Raw Glycerol Based Medium for DHA and Lipids Production, Using the Marine Heterotrophic Microalga Crypthecodinium cohnii
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. cohnii Starter Cultures
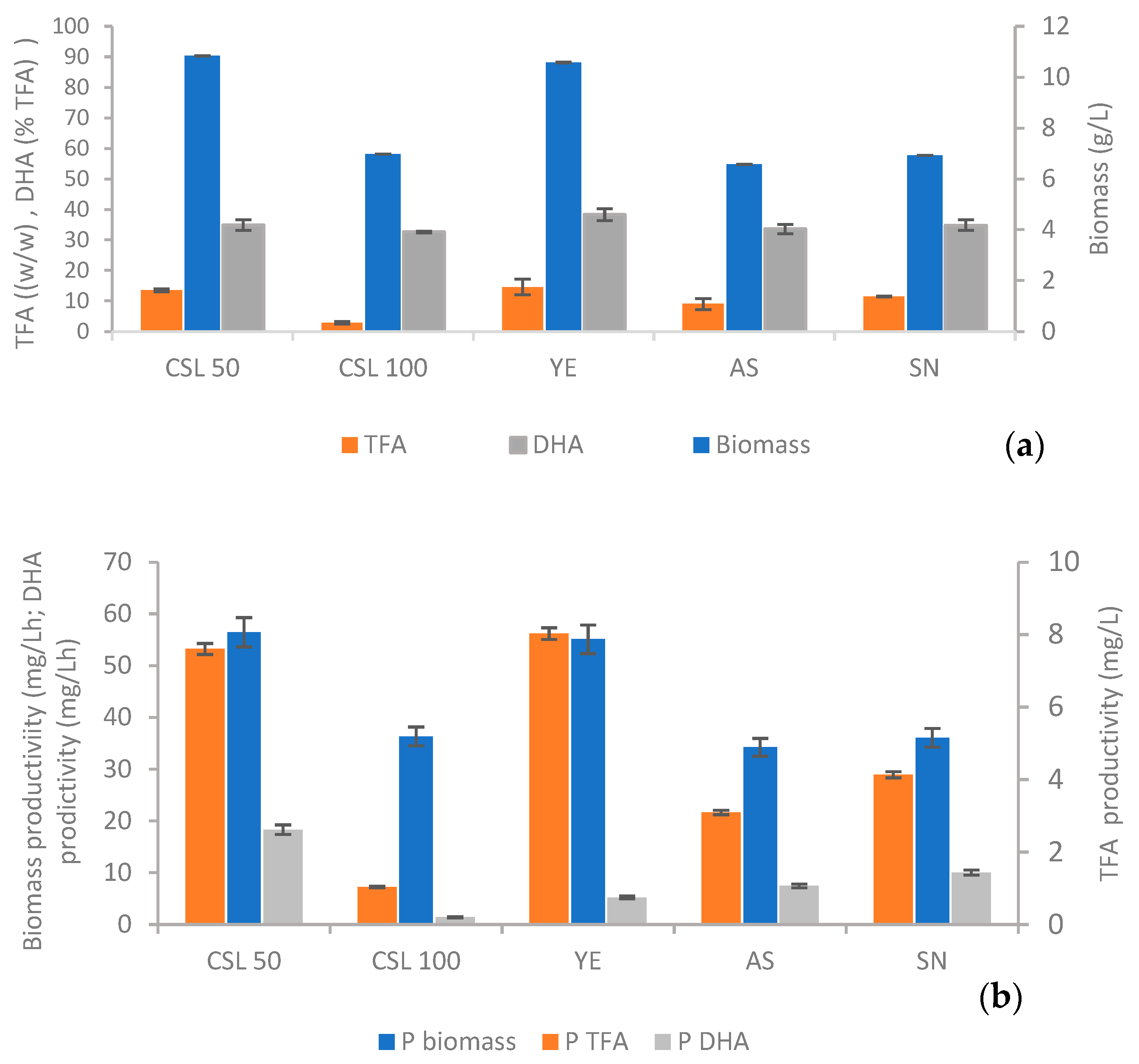
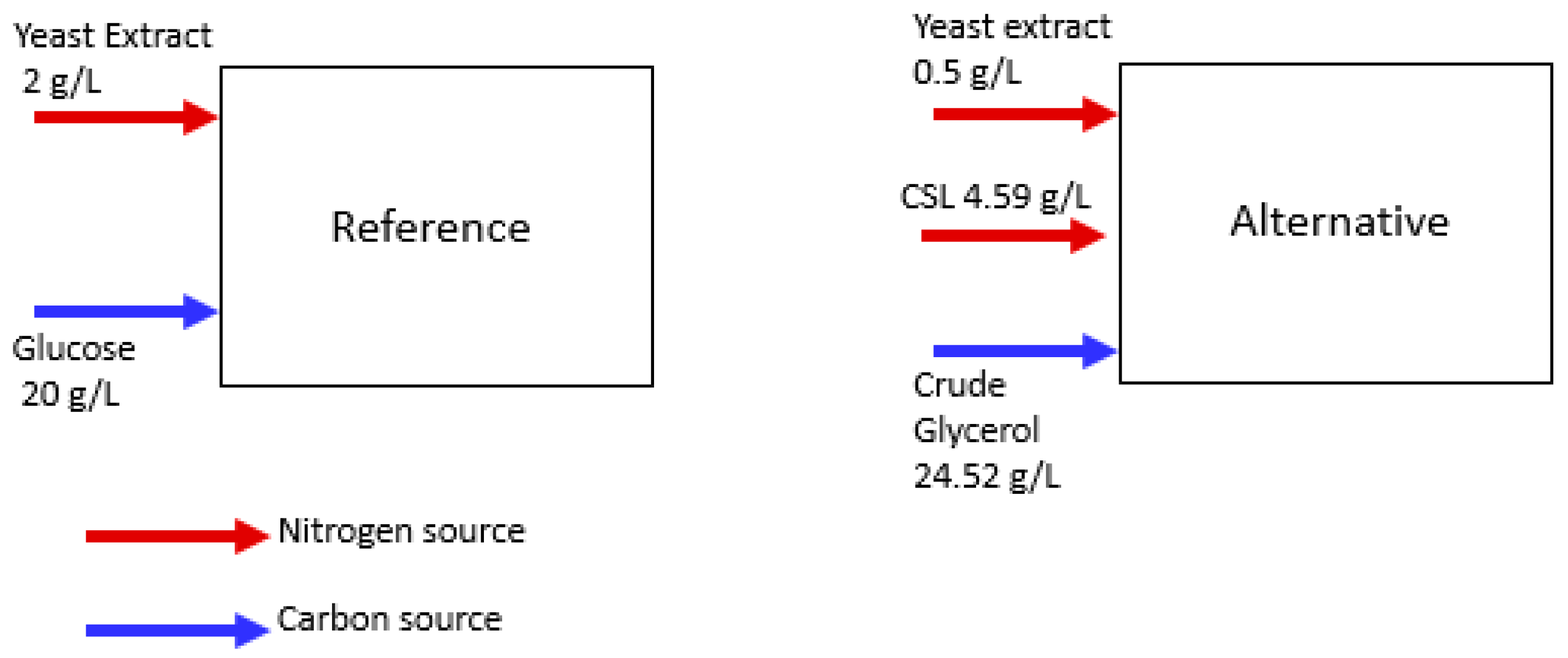
2.2. Shake Flasks Experiments—Selection of a Low-Cost Nitrogen Source
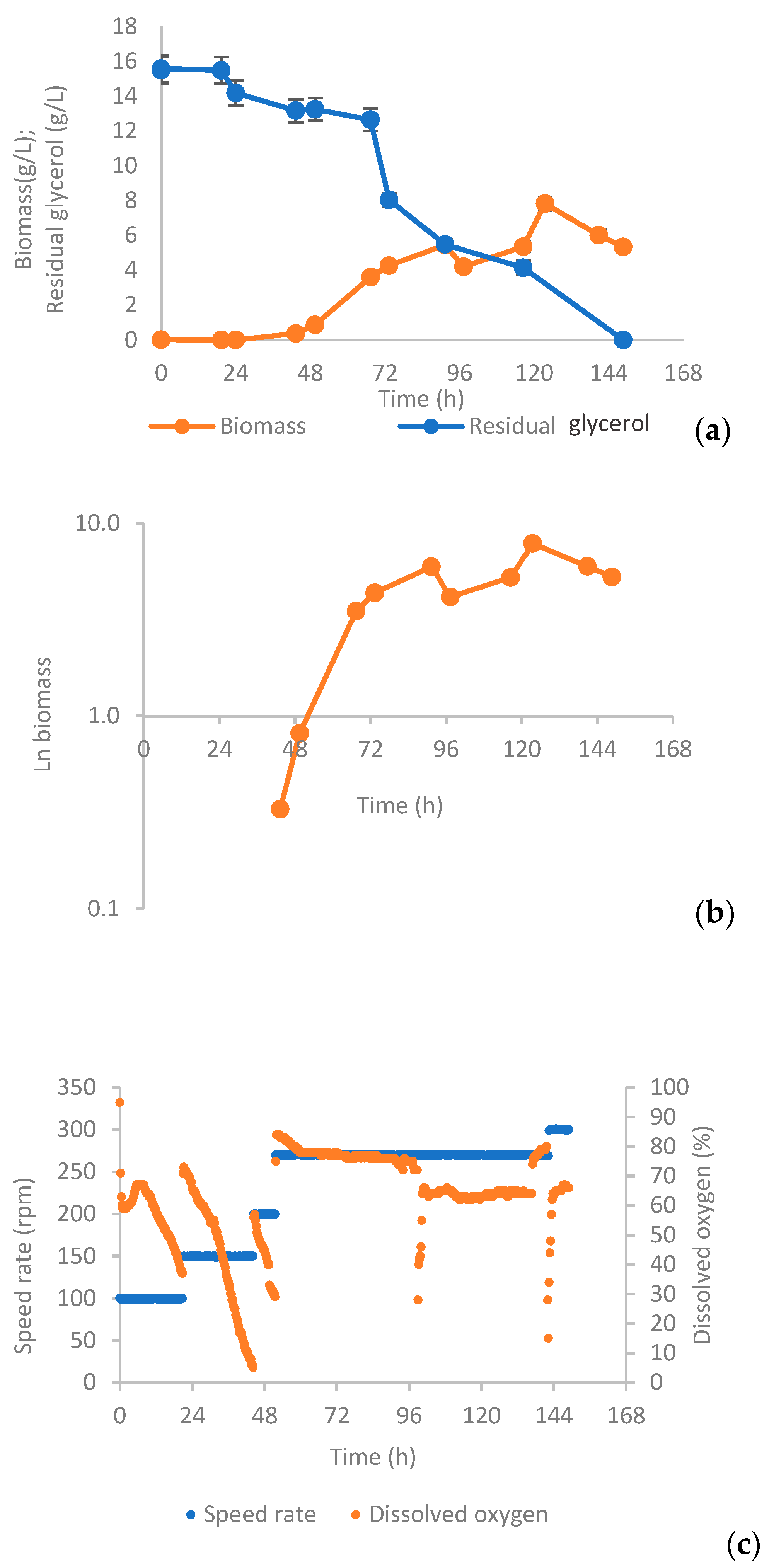
2.3. Bioreactor Experiments Using Raw Glycerol-Based Medium
2.4. Growth Measurement
