experiments. Adv. Cancer Res. 2004, 104, 1–8. [Google Scholar]
- Okada, K.; Nagashima, T.; Kameshima, Y.; Yasumori, A.; Tsukada, T. Relationship between formation conditions, properties, and crystallite size of boehmite. J. Colloid Interface Sci. 2002, 253, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Pacewska, B.; Kluk-Płoskońska, O.; Szychowski, D. Influence of aluminium precursor on physico-chemical properties of aluminium hydroxides and oxides: Part IV. Al(OH)(CH3COO)2. J. Therm. Anal. Calorim. 2007, 90, 783–793. [Google Scholar] [CrossRef]
- Pacewska, B.; Kluk-Płoskońska, O.; Szychowski, D. Influence of aluminium precursor on physico-chemical properties of aluminium hydroxides and oxides Part II. Al(ClO4)3·9H2O. J. Therm. Anal. Calorim. 2006, 86, 751–760. [Google Scholar] [CrossRef]
- Fukumoto, S.; Hookabe, T.; Tsubakino, H. Hydrolysis behavior of aluminum nitride in various solutions. J. Mater. Sci. 2000, 35, 2743–2748. [Google Scholar] [CrossRef]
- Rajendran, S. Production of ultrafine alpha alumina powders and fabrication of fine grained strong ceramics. J. Mater. Sci. 1994, 29, 5664–5672. [Google Scholar] [CrossRef]
- Sato, T. Thermal decomposition of aluminium hydroxides to aluminas. Thermochim. Acta 1985, 88, 69–84. [Google Scholar] [CrossRef]
- Shang, X.; Wang, X.; Nie, W.; Guo, X.; Zou, X.; Ding, W.; Lu, X. Facile strategy for synthesis of mesoporous crystalline γ-alumina by partially hydrolyzing aluminum nitrate solution. J. Mater. Chem. 2012, 22, 23806–23814. [Google Scholar] [CrossRef]
- Shen, L.; Hu, C.; Sakka, Y.; Huang, Q. Study of phase transformation behaviour of alumina through precipitation method. J. Phys. D Appl. Phys. 2012, 45. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jia, Y. Fluoride adsorption onto amorphous aluminum hydroxide: Roles of the surface acetate anions. J. Colloid Interface Sci. 2016, 483, 295–306. [Google Scholar] [CrossRef]
- Du, X.; Wang, Y.; Su, X.; Li, J. Influences of pH value on the microstructure and phase transformation of aluminum hydroxide. Powder Technol. 2009, 192, 40–46. [Google Scholar] [CrossRef]
- Lippens, B.C.; de Boer, J.H. Studies on pore systems in catalysts: V. The t method. J. Catal. 1965, 4, 319–323. [Google Scholar] [CrossRef]
- Hwang, K.T.; Lee, H.S.; Lee, S.H.; Chung, K.C.; Park, S.S.; Lee, J.H. Synthesis of aluminium hydrates by a precipitation method and their use in coatings for ceramic membranes. J. Eur. Ceram. Soc. 2001, 21, 375–380. [Google Scholar] [CrossRef]
- Živkovič, Z.D. Phase transformations of aluminium hydroxide in the calcination process. Thermochim. Acta 1977, 21, 391–398. [Google Scholar] [CrossRef]
- Nayar, P.; Khanna, A.; Kabiraj, D.; Abhilash, S.R.; Beake, B.D.; Losset, Y.; Chen, B. Structural, optical and mechanical properties of amorphous and crystalline alumina thin films. Thin Solid Film. 2014, 568, 19–24. [Google Scholar] [CrossRef]
- Mukhamed’yarova, A.N.; Nesterova, O.V.; Boretsky, K.S.; Skibina, J.D.; Boretskaya, A.V.; Egorova, S.R.; Lamberov, A.A. Influence of the obtaining method on the properties of amorphous aluminum compounds. Coatings 2019, 9, 41. [Google Scholar] [CrossRef]
- Macêdo, M.I.F.; Osawa, C.C.; Bertran, C.A. Sol-gel synthesis of transparent alumina gel and pure gamma alumina by urea hydrolysis of aluminum nitrate. J. Sol-Gel Sci. Technol. 2004, 30, 135–140. [Google Scholar] [CrossRef]
- Garbarino, G.; Travi, I.; Pani, M.; Carnasciali, M.M.; Busca, G. Pure vs ultra-pure γ-alumina: A spectroscopic study and catalysis of ethanol conversion. Catal. Commun. 2015, 70, 77–81. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Graham, T.R.; Pearce, C.I.; Hlushko, H.; LaVerne, J.A.; Liu, L.; Wang, S.; Zheng, S.; Zhang, Y.; et al. Crystallization and Phase Transformations of Aluminum (Oxy)hydroxide Polymorphs in Caustic Aqueous Solution. Inorg. Chem. 2021. [Google Scholar] [CrossRef]
- Digne, M.; Sautet, P.; Raybaud, P.; Euzen, P.; Toulhoat, H. Hydroxyl Groups on γ-Alumina Surfaces: A DFT Study. J. Catal. 2002, 211, 1–5. [Google Scholar] [CrossRef]
- Tago, T.; Okubo, Y.; Mukai, S.R.; Tanaka, T.; Masuda, T. Simultaneous characterization of acidic and basic properties of solid catalysts by a new TPD method and their correlation to reaction rates. Appl. Catal. A Gen. 2005, 290, 54–64. [Google Scholar] [CrossRef]
- El-Nadjar, W.; Bonne, M.; Trela, E.; Rouleau, L.; Mino, A.; Hocine, S.; Payen, E.; Lancelot, C.; Lamonier, C.; Blanchard, P.; et al. Infrared investigation on surface properties of alumina obtained using recent templating routes. Microporous Mesoporous Mater. 2012, 158, 88–98. [Google Scholar] [CrossRef]
- Landau, M.V.; de Jong, K.P. Sol-Gel Processing. In Synthesis of Solid Catalysts; Wiley-VCH: Weinheim, Germany, 2009; pp. 83–109. [Google Scholar] [CrossRef]
- Yoldas, B.E. Hydrolysis of aluminium alkoxides and bayerite conversion. J. Appl. Chem. Biotechnol. 1973, 23, 803–809. [Google Scholar] [CrossRef]
- Telnova, G.B.; Efimovskaya, T.V.; Fateeva, L.V.; Astakhova, N.M.; Ivanova, A.B. Specific features of the phase transformations occurring during the sintering process of the beta-alumina based ceramics and their properties. Solid Electrolytes Chem. Equip. 1990, 31, 146–152. [Google Scholar] [CrossRef]
- Dicks, O.A.; Shluger, A.L. Theoretical modeling of charge trapping in crystalline and amorphous Al2O3. J. Phys. Condens. Matter 2017, 29. [Google Scholar] [CrossRef]
- Karouia, F.; Boualleg, M.; Digne, M.; Alphonse, P. The impact of nanocrystallite size and shape on phase transformation: Application to the boehmite/alumina transformation. Adv. Powder Technol. 2016, 27, 1814–1820. [Google Scholar] [CrossRef]
- Mukhamed’yarova, A.N.; Egorova, S.R.; Nosova, O.V.; Lamberov, A.A. Influence of hydrothermal conditions on the phase transformations of amorphous alumina. Mendeleev Commun. 2021, 31, 385–387. [Google Scholar] [CrossRef]
- Pavlova, I.S.; Pervova, I.G.; Belov, G.P.; Khasbiullin, I.I.; Slepukhin, P.A. Ethylene oligomerization mediated by iron formazanates and organoaluminum compounds. Pet. Chem. 2013, 53, 127–133. [Google Scholar] [CrossRef]