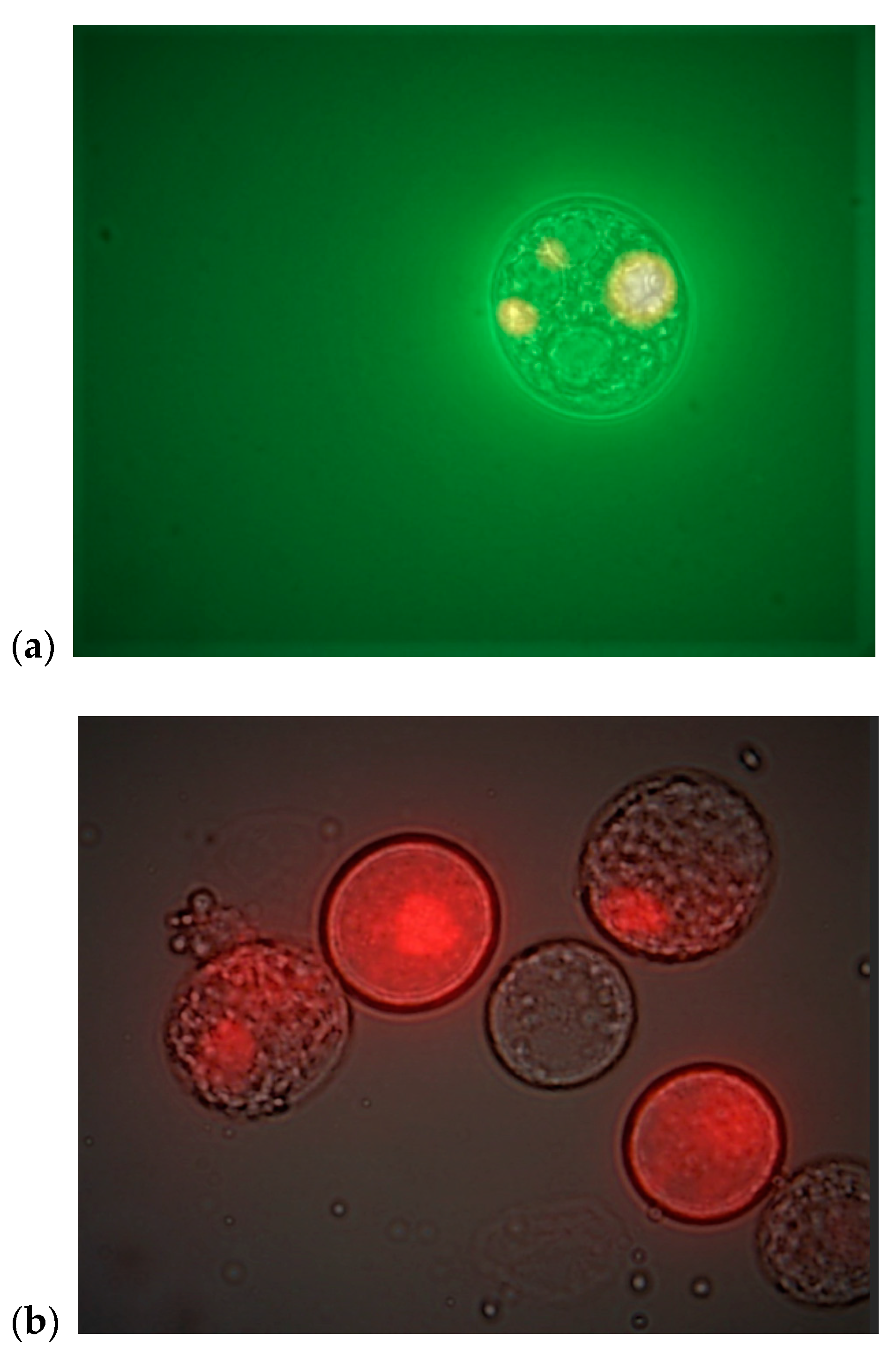
2.5. Flow Cytometry
2.6. Lipid Quantification
3. Results and Discussion
3.1. Nitrogen Source Selection Assays
3.2. Bioreactor Experiments
3.2.1. C. cohnii Cells Flow Cytometric Analysis
3.2.2. Growth
3.2.3. C. cohnii FC Analysis
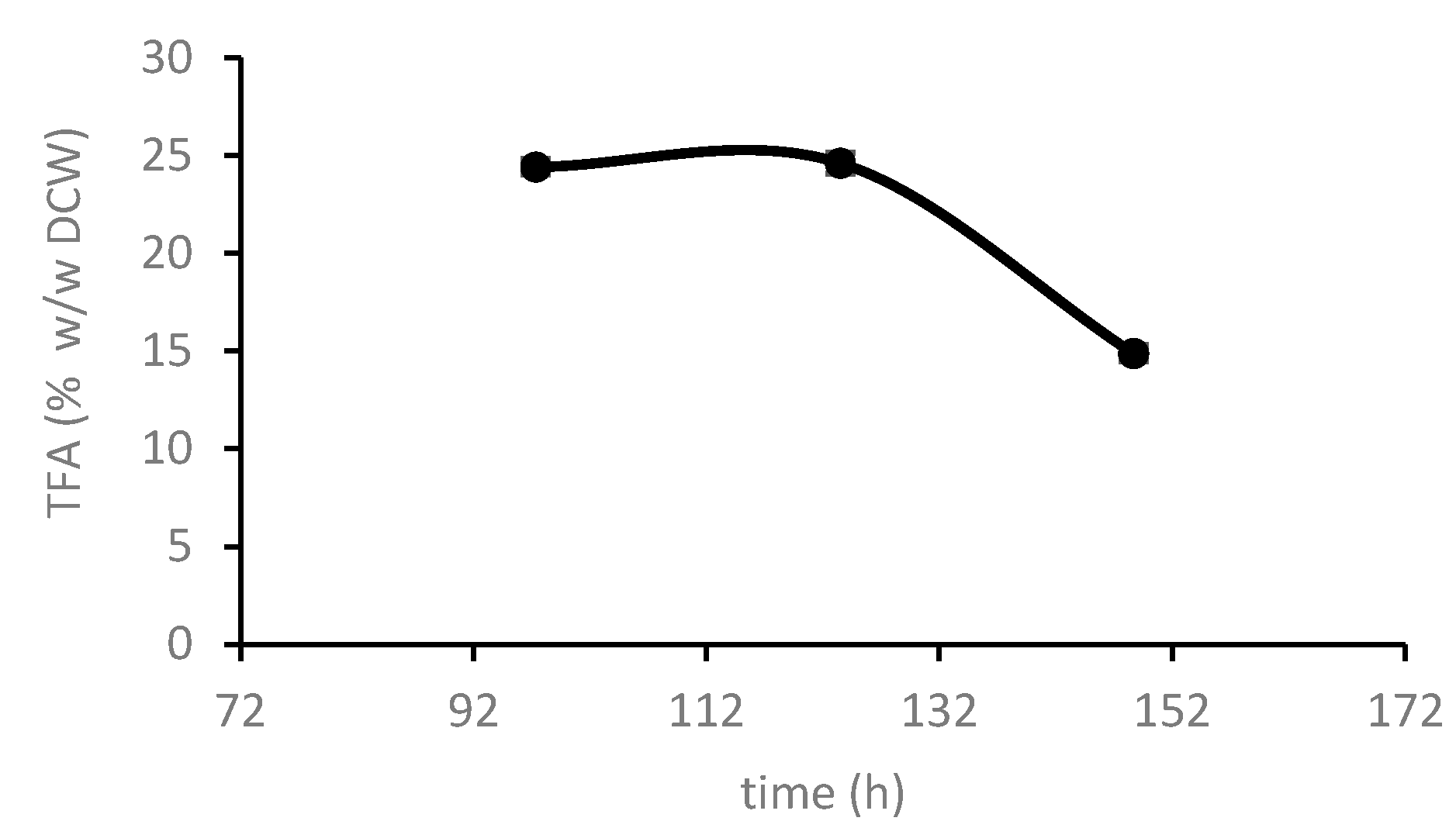
3.2.4. C. cohnii Lipid Production
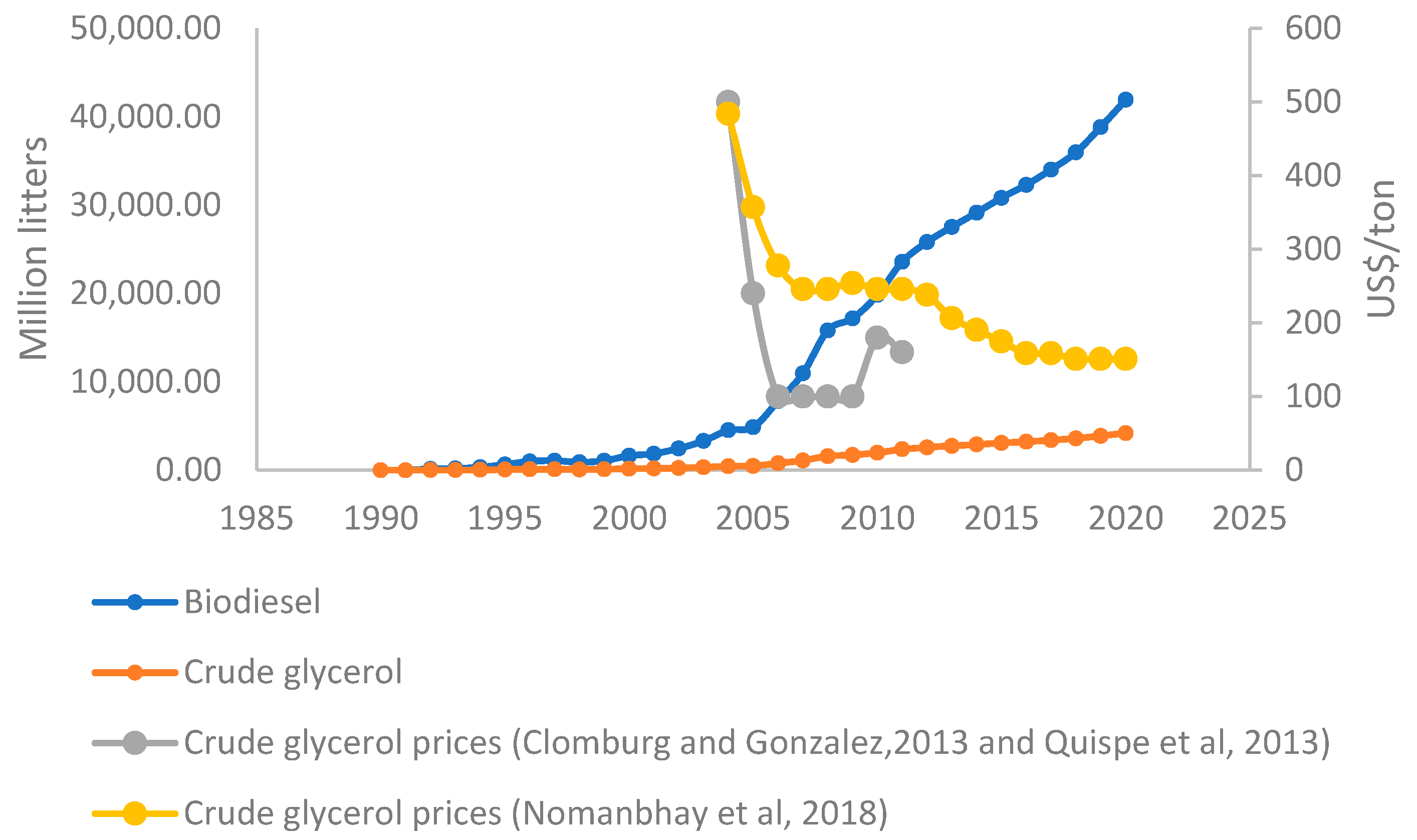
3.3. Potential Costs Impact in Up-Scaled Systems