- Sharbatdaran, M.; Amini, M.M.; Majdabadi, A. Effect of aluminium alkoxide with donor-functionalized group on texture and morphology of the alumina prepared by sol-gel processing. Mater. Lett. 2010, 64, 503–505. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, S.; Liu, H. Size-controlled synthesis of alumina nanoparticles through an additive-free reverse cation-anion double hydrolysis method. Mater. Lett. 2015, 161, 720–723. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Elsevier: Amsterdam, The Netherlands, 1990; pp. 21–297. [Google Scholar] [CrossRef]
- Thiruchitrambalam, M.; Palkar, V.R.; Gopinathan, V. Hydrolysis of aluminium metal and sol-gel processing of nano alumina. Mater. Lett. 2004, 58, 3063–3066. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Faingol’D, E.E.; Babkina, O.N.; Saratovskikh, S.L.; Panin, A.N.; Zharkov, I.V.; Fushman, E.A. Syntheses of isobutylalumoxanes by triisobutylaluminum hydrolysis and their use as activators of dimethylated zirconocene in propylene polymerization. Russ. Chem. Bull. 2013, 62, 560–567. [Google Scholar] [CrossRef]
- Maryam, K.M.; Andrus, M.B.; Burt, S.R.; Woodfield, B.F. Generalized preparation method and characterization of aluminum isopropoxide, aluminum phenoxide, and aluminum n-hexyloxide. Polyhedron 2013, 62, 18–25. [Google Scholar] [CrossRef]
- Egorova, S.R.; Mukhamed’yarova, A.N.; Kurbangaleeva, A.Z.; Zhang, Y.; Lamberov, A.A. Formation of closed mesopores in boehmite during the phase transformation of gibbsite under hydrothermal conditions. React. Kinet. Mech. Catal. 2018, 125, 873–885. [Google Scholar] [CrossRef]
- Egorova, S.R.; Mukhamed’yarova, A.N.; Nesterova, O.V.; Zhang, Y.; Skibina, J.D.; Lamberov, A.A. Formation of phases and porous system in the product of hydrothermal treatment of χ-Al2O3. Coatings 2018, 8, 30. [Google Scholar] [CrossRef]
- Tsuchida, T. Hydrothermal synthesis of submicrometer crystals of boehmite. J. Eur. Ceram. Soc. 2000, 20, 1759–1764. [Google Scholar] [CrossRef]
- Danchevskaya, M.N.; Ivakin, Y.D.; Torbin, S.N.; Muravieva, G.P. The role of water fluid in the formation of fine-crystalline oxide structure. J. Supercrit. Fluids 2007, 42, 419–424. [Google Scholar] [CrossRef]
- Danilevich, V.V.; Stolyarova, E.A.; Vatutina, Y.V.; Gerasimov, E.Y.; Ushakov, V.A.; Saiko, A.V.; Klimov, O.V.; Noskov, A.S. Optimizing the Properties of an Alumina Support of Hydrotreating Catalysts by Introducing Boron and Sulfur at the Stage of Obtaining Pseudoboehmite by Hydrothermal Treatment of the Product Produced by Flash Calcination of Gibbsite. Catal. Ind. 2019, 11, 301–312. [Google Scholar] [CrossRef]
- Hakuta, Y.; Adschiri, T.; Hirakoso, H.; Arai, K. Chemical equilibria and particle morphology of boehmite (AlOOH) in sub and supercritical water. Fluid Phase Equilib. 1999, 158–160, 733–742. [Google Scholar] [CrossRef]
- Tsukada, T.; Segawa, H.; Yasumori, A.; Okada, K. Crystallinity of boehmite and its effect on the phase transition temperature of alumina. J. Mater. Chem. 1999, 9, 549–553. [Google Scholar] [CrossRef]
- Musić, S.; Dragčević, D.; Popović, S. Hydrothermal crystallization of boehmite from freshly precipitated aluminum hydroxide. Mater. Lett. 1999, 40, 269–274. [Google Scholar] [CrossRef]
- Guzmán-Castillo, M.L.; López-Salinas, E.; Fripiat, J.J.; Sánchez-Valente, J.; Hernández-Beltrán, F.; Rodríguez-Hernández, A.; Navarrete-Bolaños, J. Active sulfated alumina catalysts obtained by hydrothermal treatment. J. Catal. 2003, 220, 317–325. [Google Scholar] [CrossRef]
- Mishra, D.; Anand, S.; Panda, R.K.; Das, R.P. Hydrothermal preparation and characterization of boehmites. Mater. Lett. 2000, 42, 38–45. [Google Scholar] [CrossRef]
- Bokhimi, X.; Sánchez-Valente, J.; Pedraza, F. Crystallization of sol-gel boehmite via hydrothermal annealing. J. Solid State Chem. 2002, 166, 182–190. [Google Scholar] [CrossRef]
- Gevert, B.S.; Ying, Z.S. Formation of fibrillar boehmite. J. Porous Mater. 1999, 6, 63–67. [Google Scholar] [CrossRef]
- Mukhambetov, I.N.; Egorova, S.R.; Mukhamed’yarova, A.N.; Lamberov, A.A. Hydrothermal modification of the alumina catalyst for the skeletal isomerization of n-butenes. Appl. Catal. A Gen. 2018, 554, 64–70. [Google Scholar] [CrossRef]
- Khan, N.A.; Hwang, J.S.; Jhung, S.H. Liquid-phase dehydration of 1-phenylethanol to styrene over an acidic resin catalyst. Bull. Korean Chem. Soc. 2011, 32, 1327–1330. [Google Scholar] [CrossRef][Green Version]
- Chaichana, E.; Boonsinvarothai, N.; Chitpong, N.; Jongsomjit, B. Catalytic dehydration of ethanol to ethylene and diethyl ether over alumina catalysts containing different phases with boron modification. J. Porous Mater. 2019, 26, 599–610. [Google Scholar] [CrossRef]
- Makgoba, N.P.; Sakuneka, T.M.; Koortzen, J.G.; Van Schalkwyk, C.; Botha, J.M.; Nicolaides, C.P. Silication of γ-alumina catalyst during the dehydration of linear primary alcohols. Appl. Catal. A Gen. 2006, 297, 145–150. [Google Scholar] [CrossRef]
- Mukhamediarova, A.N.; Boretsky, K.S.; Egorova, S.R.; Lamberov, A.A. Investigation of the catalytic properties in the dehydration of 1-phenylethanol in styrene process of an amorphous aluminum hydroxyl gel after its heat treatments. J. Phys. Conf. Ser. 2019, 1347. [Google Scholar] [CrossRef]
- Haider, M.S.; Isik, M.A.; Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Demineralization of Miscanthus Biocrude Obtained from Catalytic Hydrothermal Liquefaction: Conditioning through Acid Washing. Processes 2021, 9, 1035. [Google Scholar] [CrossRef]
- Yang, S.; Chen, G.; Guan, Q.; Xu, H.; Wang, Z.; Liu, B.; Yang, S.; Lei, T.; Zeng, X.; Lin, L. An efficient Pd/carbon-silica-alumina catalyst for the hydrodeoxygenation of bio-oil model compound phenol. Mol. Catal. 2021, 510, 111681. [Google Scholar] [CrossRef]
- Boretskaya, A.; Il’yasov, I.; Egorova, S.; Popov, A.; Lamberov, A. Modification of a phase-inhomogeneous alumina support of a palladium catalyst. Part I: Effect of the amorphous phase on the textural and acidic characteristics of alumina and methods for controlling its phase homogeneity. Mater. Today Chem. 2020, 18, 100371. [Google Scholar] [CrossRef]
- De la Cruz Parejas, R.; Moura, F.J.; de Avillez, R.R.; de Souza Mendes, P.R. Effects of Al2O3-NiO, TiO2 and (Mg,Ni)O particles on the viscosity of heavy oil during aquathermolysis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625. [Google Scholar] [CrossRef]