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendes, A.; da Silva, T.L.; Reis, A. DHA concentration and purification from the marine heterotrophic microalga Crypthecodinium cohnii CCMP 316 by winterization and urea complexation. Food Technol. Biotechnol. 2007, 45, 38–44. [Google Scholar]
- Choi, H.; Yu, S. Influence of crude glycerol on the biomass and lipid content of microalgae. Biotechnol. Biotechnol. Equip. 2015, 29, 506–513. [Google Scholar] [CrossRef]
- Isleten-Hosoglu, M.; Elibol, M. Improvement of medium composition and cultivation conditions for growth and lipid production by Crypthecodinium cohnii. Rom. Biotechnol. Lett. 2017, 22, 13086–13095. [Google Scholar]
- Isleten-Hosoglu, M.; Elibol, M. Bioutilization of Cheese Whey and Corn Steep Liquor by Heterotrophic Microalgae Crypthecodinium cohnii for Biomass and Lipid Production. Acad. Food J./Akademik GIDA 2017, 15, 233–241. [Google Scholar]
- Song, O.; Kuryatov, A.; Axelsen, P.H. A new synthetic medium for the optimization of docosahexaenoic acid production in Crypthecodinium cohnii. PLoS ONE 2020, 15, e0229556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Swaaf, M.E.; Rijk, T.C.; Eggink, G.; Sijtsma, L. Optimization of docosahexaenoic acid production in batch cultivations by Crypthecodinium cohnii. J. Biotechnol. 1999, 70, 185–192. [Google Scholar] [CrossRef]
- De Swaaf, M.E.; Pronk, J.T.; Sijtsma, L. Fed-batch cultivation of docosahexaenoic-acid-producing marine alga Crypthecodinium cohnii on ethanol. Appl. Microbiol. Biotechnol. 2003, 61, 40–43. [Google Scholar] [CrossRef]
- Ratledge, C.; Kanagachandran, K.; Abdersin, A.J.; Grantham, D.J.; Stephenson, J.C. Production of docosahexaenoic acid by Crypthecodinium cohnii with acetic acid grown in a pH-auxostat culture with acetic acid as principal carbon source. Lipids 2001, 36, 1241–1246. [Google Scholar] [CrossRef]
- Alibaba.com. Available online: www.alibaba.com (accessed on 30 June 2021).
- Taborda, T.; Moniz, P.; Reis, A.; Lopes da Silva, T. Evaluating low-cost substrates for Crypthecodnium cohnii lipids and DHA production, by flow cytomtery. J. Appl. Phycol. 2020, 33, 263–274. [Google Scholar] [CrossRef]
- Lopes da Silva, T.; Moniz, P.; Silva, C.; Reis, A. The dark side of microalgae Biotechnology: A heterotrophic biorefinery platform directed to ω-3 lipid production. Microorganisms 2019, 7, 670. [Google Scholar] [CrossRef] [Green Version]
- Yeung, P.K.K.; Wong, J.T.Y. Inhibition of cell proliferation by mechanical agitation involves transient cell cycle arrest at G1 phase in dinoflagellates. Protoplasma 2003, 9, 1–13. [Google Scholar]
- Andrade, R.; Leal, R.; Roseiro, J.; Reis, A.; Lopes da Silva, T. Monitoring Rhodosporidium toruloides NCYC 921 batch fermentations growing under carbon and nitrogen limitation by flow cytometry. World J. Microbiol. Biotechnol. 2012, 28, 1175–1184. [Google Scholar] [CrossRef]
- Safdar, W.; Zan, X.; Song, Y. Synergistic effects of pH, temperature and agitation on growth kinetics and docosahexaenoic acid production of C. cohnii cultures on different carbon sources. Int. J. Res. Agric. Sci. 2017, 4, 2348–3997. [Google Scholar]
- Lopes da Silva, T.; Santos, A.R.; Gomes, R.; Reis, A. Valorizing fish canning industry by-products to produce ω-3 compounds and biodiesel. Environm. Technol. Innov. 2018, 8, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Cavka, A.; Martín, C.; Alriksson, B.; Mörtsell, M.; Jönsson, L. Techno-economic evaluation of conditioning with sodium sulfite for bioethanol production from softwood. Bioresour. Technol. 2015, 196, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Future Markets Consultancy. Available online: https://www.futuremarketinsights.com/reports/dry-yeast-market (accessed on 14 September 2021).
- Lynch, M.D. The bioprocess TEA calculator: An online technoeconomic analysis tool to evaluate the commercial competitiveness of potential bioprocesses. Metab. Eng. 2021, 65, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, P.; Kennedy, P.; Ferreira, C. An Estimation of a Price Model of the High Fructose Corn Syrup Industry in the Unites States. Selected Paper Prepared for Presentation at the Southern Agricultural Economics Association (SAEA) Annual Meeting, Mobile, Alabama, 4–7 February 2017. Available online: https://ageconsearch.umn.edu/record/252722?ln=en (accessed on 14 September 2021).
- Galitsky, C.; Worrell, E.; Ruth, M. Energy Efficiency Improvement and Cost Saving Opportunities for the Corn Wet Milling Industry. Report Funded by the U.S. Environmental Protection Agency’s Climate Protection Partnerships Division as Part of ENERGY STAR. 2003. Available online: https://www.energystar.gov/sites/default/files/buildings/tools/LBNL-52307.pdf (accessed on 14 September 2021).
- Available online: https://www.marketintellica.com/report/MI49662-global-corn-steep-liquor-csl-market# (accessed on 14 September 2021).