- Yenumala, S.R.; Kumar, P.; Maity, S.K.; Shee, D. Hydrodeoxygenation of karanja oil using ordered mesoporous nickel-alumina composite catalysts. Catal. Today 2020, 348, 45–54. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Grigor’eva, N.G.; Danilova, I.G.; Magaev, O.V.; Vodyankina, O.V. Template-free sol-gel synthesis of catalytically active mesoporous aluminosilicates. Kinet. Catal. 2015, 56, 501–508. [Google Scholar] [CrossRef]
- Newell, A.; Thampi, K.R. Novel amorphous aluminum hydroxide catalysts for aluminum–water reactions to produce H2 on demand. Int. J. Hydrog. Energy 2017, 42, 23446–23454. [Google Scholar] [CrossRef]
- Sviridenko, N.N.; Vosmerikov, A.V.; Agliullin, M.R.; Kutepov, B.I. General Features of Catalytic Upgrading of Karmalskoe Heavy Oil in the Presence of Amorphous Aluminosilicates. Pet. Chem. 2020, 60, 384–391. [Google Scholar] [CrossRef]
- Nakano, K.; Ali, S.A.; Kim, H.J.; Kim, T.; Alhooshani, K.; Park, J.-I.; Mochida, I. Deep desulfurization of gas oil over NiMoS catalysts supported on alumina coated USY-zeolite. Fuel Process. Technol. 2013, 116, 44–51. [Google Scholar] [CrossRef]
- Salimi, M.; Tavasoli, A.; Rosendahl, L. Optimization of γ-Alumina porosity via Response Surface Methodology: The influence of engineering support on the performance of a residual oil hydrotreating catalyst. Microporous Mesoporous Mater. 2020, 299, 110124. [Google Scholar] [CrossRef]
- Yenumala, S.R.; Kumar, P.; Maity, S.K.; Shee, D. Production of green diesel from karanja oil (Pongamia pinnata) using mesoporous NiMo-alumina composite catalysts. Bioresour. Technol. Rep. 2019, 7, 100288. [Google Scholar] [CrossRef]
- Radchenko, E.D.; Melik-Akhnazarov, T.K.; Kaminskii, E.F.; Khavkin, V.A.; Nefedov, B.K.; Freiman, L.L.; Kurganov, V.M.; Bichkova, D.M.; Lochenkova, I.N. Sposo, Oblagorazhivaniya Benzinovikh Distillyatov. Patent RU 2 087 524 C1, 20 August 1997. [Google Scholar]
- Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Vakhin, A.V.; Sitnov, S.A.; Akhmadiayrov, A.A.; Varfolomeev, M.A.; Nurgaliev, D.K. Catalytic heavy oil upgrading by steam injection with using of transition metals catalysts. Oil Ind. 2017, 8, 30–34. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Gafurov, M.R.; Morozov, O.G.; Nasybullin, A.R.; Karandashov, S.A.; Ponomarev, A.A.; Krapivnitskaia, T.O.; Glyavin, M.Y.; et al. The Role of Nanodispersed Catalysts in Microwave Application during the Development of Unconventional Hydrocarbon Reserves: A Review of Potential Applications. Processes 2021, 9, 420. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-situ heavy oil aquathermolysis in the presence of nanodispersed catalysts based on transition metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Extra-heavy Oil Aquathermolysis Using Nickel-based Catalyst: Some Aspects of in-situ Transformation of Catalyst Precursor. Catalysts 2021, 11, 189. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Sharifullin, A.V.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Catalytic aquathermolysis of boca de jaruco heavy oil with nickel-based oil-soluble catalyst. Processes 2020, 8, 532. [Google Scholar] [CrossRef]
- Rajasuriyan, S.; Mohd Zaid, H.F.; Majid, M.F.; Ramli, R.M.; Jumbri, K.; Lim, J.W.; Mohamad, M.; Show, P.L.; Yuliarto, B. Oxidative Extractive Desulfurization System for Fuel Oil Using Acidic Eutectic-Based Ionic Liquid. Processes 2021, 9, 1050. [Google Scholar] [CrossRef]
- Fan, H.F.; Liu, Y.J.; Zhao, X.F.; Zhong, L.G. Studies on effect of metal ions on aquathermolysis reaction of Liaohe heavy oils under steam treatment. J. Fuel Chem. Technol. 2001, 29, 430–433. [Google Scholar]

| Precipitation Conditions | Morphology | Phase Composition | |||
|---|---|---|---|---|---|
| Initial Compounds | pH | T, °C | τ, h | ||
| Al(NO3)3 | 7 | 20 | 0 | spheres irregular shape | amorphous phase |
| 7 | 70 | 1 | small spheres | pseudoboehmite | |
| 7 | 70 | 36 | needles | pseudoboehmite | |
| NaAlO2 | 7 | 70 | 100 | needles | pseudoboehmite |
| Al(NO3)3 | 11 | 20 | 96 | rods and prisms | bayerite |
| NaAlO2 | 11 | 20 | 100 | rods and prisms | bayerite |
| pH | Aging, h (hour) | Chemical Composition | Phase Composition |
|---|---|---|---|
| 6 | 0 or 240 | Al2O3·1.77 H2O·0.50 NO3 | Amorphous |
| 7 | 0 or 100 | Al2O3·1.80 H2O·0.35 NO3 | Amorphous |
| 9 | 0 | Al2O3·2.00 H2O·0.15 NO3 | Amorphous |
| 20 | Al2O3·1.60 H2O·0.02 NO3 | Boehmite | |
| 70 | Al2O3·2.20 H2O | Boehmite/Bayerite | |
| 10 | 0 | Al2O3·2.00 H2O·0.06 NO3 | Amorphous |
| 1.5 | Al2O3·1.80 H2O·0.05 NO3 | Boehmite | |
| 70 | Al2O3·2.40 H2O | Boehmite/Bayerite | |
| 11 | 0 | Al2O3·2.00 H2O·0.01 NO3 | Amorphous |
| 3 | Al2O3·1.70 H2O·0.003 NO3 | Boehmite | |
| 70 | Al2O3·2.50 H2O | Boehmite/Bayerite |
| Mode of Production | Phase Composition,% | S, m2/g | V, sm3/g | D, nm | ||
|---|---|---|---|---|---|---|
| Pb | Gb (Br) | Am | ||||
| Aluminate-nitrate (Angarsk) | 85 | 5 | 10 | 255 | 0.58 | 4.6 |
| Nitrate (Novokuibyshevsk) | 95 | 15 | - | 190 | 0.36 | 7.7 |
| Nitrate (Ryazan) | 100 | - | - | 240 | 0.45 | 3.8 |
| Aluminate-sulfate (Angarsk) | 70 | 15 | 15 | 247 | 0.60 | 4.8 |
| Aluminate-sulfonic acid (Omsk) | 75 | 10 | 15 | 220 | 0.58 | 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhamed’yarova, A.N.; Gareev, B.I.; Nurgaliev, D.K.; Aliev, F.A.; Vakhin, A.V. A Review on the Role of Amorphous Aluminum Compounds in Catalysis: Avenues of Investigation and Potential Application in Petrochemistry and Oil Refining. Processes 2021, 9, 1811. https://doi.org/10.3390/pr9101811
Mukhamed’yarova AN, Gareev BI, Nurgaliev DK, Aliev FA, Vakhin AV. A Review on the Role of Amorphous Aluminum Compounds in Catalysis: Avenues of Investigation and Potential Application in Petrochemistry and Oil Refining. Processes. 2021; 9(10):1811. https://doi.org/10.3390/pr9101811
Chicago/Turabian StyleMukhamed’yarova, Aliya N., Bulat I. Gareev, Danis K. Nurgaliev, Firdavs A. Aliev, and Alexey V. Vakhin. 2021. "A Review on the Role of Amorphous Aluminum Compounds in Catalysis: Avenues of Investigation and Potential Application in Petrochemistry and Oil Refining" Processes 9, no. 10: 1811. https://doi.org/10.3390/pr9101811
APA StyleMukhamed’yarova, A. N., Gareev, B. I., Nurgaliev, D. K., Aliev, F. A., & Vakhin, A. V. (2021). A Review on the Role of Amorphous Aluminum Compounds in Catalysis: Avenues of Investigation and Potential Application in Petrochemistry and Oil Refining. Processes, 9(10), 1811. https://doi.org/10.3390/pr9101811