- Tan, J.P.; Jahim, J.M.; Wu, T.Y.; Harun, S.; Mumtaz, T. Use of corn steep liquor as an economical nitrogen source for biosuccinic acid production by Actinobacillus succinogenes. Int. Conf. Chem. Eng. Bioproc. Eng. 2016, 26, 01258. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.; Runcang, S. Value-added used for crude glycerol—A byproduct of biodiesel production. Biotechnol. Fuels 2012, 5, 13. [Google Scholar]
- Quispe, C.; Coronado, C.; Carvalho, J. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Ren. Sust. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Hussein, R.; Ong, M. Sustainability of biodiesel production in Malaysia by production of bio-oil from crude glycerol using microwave pyrolysis: A review. Green Chem. Let. Rev. 2017, 11, 137–157. [Google Scholar] [CrossRef]
- Clomburg, J.; Gonzalez, R. Anaerobic fermentation of glycerol: A platform for renewable fuels and chemicals. Trends Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Near-Term Transportation Sector Electrification Program. Available online: https://www.iea.org/policies/598-near-term-transportation-sector-electrification-program (accessed on 14 September 2021).


| Water Content (% w/w) | Glycerol Content (% w/w) | Ash Content (% w/w) | Methanol Content (% w/w) | Matter Organic Non-Glycerol (% w/w) | Sodium (% w/w) | Potassium (mg/kg) |
|---|---|---|---|---|---|---|
| 9.90 | 83.8 | 6.54 | 0.007 | n.d. | 1.65 | 8.35 |
| Cultivation Time (h) | |||
|---|---|---|---|
| FAME (% w/w TFA) | t = 97.33 | t = 123.50 | t = 148.67 |
| 10:0 | 1.10 ± 0.02 | 1.28 ± 0.02 | 1.03 ± 0.08 |
| 12:0 | 8.42 ± 0.18 | 8.83 ± 0.00 | 8.03 ± 0.08 |
| 14:0 | 20.18 ± 0.53 | 19.95 ± 0.07 | 19.44 ± 0.39 |
| 16:0 | 17.76 ± 0.45 | 16.71 ± 0.00 | 16.36 ± 0.54 |
| 16:1ω9 | 2.84 ± 0.10 | 3.01 ± 0.02 | 2.97 ± 0.01 |
| 18:0 | 0.65 ± 0.00 | 0.51 ± 0.00 | 0.47 ± 0.02 |
| 18:1ω9 | 10.85 ± 0.28 | 10.67 ± 0.14 | 10.90 ± 0.08 |
| 18:2ω6 | n.d. | 0.06 ± 0.030 | 0.04 ± 0.00 |
| 22:5ω3 | 0.46 ± 0.09 | 0.56 ± 0.02 | 0.64 ± 0.00 |
| 22:6ω3 (DHA) | 37.78 ± 1.48 | 39.58 ± 1.41 | 40.09 ± 0.68 |
| SFA | 48.11 | 47.28 | 45.33 |
| MUFA | 13.69 | 13,68 | 13.87 |
| PUFA | 38.24 | 40.20 | 40.77 |
| Item/Price | Min USD/ton | Max USD/ton |
|---|---|---|
| Yeast extract | 1000 | 7286 |
| Glucose | 100 | 1200 |
| Glycerol | 100 | 500 |
| CSL | 1.5 | 200 |
| Reference (USD/L Bioreactor) | Alternative (USD/L Bioreactor) | Absolute Savings (USD/L Bioreactor) | Relative Savings (%) | |
|---|---|---|---|---|
| Minimum | 0.004 | 0.01 | −0.01 | −242 |
| Maximum | 0.039 | 0.006 | 0.032 | 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moniz, P.; Silva, C.; Oliveira, A.C.; Reis, A.; Lopes da Silva, T. Raw Glycerol Based Medium for DHA and Lipids Production, Using the Marine Heterotrophic Microalga Crypthecodinium cohnii. Processes 2021, 9, 2005. https://doi.org/10.3390/pr9112005
Moniz P, Silva C, Oliveira AC, Reis A, Lopes da Silva T. Raw Glycerol Based Medium for DHA and Lipids Production, Using the Marine Heterotrophic Microalga Crypthecodinium cohnii. Processes. 2021; 9(11):2005. https://doi.org/10.3390/pr9112005
Chicago/Turabian StyleMoniz, Patrícia, Carla Silva, Ana Cristina Oliveira, Alberto Reis, and Teresa Lopes da Silva. 2021. "Raw Glycerol Based Medium for DHA and Lipids Production, Using the Marine Heterotrophic Microalga Crypthecodinium cohnii" Processes 9, no. 11: 2005. https://doi.org/10.3390/pr9112005






